Abstract
There appears to be increased use of computed tomography angiography (CTA) in the preoperative planning of autologous perforator flap breast reconstruction. Despite the advantages of providing superior anatomical detail, concerns regarding cost and radiation exposure of this technique remain. In the current study, a paper-based survey was distributed to 44 plastic surgeons with a special interest in breast reconstruction at 19 different centres across Canada to collect their perspectives and practice characteristics with respect to the use of CTA as a preoperative imaging modality in breast reconstruction. The response rate of the survey was 75%. The majority of respondents commonly use perforator flap breast reconstruction and CTA in their breast reconstruction practice. Surgeons identified particular benefits of CTA in patients who had previously undergone abdominal surgery. However, more than one-half of the overall cohort was concerned about radiation exposure associated with CTA. A review of the literature suggests that it may be worthwhile to reduce the unnecessary risks of additional radiation exposure to the breast cancer population. A prospective study may help to better define the group of patients in whom CTA will provide optimal benefits in terms of reducing perioperative microvascular morbidity.
Keywords: Breast reconstruction, Computed tomography angiography, Perforator flap
Abstract
On semble utiliser davantage l’angiographie par tomodensitométrie (ATD) lors de la planification préopératoire d’une reconstruction mammaire au moyen d’un lambeau perforant autologue. Malgré les avantages associés à une meilleure précision anatomique, on s’inquiète du coût de cette technique et de l’exposition aux radiations qu’elle entraîne. Dans la présente étude, 44 chirurgiens plasticiens ayant un intérêt pour la reconstruction mammaire provenant de 19 centres du Canada ont reçu un sondage papier afin de colliger leurs points de vue et les caractéristiques de leur pratique à l’égard de l’utilisation de l’ATD comme modalité d’imagerie en prévision d’une reconstruction mammaire. Le sondage a obtenu un taux de réponse de 75 %. La majorité des répondants utilisent souvent la reconstruction mammaire par lambeau perforant et l’ATD dans le cadre de leur pratique de reconstruction mammaire. Les chirurgiens ont souligné les avantages particuliers de l’ATD chez les patients qui avaient déjà subi une opération abdominale. Cependant, plus de la moitié de l’ensemble de la cohorte s’inquiétait de l’exposition aux radiations associée à l’ATD. D’après l’analyse bibliographique, il pourrait être avantageux de réduire les risques inutiles liés à une exposition supplémentaire aux radiations de la population atteinte d’un cancer du sein. Une étude prospective pourrait contribuer à mieux définir le groupe de patients chez qui l’ATD réduit de manière optimale la morbidité microvasculaire périopératoire.
Autologous breast reconstruction is considered by many to be the most elegant method of breast reconstruction. Over the past two decades, free tissue transfer has increasingly been used to achieve this goal; however, free tissue transfer carries the additional risk of micro-vascular compromise. Many approaches have been taken to minimize the risk of microsurgical complications including improved technique, use of the internal mammary artery as a recipient vessel and better preoperative imaging of the vascular supply of the flaps (1–4). Perforator flaps are used with increasing frequency for autologous breast reconstruction and some authors advocate that these types of flaps be the preferred approach to autologous microvascular breast reconstruction because they reduce donor site morbidity (1–4). However, achieving these benefits requires navigating the challenges of the variable anatomy of the perforator vessels (2,4). A perforator with sufficient calibre and length, minimum intramuscular course and a perpendicular fascia penetration pattern is considered to be ideal for microsurgical dissection (1,2). Until recently, Doppler ultrasonography (US) has been the standard pre-/intraoperative imaging modality used in selecting the dominant perforator(s) (2,4,5). Doppler US is a simple, noninvasive procedure that does not involve intravenous contrast or radiation exposure. Unfortunately, it is time consuming and operator dependent. In addition, Doppler US lacks the resolution to predict important vessel characteristics for flap viability and has low sensitivity for detecting vessels such as the superficial inferior epigastric perforator (5). These limitations of Doppler US led to the introduction of computed tomography angiography (CTA) in preoperative planning for flap reconstruction.
Unfortunately, the increased use of CTA is not without drawbacks. Unlike Doppler US, CTA involves the use of intravenous contrast and exposes patients to a radiation dose equivalent to approximately 6 mSV to 10 mSV, corresponding to a dose of 2.5 years of background environmental radiation or 300 to 500 chest x-rays (6). In addition, with shrinking resources in the health system, added attention should be devoted to cost-benefit aspects of each diagnostic and therapeutic intervention. As the approach to optimal perforator breast reconstruction continues to advance, developing a consensus guideline on the responsible use of CTA as a preoperative planning tool would be valuable. As a start, we report on a survey of the current practice attitudes toward CTA use among plastic surgeons in Canada who have a special interest in breast reconstruction.
METHODS
A paper-based survey regarding the use of CTA as a preoperative planning tool in perforator flap breast reconstruction was distributed to 44 plastic surgeons in 19 different cities across Canada. The survey was conducted between August and December 2011. Chiefs and/or program directors of academic centres in Canada, with the exception of Quebec, were contacted by e-mail by the senior author and asked to compile a list of surgeons in their region who have a specialty focus on autologous breast reconstruction. This list was supplemented with a review of the Canadian Society of Plastic Surgeons database and a review of Canadian surgeons who had published on breast reconstruction in the past. This final list of individuals was compiled and contacted to participate. All physicians were listed as active members in the Canadian Society of Plastic Surgeons database.
Survey
The survey was developed following a comprehensive review of the literature regarding the use of preoperative imaging modalities in autologous breast reconstruction. Questions were formulated around characterizing three main areas: surgeon demographic information, respondents’ breast reconstruction practice, and surgeon use of CTA and associated concerns regarding radiation in the preoperative planning of perforator free flaps.
Collected respondent data included location and type of practice, year of completion of residency training, fellowship training details and self-identified subspecialty affiliations in plastic surgery.
The mean percentage of time of a surgeon’s practice spent on both breast reconstruction and free flap reconstruction were identified using five-point rating scales with ranges of <10%, 11% to 30%, 31% to 50%, 51% to 70%, 71% to 90% and >90%. Surgeons were also asked to rank the order of frequency of use of different approaches to breast reconstruction. In addition, a four-point scale, including options of never, sometimes, often and always, was used to identify whether surgeons used colour Doppler US, magnetic resonance angiography or CTA in the operative planning of breast reconstruction. If CTA was never used, the participant was asked to indicate possible reasons including availability, cost and concerns about radiation exposure. An option was also provided for participants to identify specific cases in which CTA would not be appropriate for the preoperative planning of perforator flap breast reconstruction. If a surgeon used CTA in pre-operative planning, they were asked to indicate the length of time this has been a part of their practice.
Surgeons were asked to specify where CTA was used as a first-line imaging modality including the type of perforator used (eg, deep inferior epigastric perforator [DIEP], superficial inferior epigastric artery, superior gluteal artery, inferior gluteal artery, thoracodorsal artery) and the recipient reconstruction characteristics (eg, unilateral, bilateral, immediate or delayed postmastectomy, postradiation, previous failed reconstruction) in their practice. All questions had an option to respond ‘not applicable’ to their practice.
The final section of the survey asked whether surgeons were aware of the scanning protocols used at their centres and whether any modifications to these protocols have been applied to reduce radiation exposure. An open-ended question asking whether surgeons were concerned about the radiation dose associated with CTA concluded the survey.
Descriptive statistics were used to analyze the responses. All statistical analyses were performed using SPSS version 17.0 (IBM Corporation, USA).
RESULTS
Of the 44 surgeons identified as plastic surgeons with a specialty interest in breast reconstruction in Canada, 33 responses were collected, yielding a response rate of 75%. Because this response rate exceeded average response rates for mail-based surveys (7), nonresponders were not contacted for follow-up. The demographic details of the respondents are summarized in Table 1.
Table 1.
Characteristics of surgeon respondents (n=33)
| Geographical location* (Canada) | |
| West | 27.3 |
| Central | 54.5 |
| East | 18.2 |
| Practice type | |
| Academic | 63.6 |
| Community | 12.1 |
| Mixed | 24.2 |
| Fellowship training | |
| Microsurgery | 70.0 |
| Breast reconstruction | 39.4 |
| Self-affiliation | |
| Microsurgery | 66.7 |
| Breast reconstruction | 87.9 |
| Years in practice, mean (range) | 14.0 (2–31) |
| Years of using CTA in the preoperative planning of perforator flap BR, mean (range) | 1.9 (0–14) |
Data presented as % unless otherwise indicated.
West: British Columbia, Alberta; Central: Saskatchewan, Manitoba, Ontario; East: Quebec, New Brunswick, Nova Scotia, Newfoundland and Labrador. CTA Computed tomography angiography; BR Breast reconstruction
Perforator free flaps were used by the majority of the surveyed surgeons performing breast reconstruction (n=29 [87.8% of respondents]). Four of the surgeons who responded (12.0%) did not use perforator flaps in breast reconstruction. Of the perforator flaps used, the DIEP flap was most commonly selected. Sixteen respondents performed perforator free flap breast reconstruction and used CTA in their preoperative work-up. Of this subset, the majority (13 of 16 surgeons [81.3%]) identified CTA as the first-line imaging modality for DIEP flaps. In addition, one-half of this subset (eight of 16 surgeons [50.0%]) identified CTA as first-line imaging in patients who had previously undergone abdominal surgery.
Of the 13 surgeons in the cohort who never used CTA in the pre-operative planning of perforator free flaps, three stated that their reason was related to the radiation dose of CTA. The other surgeons (seven of 13 [53.8%]) cited availability, cost and no known decrease in donor/flap morbidity as their rationale for not incorporating CTA into their perforator flap breast reconstruction practice. Three surgeons (23.0%) did not cite a reason for not using CTA.
When asked to identify cases in which CTA would not be appropriate in the preoperative planning of breast reconstruction, several responses were provided. Some of these case examples included high-risk patients (diabetic, body mass index >30 kg/m2) in which a muscle-sparing transverse rectus abdominus myocutaneous flap is most likely to be used, cases in which a strong Doppler signal is present, if CTA acquisition will delay surgery, in patients with previous radiation treatment or in patients who prefer to avoid radiation. The frequency of use of different imaging modalities in the planning of perforator flaps is outlined in Figure 1.
Figure 1).
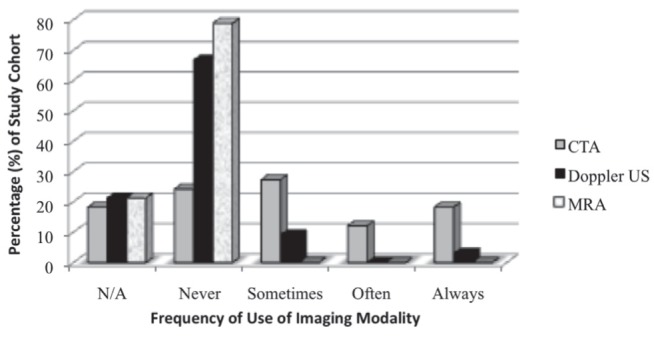
The use of imaging modalities in perforator free flaps including computed tomography angiography (CTA), Doppler ultrasound (US) and magnetic resonance imaging (MRA). N/A Not applicable
One-half of the respondents (17 of 33 [51.5%]) were concerned about the radiation that CTA exposes their patients to, whereas a smaller portion (seven of 33 [21.2%]) were not concerned while the remainder did not respond to this question. Figure 2 shows the breakdown between surgeons’ use of CTA in perforator flap reconstruction and their concern about radiation. Only five surgeons were aware of what CTA scanning protocol was used at their centre. In addition, only three surgeons were aware of any modifications to CTA protocols designed to reduce radiation exposure of CTA in the preoperative planning of breast reconstruction at their centres.
Figure 2).
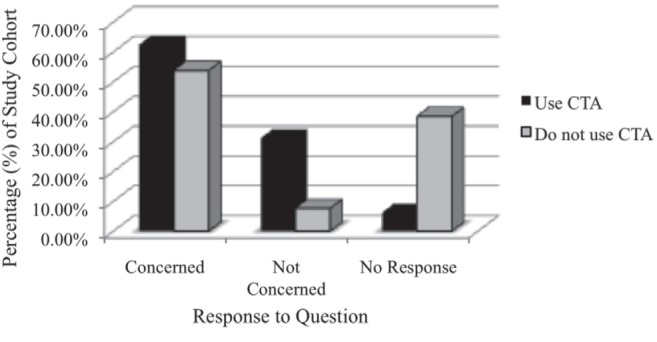
Concern regarding radiation exposure associated with the use of computed tomography angiography (CTA) in preoperative planning of perforator flap breast reconstruction compared between those who use CTA (n=16) and those who do not use CTA (n=13) in their practice
DISCUSSION
It appears that CTA is rapidly becoming the diagnostic modality of choice in delineating the vascular anatomy of perforator flaps in breast reconstruction.
The objective of the present study was to describe the prevalence of the use of CTA as a preoperative planning tool in perforator flap breast reconstruction in Canada. In addition, the perspectives of the surgeons regarding the impact of radiation exposure and choice of imaging modality were sought. We described the responses of 33 of 44 surgeons who were identified as having a practice with a specialty focus on breast reconstruction.
Of the surgeons who used perforator free flaps in their practice, one-half of the cohort (16 of 29 [55.2%]) used CTA in the preoperative planning of breast reconstruction. It is, therefore, clear that among surgeons with a specialty interest in breast reconstruction, there is variability in the standard preoperative work-up. Interestingly, most of the surgeons who used CTA (10 of 16 [62.5%]) were concerned about the radiation exposure from CTA but few were aware of either the scanning protocol used at their centres or any attempts to alter protocol design to reduce radiation.
Most of the literature regarding the use of computed tomography scanning and radiation exposure focuses on the stochastic risk of developing a fatal cancer as a result of the increased radiation for diagnostic purposes (8–10). However, over the past decade, the applications of multidectector CTA of the abdomen have expanded beyond a solely diagnostic role. Aside from the evaluation of perforators for autologous breast reconstruction, CTA is used in the preoperative planning of hepatic resections and liver transplants, the evaluation of Crohn disease or suspected mesenteric ischemia (11), as well as for the surveillance of endovascular abdominal aortic aneurysm repair (12). With the increased use of CT across different patient populations, questions regarding associated risk permeate the literature. It is now well accepted that the largest single source of population-based radiation exposure is from ionizing radiation for medical purposes (13).
The potential risk associated with radiation depends on many factors including the age and sex of the patient, the type of irradiation and the tissue being irradiated (8–10,14). The effective radiation dose describes the amount of radiation to exposed organs and each particular organ’s sensitivity to develop cancer from radiation (10). Computed tomography examinations of the abdomen include a large number of radiosensitive organs in the exposed field. Females are also known to be at an increased risk to develop a cancer secondary to radiation (9,10). This is particularly a concern for preoperative CTA used in the breast reconstruction population. Nevertheless, the proposed risk of radiation from CTA to the population of women receiving autologous breast reconstruction following mastectomy is subject to debate. This patient cohort has already developed a primary cancer in addition to routinely receiving much larger radiation doses, approximately 10,000 times greater than a single CTA study, as part of their treatment regimen (9). Thus, the added exposure from a CTA study for the purposes of breast reconstruction may be perceived as a relatively small addition in this population. On the other hand, although the radiation risk associated with a single imaging test is small, albeit not insignificant, radiation risk is cumulative across a patient’s lifetime. It has been calculated that each abdominal/pelvic CTA contains an effective radiation dose of 6 mSV to 10 mSV. This effective radiation dose would translate to the induction of one extra fatal malignancy for every 2000 investigations (14). Interestingly, only three surgeons in the current study cited previous radiation treatment as a reason for not proceeding with CTA as part of the preoperative evaluation for breast reconstruction. Tracking the radiation exposure of the breast cancer population may be one way to guide decisions regarding the use of further discretionary imaging.
Most surgeons in the current study (24 of 29 [82.7%]) were not aware of the CTA protocols used at their respective institutions, or of attempts made to modify or reduce radiation exposure. From investigations in other countries, it is clear that protocols can differ widely among different institutions. For example, in a retrospective cross-sectional study of 1119 patients by Smith-Bindman et al (10), the radiation dose of the 11 most common types of diagnostic computed tomography studies were compared across four different centres in the United States. This study revealed that the radiation dose for a single study type varied up to 13-fold across the various institutions. This finding was echoed by a study performed in the United Kingdom (9), which found that large variations in effective radiation dose for a specific imaging test across different centres exist. Rozen et al (15) offered some suggestions for techniques to decrease radiation exposure including scanning in a caudocranial direction, using a 64-multidetector row protocol and using the common femoral artery as a site for bolus triggering to maximize arterial perforator filling. Whether these modifications have been adopted at Canadian institutions is not known. Therefore, a national interdisciplinary effort between radiologists and plastic surgeons may be able to help in formulating an optimal scanning protocol for the use of CTA in breast reconstruction in Canada.
Undoubtedly, CTA offers a detailed comprehensive evaluation of perforator vessels available for breast reconstruction (1,2,4,15). In previous work, the superiority of CTA compared with Doppler US with respect to providing detailed anatomical data (2,4,5,15) reduced surgical time (16,17), and fewer complications have been reported (2,4,5,15). In a prospective study of 30 abdominal flap breast reconstructions, Scott et al (5) demonstrated that colour Doppler US was only able to predict 66.3% of dominant DIEP vessels identified by CTA. In contrast, Cina et al (2) cite an equivalent accuracy between the two imaging techniques in identifying a perforator vessel of adequate calibre. Most studies agree that CTA is superior to Doppler US in revealing detailed anatomical data such as the presence of superficial venous communication and the intramuscular course of the perforator (1,2,4,15). The risk for flap morbidity is increased in the absence of superficial venous communication (2). In a cohort study of 104 breast reconstructions, the use of CTA was associated with a significant decrease in the incidence of partial flap losses and donor site morbidity (2,4). Less error in perforator selection is also of particular importance in ensuring adequate blood supply to cases of bilateral breast reconstruction (5,15). The respondents in the current study identified a particular benefit for the use of CTA in patients who have previously undergone abdominal surgery, which is known to alter vascular and soft tissue anatomy (15). However, even before the routine use of CTA in DIEP breast reconstruction, complication rates were low. A 10-year retrospective review of 758 DIEP flaps (18) found that total or partial flap loss occurs in <2.5% of cases and donor site morbidity was <5%. The most frequent complication was fat necrosis (13%) (18). Because of the overall high success rates of contemporary free flap surgery (>95% to 98% success rates), prospective studies investigating the contribution of preoperative CTA to increased flap survival rates would require an exceedingly high number of study participants.
In the current climate of shrinking resources, the use of CTA may still have an overall benefit in terms of shortening dissection times for DIEP flap harvesting. Some studies have argued that the cost of the CTA is justified by the savings acquired by a reduction in operating time (16,17). However, overall, the literature is inconclusive regarding whether findings of faster operating times are truly significant (19). Our questionnaire was not designed to investigate this aspect and, because of its methodology, would have had to rely on surgeon recall. We, therefore, are not able to draw any conclusions as to whether CTA shortens intraoperative dissection time. However, it remains to be determined whether the increased costs of the test in combination with the increased exposure to radiation outweighs this benefit. Prospective studies examining flap dissection times and reduction of peri- and postoperative complications with the use of CTA are needed.
As with any study, the present study also had some limitations, which included both nonresponse and recall biases. A 75% response rate among surgeons with an established interest in breast reconstruction does not reflect the use of CTA among the entire population of plastic surgeons in Canada. These high-volume breast reconstructive surgeons may use a protocol-like approach to their work-up of their patients. Plastic surgeons who perform breast reconstructions on an occasional basis may use fewer perforator flaps and, thus, not feel a need for the use of CTA. Despite this, it appears that even among surgeons with a recognized specialty interest in breast reconstruction and with a preference to use perforator flaps, there is variability in the use of CTA in preoperative planning. Our results also show that among surgeons who commonly use CTA for the preoperative planning of perforator flap breast reconstruction, concerns exist regarding the associated radiation exposure to patients as well as the cost of this modality. Finally, our results show that among surgeons, there is an inadequate understanding of the specific CTA protocol used in their centre. A national interdisciplinary effort to develop an optimal scanning protocol with the lowest possible radiation exposure may help in reducing radiation risk to patients. In addition, the development of a consensus guideline establishing a narrower spectrum of patients who may truly benefit from the advantages offered by preoperative CTA may be helpful in guiding preoperative planning decisions.
REFERENCES
- 1.Alonso-Burgos A, García-Tutor E, Bastarrika G, Cano D, Martínez-Cuesta A, Pina LJ. Preoperative planning of deep inferior epigastric perforator flap reconstruction with multi-slice-CT angiography: Imaging findings and initial experience. J Plast Reconstruct Aesthet Surg. 2006;59:585–93. doi: 10.1016/j.bjps.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Cina ASM, Barone-Adesi L, Rinaldi P, Bonomo L. Planning breast reconstruction with deep inferior epigastric artery perforating vessels: Multidetector CT angiography versus color Doppler US. Radiology. 2010;255:979–87. doi: 10.1148/radiol.10091166. [DOI] [PubMed] [Google Scholar]
- 3.Granzow JW, Levine JL, Chiu ES, Allen RJ. Breast reconstruction using perforator flaps. J Surg Oncol. 2006;94:441–54. doi: 10.1002/jso.20481. [DOI] [PubMed] [Google Scholar]
- 4.Rozen WM, Anavekar NS, Ashton MW, et al. Does the preoperative imaging of perforators with CT angiography improve operative outcomes in breast reconstruction. Microsurgery. 2008;28:516–23. doi: 10.1002/micr.20526. [DOI] [PubMed] [Google Scholar]
- 5.Scott JR, Liu D, Said H, Neligan PC, Mathes DW. Computed tomographic angiography in planning abdomen-based microsurgical breast reconstruction: A comparison with color duplex ultrasonography. Plast Reconstr Surg. 2010;125:446–53. doi: 10.1097/PRS.0b013e3181c82d24. [DOI] [PubMed] [Google Scholar]
- 6.Rozen W, Whitaker IS, Stella DL, Acosta R, Ashton MW. The radiation exposure of computed tomographic angiography (CTA) in DIEP flap planning: Low dose high impact. J Plast Reconstruct Aesth Surg. 2009;62:e654–e655. doi: 10.1016/j.bjps.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Asch DA, Jedrziewski MK, Christakis NA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–36. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- 8.Roobottom CA, Mitchell G, Morgan-Hughes G. Radiation-reduction strategies in cardiac computed tomographic angiography. Clin Radiol. 2010;65:859–67. doi: 10.1016/j.crad.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Tsalafoutas IA, Koukourakis GV. Patient dose considerations in computed tomography examinations. World J Radiol. 2010;2:262–8. doi: 10.4329/wjr.v2.i7.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–86. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Güven K, Acunaş B. Multidetector computed tomography angiography of the abdomen. Eur J Radiol. 2004;52:44–55. doi: 10.1016/j.ejrad.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Perini P, Sediri I, Midulla M, et al. Single centre prospective comparison between contrast-enhanced ultrasound and computed tomography angiography after EVAR. Eur J Vasc Endovasc Surg. 2011;42:797–802. doi: 10.1016/j.ejvs.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 13.United Nations Scientific Committee on the Effects of Atomic Radiation . UNSCEAR 2008 Report to the General Assembly with Scientific Annexes Volume II. New York: United Nations Press; 2008. Sources and Effects of Ionizing Radiation. [Google Scholar]
- 14.Tsapaki V, Rehani M, Saini S. Radiation safety in abdominal computed tomography. Semin Ultrasound CT MR. 2010;31:29–38. doi: 10.1053/j.sult.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Rozen WM, Ashton MW, Grinsell D, Stella DL, Phillips TJ, Taylor GI. Establishing the case for CT angiography in the preoperative imaging of abdominal wall perforators. Microsurgery. 2008;28:306–13. doi: 10.1002/micr.20496. [DOI] [PubMed] [Google Scholar]
- 16.Smit JM, Dimopoulou A, Liss AG, et al. Preoperative CT angiography reduces surgery time in perforator flap reconstruction. J Plast Reconstruct Aesth Surg. 2009;62:1112–7. doi: 10.1016/j.bjps.2007.12.090. [DOI] [PubMed] [Google Scholar]
- 17.Minqiang X, Lanhua M, Jie L, Dali M, Jinguo L. The value of multidetector-row CT angiography for pre-operative planning of breast reconstruction with deep inferior epigastric arterial perforator flaps. Br J Radiol. 2010;83:40–3. doi: 10.1259/bjr/29140440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. 2004;113:1153–60. doi: 10.1097/01.prs.0000110328.47206.50. [DOI] [PubMed] [Google Scholar]
- 19.Fansa H, Schirmer S, Frerichs O, Gehl HB. [Significance of abdominal wall CT-angiography in planning DIEA perforator flaps, TRAM flaps and SIEA flaps.] Handchir Mikrochir Plast Chir. 2011;43:81–7. doi: 10.1055/s-0030-1262844. [DOI] [PubMed] [Google Scholar]


