Abstract
This study is aimed to investigate the clinical significance and the short-term prognostic value of fragmented QRS (fQRS) for patients with acute myocardial infarction (AMI). Three hundred patients with AMI were tested with retrospective analysis on the patients’ clinical information, hospitalized treatment, fQRS onset time, location of lesions, and other relevant data, in order to assess the relationship between the presence of fQRS and its prognosis. The rates of malignant cardiac arrhythmia, left ventricular systolic dysfunction (LVSD), and mortality in the positive fQRS group were 13.6%, 29.2%, and 23.7%, respectively, with all showing a p value <0.05. For the ST segment elevation myocardial infarction (STEMI) subgroup, all the rates showed significant differences with a p value <0.01, while for the non-STEMI (NSTEMI) subgroup showed no significant differences. In patients with a positive fQRS, there were no differences in malignant cardiac arrhythmia between patients with and without percutaneous coronary intervention (PCI) (p>0.05). As for the LVSD and mortality, the p values between patients with and without PCI were 0.031 and 0.000, respectively, suggesting statistical significance. The results imply that AMI patients with positive fQRS especially for the patients with STEMI had higher rates of malignant cardiac arrhythmia, LVSD, and mortality than the non-fQRS group. Patients of AMI with positive fQRS, who underwent early revascularization, could lower the incidence of the cardiovascular event. In addition, the presence of fQRS could be used as an indication of early intervention treatment for patients.
Keywords: Fragmented QRS, Acute myocardial infarction, Short-term prognosis
1. Introduction
Acute myocardial infarction (AMI) is due to the onset of stenosis or occlusion in the coronary artery that can cause severe ischemia and necrosis of myocardium and consequently result in a high mortality rate and poor prognosis. It has been shown that the primary causes of death are arrhythmia and heart failure. Malignant arrhythmia (mainly ventricular fibrillation) is usually the primary manifestation and accounts for 50% of the mortality (Wang and Zhao, 2011) while heart failure is commonly seen in patients with a large area of myocardial infarction (>40%) and sometimes results in cardiogenic shock. As a new electrocardiographic index, fragmented QRS (fQRS) has been proved to be a valuable tool for detecting myocardial infarction (France et al., 1990; Michael et al., 2007; Xu and Qi, 2009), as well as predicting the incidence of malignant cardiac arrhythmia and left ventricular systolic dysfunction (LVSD) (Guo, 2008; Cheema et al., 2010). However, the correlation between incidence of malignant cardiac arrhythmia, LVSD, or mortality and factors such as fQRS onset and duration, fQRS distribution in electrocardiography (ECG) leads, coronary lesion, ST segment elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), and percutaneous coronary intervention (PCI), is still poorly understood as there are only a few studies available on these subjects. This study focuses on the investigation of the clinical significance and the short-term prognostic value of fQRS for patients with AMI.
2. Materials and methods
2.1. General information
ST-segment and T-wave abnormalities and Q waves were defined per standard definitions recommended by consensus documents of the Guideline for Diagnosis and Treatment of Patients with Acute Myocardial Infarction (Gao, 2001) and the universal definition of myocardial infarction (MI) based on patients’ symptoms, analysis of the standard 12-lead electrocardiogram, biomarkers, cardiac catheterization results as well as clinical data. Three hundred patients with AMI under continuous treatment in the coronary care unit and the general wards of the Peking University First Hospital during January 2010 to September 2011 were selected for the study. Patients with the following conditions would be excluded: (1) severe valvular disease, congenital heart disease, or pacemaker implantation; (2) other severe organ disease or dysfunction such as cancer, liver or kidney failure, neurological or mental disease.
2.2. Experiment procedure
2.2.1. Routine ECG
All admitted patients would receive 12-lead ECG as a part of a routine examination (filter range, 0.5–100 Hz; AC filter, 25 mm/s, 10 mm/mV). ECG monitoring was required for every patient upon admission to the hospital. Serial ECG was performed right after the patients were administered in the hospital. To minimize the omission of fQRS, patients would have their ECG rechecked after administered in 1, 3, 6, 12, and 24 h segments. Every patient would have an ECG performed every day and additional ECG would be performed when presented with any related discomfort until they were discharged or died. All the patients would have an ECG rechecked after a PCI. Every patient would have their Holter ECG and echocardiogram on the third day of admission as well.
2.2.2. Diagnostic standard for fQRS (Das et al., 2006; 2007)
Narrow fQRS: fQRS includes various RSR’ patterns with different morphologies of the QRS complexes except the bundle branch block, with or without the Q wave on the regular 12-lead ECG shown in at least two contiguous leads, corresponding to a myocardial territory.
Fragmented wide QRS (f-wQRS): For a wide QRS wave of bundle branch block (QRS>120 ms), R wave or S wave with more than two notches is observed in two sequential leads (f-LBBB QRS, f-RBBB QRS); for a wide QRS complex of premature ventricular contractions (PVCs), R waves will show two notches and it is required to show time intervals of greater than 40 ms between two notches of the QRS waves in two or more sequential leads (f-PVCs). Electrocardiograms were compared with previous electrocardiograms if available to confirm fQRS or that pathologic Q waves were of a new onset.
2.3. Follow-up and observation endpoint
An echocardiogram was performed for all the patients. A patient with a left ventricular ejection fraction (LVEF), which evaluated the heart function, shown to be <40% had LVSD (Paulus et al., 2007). Malignant cardiac arrhythmia included polymorphic ventricular extrasystoles, ventricular tachycardia, and ventricular fibrillation. The patients testing would end with either a discharge after recovery or an all-cause death.
2.4. Statistical analysis
All quantitative data would be shown as mean±standard deviation (SD). The t-test was for comparison of averages. The Chi-square test was for the comparison of rates. The 95% confidence interval (CI) of relative risk (RR) suggested the presence of clinically significant risk factor. A p value <0.05 indicated statistical significance. The Kaplan-Meier method was used to estimate survival functions of all-cause mortality and a Cox regression model was used to estimate the hazard ratios of variables of interest while controlling for other covariates. All statistical analyses would be completed using the SPSS 16.0 software.
3. Results
3.1. Basic information of selected patients
The mean follow-up period of the patients studied was (15.09±0.813) d and the average age of the 300 patients was (68.48±0.71) years. The study included 204 male patients and 96 female patients, 99 STEMI patients and 201 NSTEMI patients, and 184 patients received PCI treatment during hospitalization. We found 169 patients with fQRS and the other 131 patients without fQRS, and there was no statistical significance in comparison of age, sex, and location of the infarct between the groups with or without fQRS.
3.2. Information of fQRS
3.2.1. Onset time of fQRS
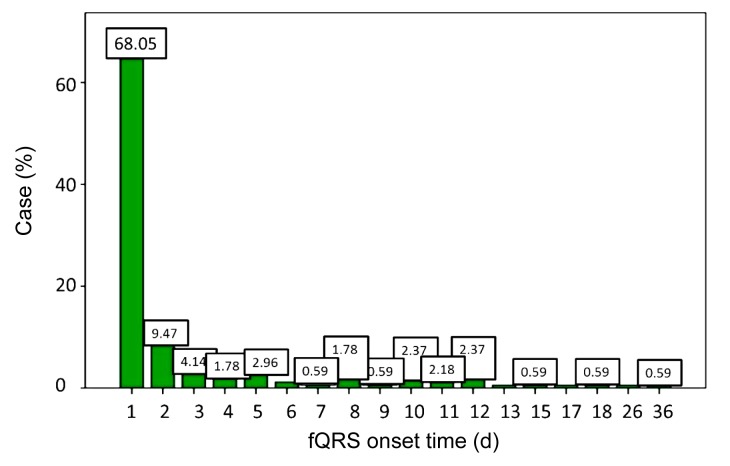
The average fQRS onset time was (2.91±0.35) d after AMI. Sixty-eight percent of the patients had fQRS within 24 h after AMI (115/169), 9.5% patients showed fQRS in 2 d (16/169), and 4.1% on the third day (7/169) (p<0.01). Approximately 88.2% of all patients had fQRS by the first week of testing after AMI (149/169) (Fig. 1).
Fig. 1.
Onset time of fQRS of selected patients
Onset time of fQRS of 68.05% patients was within 2 d after AMI
3.2.2. Duration of fQRS
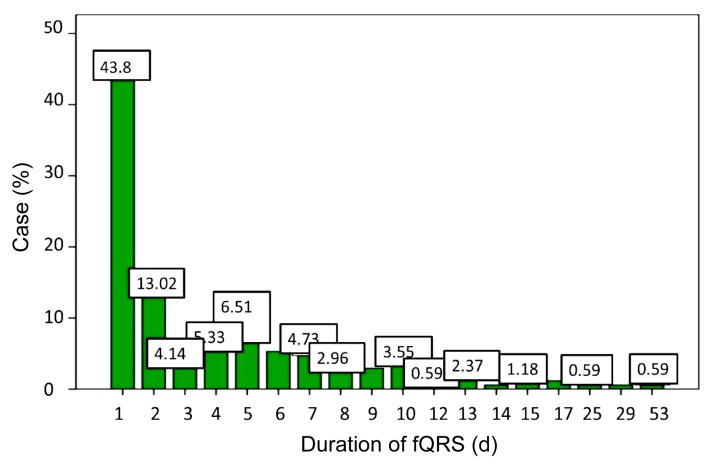
The average duration of fQRS was (4.34±0.45) d. Most of all patients, 43.8% had persistence of fQRS for 1 d (74/169), 13.0% showed fQRS for 2 d (22/169), and 4.1% showed fQRS for 3 d (7/169) (p<0.01). Approximately 4.7% of the patients had fQRS for as long as a period of 7 d (8/169). For a duration of fQRS longer than two weeks, 4.7% of patients fell into this category (8/169) (Fig. 2).
Fig. 2.
Duration of fQRS of selected patients
Duration of fQRS over 50% patients was 2 d
3.3. Short-term prognostic outcome for AMI patients with fQRS
3.3.1. fQRS and malignant cardiac arrhythmia
Retrospective analysis on ECGs for the 300 hospitalized AMI patients showed that 30 patients developed malignant cardiac arrhythmia. Based on the presence of fQRS, 300 patients were put into different groups and the correlation between fQRS and malignant cardiac arrhythmia would then be observed and analyzed. From Table 1, it was seen that in 30 patients, who later developed malignant cardiac arrhythmia, 76.7% had positive fQRS (23/30) and 23.3% showed a negative result for fQRS (7/30). In the positive fQRS group, including 169 patients, 13.6% of the patients developed malignant cardiac arrhythmia (23/169); in 131 cases for the non-fQRS group, only 5.3% showed this condition (7/131) (p<0.05). Univariate electrocardiographic predictors of mortality on the basis of the Cox regression model were fQRS and Q waves. After controlling for age, diabetes, hypertension, hypercholesterolemia, family history of coronary artery disease, and history of smoking, fQRS was shown to be a significant predictor of malignant cardiac arrhythmia, whereas Q waves did not predict malignant cardiac arrhythmia (Table 2).
Table 1.
Correlation between fQRS and malignant cardiac arrhythmia
| Group | Number |
p | ||
| Total | Positive fQRS | Negative fQRS | ||
| With malignant cardiac arrhythmia | 30 | 23 | 7 | 0.018 |
| Without malignant cardiac arrhythmia | 270 | 146 | 124 | |
Table 2.
Cox regression analysis
| Electrocardiographic sign | p | Hazard ratio* (95% CI) |
| fQRS | 0.024 | 0.393 (0.174–0.887) |
| Q wave | 0.419 | 0.720 (0.324–1.597) |
Derived after controlling for age, diabetes, hypertension, hypercholesterolemia, family history of coronary artery disease, and smoking
3.3.2. fQRS and LVSD
In the hospitalized AMI patients, 283 of them completed echocardiography and were grouped according to LVEF. There were 219 patients with LVEF≥40% and 64 patients with LVEF <40% suggesting LVSD. As shown in Table 3, there were a total of 64 patients with LVSD, in which 73.4% also had a positive fQRS (47/64) and 26.6% had a negative result of fQRS (17/64). In the positive fQRS group including 161 patients, 29.2% of the patients developed LVSD (47/161), and in 122 cases of the non-fQRS group, only 13.9% showed this condition (17/122) (p<0.05).
Table 3.
Correlation between fQRS and LVSD
| Group | Number |
p | ||
| Total | Positive fQRS | Negative fQRS | ||
| LVSD | 64 | 47 | 17 | 0.013 |
| Non-LVSD | 219 | 114 | 105 | |
3.3.3. fQRS and mortality
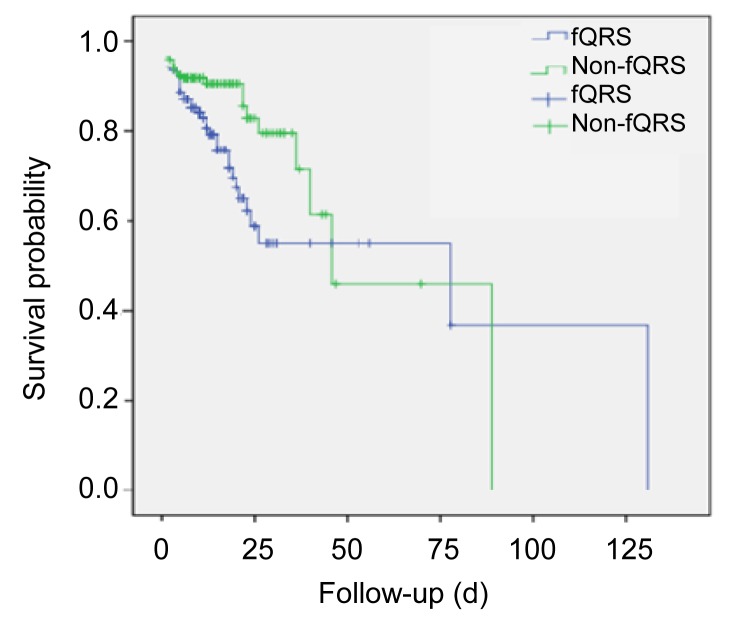
During a mean follow-up period of (15.09±0.813) d, 19.7% of the patients died. Mortality was significantly higher in the fQRS group than in the non-fQRS group. By observing the prognoses of patients in relation to the presence of fQRS, it was found that in 169 cases of positive fQRS, the mortality rate was 23.7% (40/169); the 131 patients of the non-fQRS group showed a 14.5% mortality rate (19/131) (odd ratio (OR) 1.828, 95% CI 1.001–3.337, p=0.048). Kaplan-Meier survival analysis revealed significantly decreased survival for the fQRS group compared to the non-fQRS group (p=0.013) (Fig. 3).
Fig. 3.
Kaplan-Meier survival analysis of fQRS and non-fQRS groups for all-cause mortality
3.4. Correlation between fQRS and the short-term prognosis for patients with STEMI
There were 99 STEMI patients (33.0%) and 201 NSTEMI patients (67.0%). For the two groups, an analysis on the correlation between fQRS and a condition of malignant cardiac arrhythmia, LVSD, or prognosis was conducted. As shown in Table 4, the STEMI patients of positive fQRS had four times the incidence of malignant cardiac arrhythmia in comparison to the non-fQRS group (p<0.01). The rate of LVSD of fQRS group was 7.5 times higher than that of the non-fQRS group (p<0.01). The mortality rate of fQRS group was 2.4 times greater than that of the non-fQRS group (OR 2.44, 95% CI 1.044–5.702, p=0.006).
Table 4.
Correlation between fQRS and the short-term prognosis for patients with STEMI
| Prognosis | Number (percentage) |
p | |
| fQRS | Non-fQRS | ||
| Malignant cardiac arrhythmia | 16 (31.4%) | 4 (8.3%) | 0.004 |
| LVSD | 25 (53.2%) | 3 (7.1%) | 0.000 |
| Death | 24 (47.1%) | 10 (20.8%) | 0.006 |
3.5. Correlation between fQRS and the short-term prognosis for patients with NSTEMI
Between the subgroups of fQRS and non-fQRS in the NSTEMI patients, there was no statistical significance in malignant cardiac arrhythmia, LVSD, and mortality (Table 5).
Table 5.
Correlation between fQRS and the short-term prognosis for patients with NSTEMI
| Prognosis | Number (percentage) |
p | |
| fQRS | Non-fQRS | ||
| Malignant cardiac arrhythmia | 7 (5.9%) | 3 (3.6%) | 0.457 |
| LVSD | 22 (19.3%) | 14 (17.5%) | 0.842 |
| Death | 16 (13.6%) | 9 (10.8%) | 0.566 |
3.6. Correlation between PCI and the short-term prognosis for patients with positive fQRS
Out of 169 cases with positive fQRS, 101 patients had coronary angiography, including 10 patients for the coronary bypass surgery, and 90 patients for PCI treatment. A total of 68 patients did not receive a coronary angiography because of the patients’ health conditions, economics, etc. The correlation analysis between PCI treatment and the development of malignant cardiac arrhythmia, LVSD, or mortality in patients with positive fQRS is shown in Table 6. In comparison to the PCI group, there was no difference in development of malignant cardiac arrhythmia in the non-PCI group, but as for the LVSD and mortality rate, the non-PCI group had significantly higher incidences (p<0.05).
Table 6.
Correlation of PCI and clinical outcome in patients with fQRS
| Prognosis | Number |
p | |
| PCI | Non-PCI | ||
| Malignant cardiac arrhythmia | 11 | 12 | 0.209 |
| LVSD | 23 | 24 | 0.031 |
| Death | 14 | 26 | 0.000 |
4. Discussion
AMI is a high-risk disease whose primary cause of death is malignant cardiac arrhythmia, accounting for 50% of all deaths. Heart failure is commonly found in patients with a large area of myocardial infarction. Therefore, the objective of risk evaluation immediately after admission is to provide early risk stratification of patients with AMI and to identify high-risk patients and predict complications. Thus, it plays an important role to assess the short-term and long-term prognostic outcomes, as well as preventing adverse events and improving prognosis for patients (Das et al., 2006; 2009). More researchers are now focusing on clinical application of fQRS (Carey et al., 2010). Currently, there are many theories on the mechanism of fQRS (Flowers et al., 1969; Frustaci et al., 2005; Zhang et al., 2011).
In our study, the frequent onset time of fQRS of approximately 77.5% of the patients was 24 to 48 h after AMI, and the fQRS duration of 56.8% of the patients who developed fQRS was 24 to 48 h. It takes approximately two to four weeks for cardiac muscle remodeling after infarction and the replacement of tissues by fibroblasts is completed by the 6th and the 8th weeks. The myocardial scarring becomes the basis of the ventricular remodeling process. It was thus postulated that the formation of fQRS in early myocardial ischemia might be due to the asynchronous conduction during infarction (Wang and Zhao, 2011). Most of the studies related to fQRS spent more time to evaluate the outcomes of the MI patients after more than 30 d. Many early validation studies had shown that fQRS was a remote surrogate marker of the myocardial scar (Gardner et al., 1985; Das et al., 2008). In our study, a large portion of the patients developed fQRS in the acute phase. This phenomenon showed that fQRS might be a remark for the electrocardiographic predictors in the acute phase of MI. Can fQRS be induced for the same reasons that cause electrical storm in the early phase of AMI patients? This may require further study to investigate the etiology of fQRS. This study also showed that there was an 81.1% incidence rate of fQRS in the inferior lead (137/169), which was significantly higher than the rates observed at the anterior and the lateral chest wall. However, the analysis on fQRS in correspondence with coronary lesions found no statistical significance between the two. The attempt to link fQRS of a lead to the number of coronary lesions showed that there were variations in the number of coronary lesions (either single or multiple) when fQRS occurred in the inferior lead (p<0.05). On the other hand, there was no statistical significance between the incidence of fQRS in the anterior or the lateral leads and the number of coronary lesions. Yao et al. (2011) studied the relationship between fQRS in the inferior lead and the coronary lesion in 128 patients with coronary heart disease. They arrived at the same conclusion as this study that the incidence rate of fQRS was significantly higher in the inferior lead than in the other two leads (anterior and lateral). Furthermore, they pointed out that fQRS in the II, III and the aVF-lead could not be used to determine the number of coronary lesions (either to be single or multiple). Apoptosis appears to play a more important role in causing arrhythmogenic right ventricular cardiomyopathy (ARVC). Coincidently, the apoptosis usually occurs in the right ventricular segments adjacent to the diaphragm, as well as the apical (known as the triangle of dysplasia). Therefore, this might in some aspect explain the high incidence rate of fQRS located in the inferior lead. However, it is still unclear why the incidence rate of fQRS is notably higher in the inferior lead and further investigation is required to understand if it is related to the anatomical uniqueness where blood supplies from both directions of coronary arteries overlap or is simply due to the average level of cardiac electric axis.
It is well known that fQRS can be the indicator of the upcoming malignant arrhythmia, which is consistent with our results. However, in our study, Q waves were significant predictors of mortality (OR 1.934, 95% CI 1.121‒3.335, p=0.018) compared to fQRS (OR 1.687, 95% CI 0.978‒2.910, p=0.060). This indicates that Q wave still can be a valuable predictor of mortality while fQRS can assist physicians to evaluate the condition of their AMI patients with its high predict value of malignant arrhythmia or when there is no evidence of Q waves existing.
This study also showed that the positive fQRS group had higher rates in malignant cardiac arrhythmia, LVSD, and mortality than the non-fQRS group (p<0.05). The STEMI patients of positive fQRS had four times the incidence of malignant cardiac arrhythmia in comparison to the non-fQRS group (p<0.01). The rate of LVSD of the fQRS group was 7.5 times higher than that of the non-fQRS group (p<0.01). The mortality rate of the fQRS group was 2.4 times greater than that of the non-fQRS group (OR 2.44, 95% CI 1.044‒5.702, p=0.006). Between the subgroups of fQRS and non-fQRS in the NSTEMI patients, there was no statistical significance in malignant cardiac arrhythmia, LVSD, or mortality. It suggests that the presence of fQRS in STEMI patients might be strongly correlated to a higher incidence of cardiovascular events. Statistical analysis showed no significant variation in the incidence rates of fQRS between the STEMI and the NSTEMI patients (p>0.05), implying that fQRS was not correlated to the type of myocardial infarction. The result was probably due to the small sample size and further research with a larger population of subjects might be required to prove it. To understand if PCI was capable of reducing rates of malignant cardiac arrhythmia, LVSD, and mortality in patients with positive fQRS, the experimental results showed no difference in the number of malignant cardiac arrhythmia between the groups with and without PCI treatment (p=0.209). As for the LVSD and mortality, the p-values between the patients with and without PCI were 0.031 and 0.000, respectively. There were many reports using fQRS as an index to detect various cardiac structural abnormalities such as the arrhythmogenic right ventricular dysplasia (ARVD) (Peters et al., 2008; Das and El Masry, 2010; Zhou et al., 2011). And, many researchers have already proven a strong correlation between fQRS and malignant cardiac arrhythmia (Morita et al., 2008; Sha et al., 2011). In the study, results suggested that the incidence rate of malignant cardiac arrhythmia in the positive fQRS group was not related to revascularization (Erdoğan et al., 2012). It was postulated that the sample size with malignant cardiac arrhythmia was too small and further investigation with more subjects might be required. For patients with positive fQRS, early revascularization treatment can greatly reduce the incidence rates of LVSD and mortality. Liu et al. (2010) studied the effect of revascularization treatment at different periods for AMI patients and pointed out that early therapy could indeed reduce the fQRS rate and even improve the LVEF for patients. Therefore, by identifying fQRS, it can guide physicians in prescribing early revascularization treatment for patients and can improve their life quality, as well as the survival rate of AMI.
5. Conclusions
In summary, fQRS plays an important role in cardiovascular disease. To demonstrate the relation between fQRS and short-term prognosis of patients with AMI, we compared malignant cardiac arrhythmia, LVSD, and mortality rates between STEMI and NSTEMI patients. Early revascularization in patients with positive fQRS could lower the incidence of the cardiovascular events. Further studies are needed to clarify these interrelations.
Footnotes
Project supported by the National Science & Technology Pillar Program during the 12th Five-Year Plan Period (No. 2011BAI11B06), China
Compliance with ethics guidelines: Qin-hui SHENG, Chih-Chi HSU, Jian-ping LI, Tao HONG, and Yong HUO declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study. Additional informed consent was obtained from all patients for which identifying information is included in this article.
References
- 1.Carey MG, Luisi AJ, Jr, Baldwa S, et al. The Selvester QRS Score is more accurate than Q waves and fragmented QRS complexes using the Mason-Likar configuration in estimating infarct volume in patients with ischemic cardiomyopathy. J Electrocardiol. 2010;43(4):318–325. doi: 10.1016/j.jelectrocard.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheema A, Khalid A, Wimmer A, et al. Fragmented QRS and mortality risk in patients with left ventricular dysfunction. Circ Arrhythm Electrophysiol. 2010;3(4):339–344. doi: 10.1161/CIRCEP.110.940478. [DOI] [PubMed] [Google Scholar]
- 3.Das MK, El Masry H. Fragmented QRS and other depolarization abnormalities as a predictor of mortality and sudden cardiac death. Curr Opin Cardiol. 2010;25(1):59–64. doi: 10.1097/HCO.0b013e328333d35d. [DOI] [PubMed] [Google Scholar]
- 4.Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113(21):2495–2501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 5.Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12-lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4(11):1385–1392. doi: 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12-lead ECG: a sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol. 2008;1(4):258–268. doi: 10.1161/CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 7.Das MK, Michael MA, Suradi H, et al. Usefulness of fragmented QRS on a 12-lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol. 2009;104(12):1631–1637. doi: 10.1016/j.amjcard.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Erdoğan T, Kocaman SA, Çetin M, et al. Relationship of fragmented QRS complexes with inadequate coronary collaterals in patients with chronic total occlusion. J Cardiovasc Med (Hagerstown) 2012;13(8):499–504. doi: 10.2459/JCM.0b013e328353683c. [DOI] [PubMed] [Google Scholar]
- 9.Flowers NC, Horan LG, Thomas JR, et al. The anatomic basis for high-frequency components in the electrocardiogram. Circulation. 1969;39(4):531–539. doi: 10.1161/01.CIR.39.4.531. [DOI] [PubMed] [Google Scholar]
- 10.France RJ, Formolo JM, Penney DG. Value of notching and slurring of the resting QRS complex in the detection of ischemic heart disease. Clin Cardiol. 1990;13(3):190–196. doi: 10.1002/clc.4960130309. [DOI] [PubMed] [Google Scholar]
- 11.Frustaci A, Priori SG, Pieroni M, et al. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation. 2005;112(24):3680–3687. doi: 10.1161/CIRCULATIONAHA.105.520999. [DOI] [PubMed] [Google Scholar]
- 12.Gao RL. Guideline for the management of acute myocardial infarction. Chin Circ J. 2001;16(6):407–422. (in Chinese) [Google Scholar]
- 13.Gardner PI, Ursell PC, Fenoglio JJ, Jr, et al. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72(3):596–611. doi: 10.1161/01.CIR.72.3.596. [DOI] [PubMed] [Google Scholar]
- 14.Guo JH. Fragmented QRS. J Clin Electrocardiol. 2008;17(1):60–68. (in Chinese) [Google Scholar]
- 15.Liu JC, Zhang LY, Kou LY, et al. Influence of emergency PCI in different time windows to cardiac scar and cardiac function after myocardial infarction. Clin Med Chin. 2010;26(12):1277–1279. doi: 10.3760/cma.j.issn.1008-6315.2010.12.017. (in Chinese) [DOI] [Google Scholar]
- 16.Michael MA, El Masry H, Khan BR, et al. Electrocardiographic signs of remote myocardial infarction. Prog Cardiovasc Dis. 2007;50(3):198–208. doi: 10.1016/j.pcad.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118(17):1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 18.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 19.Peters S, Trümmel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia-cardiomyopathy. Heart Rhythm. 2008;5(10):1417–1421. doi: 10.1016/j.hrthm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Sha J, Zhang S, Tang M, et al. Fragmented QRS is associated with all-cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol. 2011;16(3):270–275. doi: 10.1111/j.1542-474X.2011.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Zhao HN. Investigation of changing and significance of connexin43, [Ca2+]i and apoptosis in early phase of acute myocardial ischemia. Chin J Cardiac Pacing Electrophysiol. 2011;4(25):349–352. (in Chinese) [Google Scholar]
- 22.Xu AG, Qi XQ. The contrast analysis on the prognostic values of fragmented QRS and pathologic Q-wave for old myocardial infarction. Shandong Med J. 2009;49(9):50–51. (in Chinese) [Google Scholar]
- 23.Yao JD, Shen B, Lu CX, et al. Clinical significance of fragmented QRS complex in II, III, aVF leads with coronary artery disease. Clin J Med Officer. 2011;39(2):214–216. (in Chinese) [Google Scholar]
- 24.Zhang Y, Liu XP, Yan Q, et al. QRS complexes in healthy adults: prevalence and implications. J Clin Cardiol. 2011;27(4):299–302. (in Chinese) [Google Scholar]
- 25.Zhou XJ, Yang B, Wang J, et al. Diagnostic value of fragmented QRS complex in patients with arrhythmogenic right ventricular cardiomyopathy. Chin J Cardiac Arrhyth. 2011;15(1):23–26. doi: 10.3760/cma.j.issn.1007-6638.2011.01.004. (in Chinese) [DOI] [Google Scholar]