Abstract
BACKGROUND/OBJECTIVE:
Bupivacaine and lidocaine are often used concurrently, in theory, to combine the more rapid onset of lidocaine and the longer duration of bupivacaine. The purpose of this study was to evaluate this concept.
METHODS:
Twenty-five subjects were enrolled in a double-blinded, randomized block design study to evaluate the onset and duration of four different mixtures of lidocaine and bupivacaine with epinephrine. The study was designed to achieve 80% power to detect an effect size of 0.37 at 5% overall significance. The four mixtures tested were: 0.25% bupivacaine with epinephrine (1:200,000); 1% lidocaine with epinephrine (1:100,000); 0.125% bupivacaine and 0.5% lidocaine with epinephrine (1:150,000); and 0.25% bupivacaine and 1% lidocaine with epinephrine (1:150,000). Four intradermal injections were made in the volar forearms of each participant. Time to effect and duration were measured by sensation of a sharp skin prick.
RESULTS:
Mean time to onset ranged from 12 s to 29 s without statistical significance across all tested solutions (P=0.891). Mean duration of effect ranged from 6 h 38 min to 7 h 25 min with a statistically significant difference across the tested solutions (P=0.036).
CONCLUSIONS:
No statistical benefit was measured when comparing lidocaine with epinephrine, bupivacaine with epinephrine, and mixtures of these local anesthetics with regard to onset of action. While a statistical difference was observed in duration of effect, the clinical benefit measured was narrow.
Keywords: Bupivacaine, Duration, Lidocaine, Local anesthetic, Onset
Abstract
HISTORIQUE ET OBJECTIF :
La bupivacaïne et la lidocaïne sont souvent utilisées conjointement, en théorie, pour associer le délai d’action plus rapide de la lidocaïne à la durée d’action plus longue de la bupivacaïne. La présente étude visait à évaluer ce concept.
MÉTHODOLOGIE :
Vingt-cinq sujets ont participé à une étude aléatoire par bloc à double insu conçue pour évaluer le délai et la durée d’action de quatre mélanges différents de lidocaïne et de bupivacaïne associés à l’adrénaline. L’étude était conçue pour atteindre une puissance de 80 % afin de déceler une ampleur de l’effet de 0,37, selon une signification globale de 5 %. Les quatre mélanges à l’essai étaient la bupivacaïne 0,25 % associée à l’adrénaline (1 sur 200 000); la lidocaïne 1 % associée à l’adrénaline (1 sur 100 000); la bupivacaïne 0,125 % et la lidocaïne 0,5 % associées à l’adrénaline (1 sur 150 000); et la bupivacaïne 0,25 % et la lidocaïne 1 % associées à l’adrénaline (1 sur 150 000). Chaque participant a reçu quatre injections intradermiques dans l’avant-bras palmaire. Les chercheurs ont mesuré le délai et la durée d’action au moyen de la sensation d’une piqûre épidermique.
RÉSULTATS :
Le délai d’action moyen variait de 12 secondes à 29 secondes, sans signification statistique entre les diverses solutions à l’essai (P=0,891). La durée d’action moyenne variait de 6 h 38 min à 7 h 25 min et s’associait à une différence statistiquement significative entre les solutions à l’essai (P=0,036).
CONCLUSIONS :
Les auteurs n’ont mesuré aucun avantage statistique lorsqu’ils ont comparé le délai d’action de la lidocaïne associée à l’adrénaline, de la bupivacaïne associée à l’adrénaline et des mélanges de ces anesthésiques locaux. Ils ont constaté une différence statistique quant à la durée d’action, mais l’avantage clinique mesuré était minime.
Physicians commonly use local anesthetics to perform minor procedures and to improve analgesia for procedures performed under general anesthesia. Local anesthetics improve postoperative pain control and reduce the amount of postoperative narcotic required (1,2). This, in turn, can reduce postoperative nausea, emesis and delayed return to bowel function (2–5). An ideal local anesthetic provides complete sensory blockade on application and has an adequate duration of effect to include the procedure and a generous postoperative period. Bupivacaine and lidocaine are two commonly used local analgesics. The two amino amides are often used concurrently to combine the more rapid onset of lidocaine and the longer duration of bupivacaine. They may be used for regional blockades but are often administered as intradermal field blocks. Many formulations include epinephrine, which acts as a vaso-constrictor improving hemostasis and reducing the rate of absorption, thereby prolonging duration and decreasing toxicity.
Compared with lidocaine, bupivacaine has a significantly longer duration of action and slower time to onset (6,7). Bupivacaine has an onset of 5 min, a duration of 2 h to 4 h and maximum dose of 2 mg/kg. The addition of epinephrine has an unknown effect on onset while increasing duration and maximum dose to 3 h to 7 h and 3mg/kg, respectively (6,7). Lidocaine is known to have an onset <2 min, a duration of 1 h to 2 h, and a maximum dose of 5 mg/kg, which improves to an onset <2 min, a duration of 2 h to 6 h and toxicity of 7mg/kg with the addition of epinephrine (6,7). Each characteristic is dependent on multiple factors including volume and concentration infused, location of administration and tissue pH (6).
The two medications are often used as a mixture to theoretically provide the longer action of bupivacaine and the faster onset of lidocaine. Previous studies have examined safety, demonstrating no increased toxicity with mixtures of bupivacaine and lidocaine (8). Research has also examined the use of multiple combinations of local anesthetics including bupivacaine and lidocaine in nerve and spinal blocks, with mixed results depending on the anesthetics used and location of the block (9–17). In studies comparing mixtures of chloroprocaine and lidocaine for epidural infusion, a decreased duration was experienced, leading some experts to recommend avoiding the practice of local anesthetic mixtures (6). A separate study found no benefit of lidocaine and bupivacaine with epinephrine when compared with bupivacaine with epinephrine alone when used in a ring block (18).
A literature review yielded a single study comparing bupivacaine, lidocaine and a mixture administered intradermally without epinephrine (17). The study involved 20 participants and demonstrated no measurable difference in onset and a statistically significant difference in duration. The authors concluded that mixtures of bupivacaine and lidocaine work well and may have possible benefit in clinical applications.
The present study was the first to evaluate the use of intradermal mixtures of bupivacaine and lidocaine with epinephrine. The hypotheses were that no difference would be measured with respect to onset among the different mixtures and that duration of action would increase proportionately with the amount of bupivacaine infused.
METHODS
The present study was evaluated and approved by an internal review board at Scott & White Hospital (Texas, USA) and registered with ClinicalTrials.gov. A sample size calculation using a randomized block design to achieve 80% power to detect differences in mean duration time (effect size of 0.37) and mean time to onset (effect size of 0.37) at 5% overall significance level yielded 25 participants. A double-blinded, randomized block design study was performed using 25 healthy volunteers, after receiving verbal and written informed consent, at Scott & White Hospital from July 24 to August 28, 2010. Volunteers completed a basic health questionnaire and were excluded from the study if they experienced any previous adverse reaction to lidocaine, bupivacaine or epinephrine, were pregnant or had a history significant for heart disease, peripheral vascular disease, diabetes mellitus, Raynaud’s syndrome or immune dysfunction. The 25 enrolled subjects had a mean (± SD) age of 36±9.7 years, with 18 female and seven male participants. Six participants had a diagnosis of hypertension, three had hypercholesterolemia, one had asthma and one had hypothyroidism.
Each volunteer was evaluated using four different mixtures of local anesthetic infused into the dermis of the volar forearm. Two injections were placed into each forearm so that >10 cm of unanesthetized skin was maintained between infusion sites. A total of 0.2 mL of solution was injected at each site using different 27-gauge needles and 1 mL syringes. The four mixtures tested were 0.25% bupivacaine with epinephrine (1:200,000); 1% lidocaine with epinephrine (1:100,000); 0.125% bupivacaine and 0.5% lidocaine with epinephrine (1:150,000); and 0.25% bupivacaine and 1% lidocaine with epinephrine (1:150,000).
Each site was tested before administration of the medication to ensure normal sensation. Sensation was evaluated at the central site of infusion with an alcohol-sterilized safety pin. Sensation was defined as the ability to sense sharp when the safety pin depressed but did not puncture the skin. Onset was recorded as the time from infusion until loss of sharp sensation measured in 15 s increments. Duration of effect was the time from onset until return of sharp sensation measured in 15 min intervals. Treatments were assigned to each patient and infusion site using a random number generator. Treatments were assigned a unique serial number that ensured participants and investigators were blinded during the trial.
Statistical analysis of the results was performed with all variables of interest summarized using descriptive statistics: mean ± SD for continuous variables and frequency and percentage for categorical variables. Time to onset of action and duration of effect were summarized according to treatment group (0.25% bupivacaine, 1% lidocaine, 0.125% bupivacaine + 0.5% lidocaine, 0.25% bupivacaine + 1% lidocaine) using mean ± SD and median (minimum-maximum). Treatment means were compared using ANOVA for randomized block design. Friedman’s test and pairwise comparisons were also performed; P=0.05 was used to indicate statistical significance.
RESULTS
Onset of action had a mode of 0 s for all treatment groups. No statistical difference was measured between groups when evaluating time to effect (P=0.891) (Table 1).
TABLE 1.
Onset of action for local anesthetic
| Anesthetic or mixture |
Onset of effect, s
|
|
|---|---|---|
| Mean ± SD | Median (Min–Max) | |
| 0.25% bupivacaine | 19±41 | 0 (0–180) |
| 1% lidocaine | 29±49 | 0 (0–180) |
| 0.125% bupivacaine and 0.5% lidocaine | 26±46 | 0 (0–120) |
| 0.25% bupivacaine and 1% lidocaine | 12±30 | 0 (0–120) |
P=0.891. Max Maximum; Min Minimum
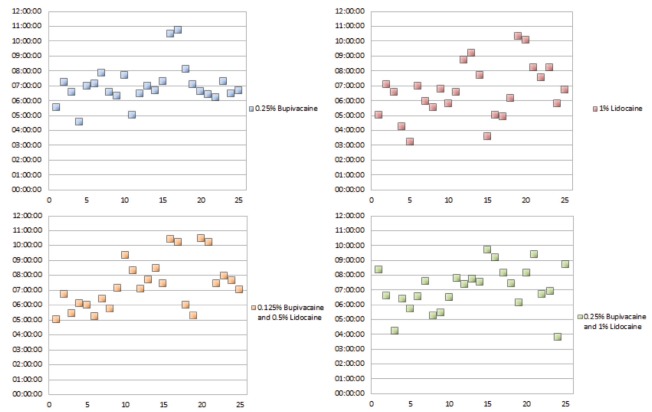
The mean duration of effect ranged from 6 h 38 min to 7 h 25 min, with significant statistical differences noted (P=0.036) (Table 2). Times varied most widely for the 1% lidocaine group and were most consistent in the 0.25% bupivacaine group, with SDs of 1 h 51 min and 1 h 21 min, respectively (Figure 1). The longest mean duration was measured in the 0.125% bupivacaine and 0.5% lidocaine group, while the longest individual effect was measured in the 0.25% bupivacaine group.
TABLE 2.
Duration of action for local anesthetic mixtures
| Anesthetic or mixture |
Duration of effect, h
|
|
|---|---|---|
| Mean ± SD | Median (Min–Max) | |
| 0.25% bupivacaine | 7.02±1.46 | 6.70 (4.58–10.75) |
| 1% lidocaine | 6.63±1.85 | 6.58 (3.22–10.32) |
| 0.125% bupivacaine + 0.5% lidocaine | 7.48±1.76 | 7.15 (5.02–10.5) |
| 0.25% bupivacaine + 1% lidocaine | 7.16±1.60 | 7.37 (3.82–9.68) |
P=0.036. Max Maximum; Min Minimum
Figure 1).
Duration of effect for all treatments
Additional analysis was performed to evaluate the significant difference identified in the mean duration of effect. Post hoc pairwise comparison was performed using ANOVA on rank of the onset of action times. Significant difference was measured between the 1% lidocaine group and the 0.125% bupivacaine and 0.5% lidocaine group.
DISCUSSION
The present study demonstrated a statistically significant difference for duration of effect for the different local anesthetic mixtures assigned. The difference did not correlate with the concentrations of either bupivacaine or epinephrine as initially expected. Furthermore, 1% lidocaine with epinephrine (1:100,000) performed well, although it is traditionally believed to have a much shorter duration of action than bupivacaine. While bupivacaine produced a more consistent duration of anesthetic relative to lidocaine alone or in mixtures, mean and median durations varied narrowly within 1 h for all groups.
The present study was powered to detect a difference based on the available pharmacokinetics for bupivacaine and lidocaine and clinical relevance. Nevertheless, no statistical difference was measured for the onset of action between treatment groups. A larger study designed to measure a statistical difference in this variable would be unlikely to demonstrate a clinically significant difference given the rapid onset observed in all formulations.
The concentration of epinephrine in solution was based on the availability of prefabricated local anesthetics with epinephrine. Epinephrine produced a dramatic effect on both duration and onset of action when compared with the study by Sweet et al (17) and pharmacology reference books (6,7). Although the study did not compare formulations with epinephrine to those without, we achieved longer durations and more rapid onset for both lidocaine and bupivacaine than reported values.
The present study was performed using commercially available 1% lidocaine with epinephrine (1:100,000), 2% lidocaine with epinephrine (1:100,000), 0.25% bupivacaine with epinephrine (1:200,000), and 0.5% bupivacaine with epinephrine (1:200,000), either alone or in a 50:50 solution. Therefore, different infusion sites received different concentrations of epinephrine with the greatest difference between the 0.25% bupivacaine and 1% lidocaine (1:200,000 compared with 1:100,000). To avoid different concentrations, mixtures could be created using individual components and equal amounts of epinephrine. We elected to mix commercially available solutions despite this possible confounding variable because physicians using this technique will typically mix bupivacaine and lidocaine in this manner at our institution.
The characteristics of bupivacaine and lidocaine measured in the present study are clinically similar. There are numerous reasons apart from onset and duration physicians choose different anesthetics. Clinicians should use toxicity, amount needed to infuse, location of infusion and availability to guide selection of local anesthetic when choosing between bupivacaine, lidocaine or a mixture of the two. Based on the results of the present study, clinicians may see less difference between bupivacaine and lidocaine than expected, resulting in a smaller potential gain when mixing formulations or selecting one for longer duration or shorter onset of action.
CONCLUSIONS
No statistical benefit was measured when comparing lidocaine with epinephrine, bupivacaine with epinephrine, and mixtures of these local anesthetics with regard to onset of action using commercially available formulations for an intradermal field block. While a statistical difference was observed in duration of effect, the clinical benefit was narrow and individual physicians should use other clinically relevant factors to determine the composition of their local anesthetic.
Footnotes
DISCLOSURES: The authors do not have any commercial associations or financial disclosures that might pose or create a conflict of interest with the information presented in this article.
FUNDING: Scott and White, Research Grants Proposal Support $2,620 (internal funding).
ROLES AND CONTRIBUTIONS: James Collins: design, grant proposal, study execution, data analysis and manuscript writing. Juhee Song: design, data analysis and manuscript editing. Raman C Mahabir: original idea, design, manuscript editing and corresponding author.
CLINICAL TRIALS REGISTRATION INFORMATION: Brief title: Combination Local Anesthetics (CA), Protocol ID: 090520, Identification Number: NCT01243112, Date Registered: 4/13/2010, Registry URL: http://clinicaltrials.gov/archive/NCT01243112, Level of Evidence Rating: 1.
REFERENCES
- 1.Partridge BL, Stabile BE. The effects of incisional bupivacaine on postoperative narcotic requirements, oxygen saturation and length of stay in the post-anesthesia care unit. Acta Anesthesiol Scand. 1990;34:486–91. doi: 10.1111/j.1399-6576.1990.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 2.Golembiewski J, Chernin E, Chopra T. Prevention and treatment of postoperative nausea and vomiting. Am J Health Syst Pharm. 2005;62:1247–60. doi: 10.1093/ajhp/62.12.1247. [DOI] [PubMed] [Google Scholar]
- 3.Andersen R, Krohg K. Pain as a major cause of postoperative nausea. Can Anaesth Soc J. 1976;23:366–9. doi: 10.1007/BF03005916. [DOI] [PubMed] [Google Scholar]
- 4.Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg. 2003;138:206–14. doi: 10.1001/archsurg.138.2.206. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MD, Walsh RM. Current therapies to shorten postoperative ileus. Cleve Clin J Med. 2009;76:641–8. doi: 10.3949/ccjm.76a.09051. [DOI] [PubMed] [Google Scholar]
- 6.Cousins MJ, Bridenbaugh PO. Neural blockade in clinical anesthesia and management of pain. 4th edn. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 7.Drug Facts and Comparisons 2011. 65th edn. St Louis: Lippincott Williams & Wilkins; 2010. Facts & Comparisons. [Google Scholar]
- 8.de Jong RH, Bonin JD. Mixtures of local anesthetics are no more toxic than the parent drugs. Anesthesiology. 1981;54:177–81. doi: 10.1097/00000542-198103000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Brodsky JB, Brock-Utne JG. Mixing local anaesthetics. Br J Anaesth. 1978;50:1269. doi: 10.1093/bja/50.12.1269. [DOI] [PubMed] [Google Scholar]
- 10.Bromage PR, Gertel M. Improved brachial plexus blockade with bupivacaine hydrochloride and carbonated lidocaine. Anesthesiology. 1972;36:479–87. doi: 10.1097/00000542-197205000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SE, Thurlow A. Comparison of a chloroprocaine-bupivacaine mixture with chloroprocaine and bupivacaine used individually for obstetric epidural analgesia. Anesthesiology. 1979;51:288–92. doi: 10.1097/00000542-197910000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham NL, Kaplan JA. A rapid-onset, long-acting regional anesthetic technique. Anesthesiology. 1974;41:509–11. doi: 10.1097/00000542-197411000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Kim JM, Goto H, Arakawa K. Duration of bupivacaine intradermal anaesthesia when the bupivacaine is mixed with chloroprocaine. Anaesth Analg. 1979;58:364. doi: 10.1213/00000539-197909000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Lee-Elliott CE, Dundas D, Patel U. Randomized trial of lidocaine vs lidocaine/bupivacaine periprostatic injection on longitudinal pain scores after prostate biopsy. J Urol. 2004;171:247–50. doi: 10.1097/01.ju.0000098688.12631.a0. [DOI] [PubMed] [Google Scholar]
- 15.Magee DA, Sweet PT, Holland AJ. Epidural anaesthesia with mixtures of bupivacaine and lidocaine. Can Anaesth Soc J. 1983;30:174–8. doi: 10.1007/BF03009348. [DOI] [PubMed] [Google Scholar]
- 16.Seow LT, Lips FJ, Cousins MJ, Mather LE. Lidocaine and bupivacaine mixtures for epidural blockade. Anesthesiology. 1982;56:177–83. doi: 10.1097/00000542-198203000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Sweet PT, Magee DA, Holland AJ. Duration of intradermal anaesthesia with mixtures of bupivacaine and lidocaine. Can Anaesth Soc J. 1982;29:481–3. doi: 10.1007/BF03009413. [DOI] [PubMed] [Google Scholar]
- 18.Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490–2. doi: 10.1016/s0196-0644(96)70239-1. [DOI] [PubMed] [Google Scholar]