Abstract
Background: Idiopathic Membranous Nephropathy (iMN) is the most common cause of nephrotic syndrome in adults. Approximately one third of patients with iMN progress to end-stage renal disease. Anti-phospholipase A2-receptor (anti-PLA2R) antibodies are present in patients with iMN and appear to play a role in the pathogenesis of iMN. Objectives: In this study, we explored the prevalence of anti-PLA2R antibodies in a cohort of patients with iMN in Iran. We also sought to determine circulating levels of anti-secretory PLA2 (anti-sPLA2) antibodies in those with anti-PLA2R antibodies.
Patients and Methods: Using an indirect immunofluorescence assay, we measured anti-PLA2R antibodies in a group of patients with iMN in Iran. The serum levels of anti-sPLA2 antibodies were also measured in those with positive results for anti-PLA2R antibodies.
Results: We studied 23 patients with iMN (M/F 12/11, 34±9.8 year), two patients with secondary MN and five patients with the nephrotic syndrome of other causes.Anti-PLA2R antibodies were detected in 17/23 (74%) of patients with iMN, but not in those with secondary MN or other forms of primary glomerular diseases. We found no correlation between anti-PLA2R antibody titer and the degree of proteinuria. We found high titers of anti-sPLA2 antibodies in a subset of patients with high levels of anti-PLA2R antibodies.
Conclusions: Anti-PLA2R antibodies are specific for iMN. Proteinuria may also reflect glomerular structural damage rather than immunological activity of the disease. The preliminary idea of any presumptive role of anti-sPLA2antibodies in iMN needs further investigation.
Keywords: Idiopathic membranous nephropathy, Anti-phospholipase A2-receptor antibodies, Anti-phospholipase A2 antibodies nephrotic syndrom, End-stage renal disease
1. Background
Idiopathic membranous nephropathy (iMN) is the most common cause of nephrotic syndrome in adults. Approximately one third of patients with iMN progress to end-stage renal disease (1).In the past half century, significant effort has been made to identify the target antigens in iMN. In Heymann nephritis, a rat model of membranous nephropathy, megalin was shown to be the target antigen. However, megalin is not expressed in human podocytes (1). In 2002, neutral endopeptidase was identified as the responsible antigen in a rare subset of infants with alloimmune antenatal membranous nephropathy (2).
In 2009, the landmark study by Beck and colleagues demonstrated that the target antigen in iMN is mainly a M-type trans membrane phospholipase A2 receptor (PLA2R) located on podocytes and circulating antibody is mainly a non-complement binding-IgG4 antibody subclass. Beck et al. found that serum from 26 of 37 patients (70%) with iMN, but not secondary MN, specifically identified PLA2R on podocytes (1). In a Chinese study, 49 out of 60 patients (82%) with iMN demonstrated anti-PLA2R antibodies in their serum, using the Western blot assay (3). In a European cohort, 14 out of 18 patients (78%) with iMN showed anti-PLA2R antibodies. Serum levels of anti-PLA2R antibodies decreased significantly during remission and increased again with relapses (4). Anti-PLA2R antibodies were also found in 5 out of 10 patients with iMN who received renal transplantation (5). Recently, Hoxha and co-workers presented a new indirect immunofluorescence test to detect anti-PLA2R antibodies in serum. In their report, 52% of patients with biopsy-proven iMN, but none of those with secondary MN, had positive results for anti-PLA2R antibodies in their serum (6). PLA2R has been accepted as the major target antigen in iMN, however the triggers of antibody production and its mechanisms of action are still unknown. The super family of PLA2 is comprised of a heterogeneous group of proteins and enzymes, including secreted PLA2 (sPLA2), cytoplasmicPLA2 (cPLA2), and lysosomal PLA2 (lPLA2) (7). They occur ubiquitously in nature as both intracellular and extracellular forms and hydrolyze various phospholipids. They play pivotal roles in various biological activities and many are toxic (7,8).
2. Objectives
In this study, we examined the presence of anti-PLA2R antibodies in an Iranian cohort of patients with iMN. We also studied the serum levels of anti-sPLA2 antibodies in those with positive results for anti-PLA2R antibodies.
3. Patients and Methods
3.1. Patient selection
All patients with MN were diagnosed by renal biopsy and had greater than 3.5 g/day proteinuria at the time of diagnosis. MN was considered to be idiopathic (iMN) when no secondary cause of MN was suspected. Lupus MN was diagnosed on the basis of clinical diagnosis and the presence of antibodies directed against nuclear antigens (ANA) including double-stranded DNA, Smith, Ro (SSA) and La (SSB) antigens. Hepatitis B (HBV) and hepatitis C(HCV)-associated MN were diagnosed by HBV and HCV serologic tests as well as HBV-DNA and HCV-RNA tests. Solid tumor-associated MN was ruled out on clinical ground. All patients were known cases of MN for a long period.
3.2. Ethical issues
This project was approved by the ethic comity of Tabriz University of Medical Sciences.
3.3. Serum samples
Serum samples were collected at outpatient clinics and remained frozen until the time of serologic studies. Patients were categorized as active iMN when proteinuria was greater than 3.5 g/day and serum albumin was less than 3.0 g/dl; in remission when urinary protein was less than 1.0 g/day; and in relapse when proteinuria increased again to greater than 3.5 g/day after a period of remission. We also examined sera from patients with secondary MN and other forms of primary glomerular diseases.
3.3.1. Measurement of anti-PLA2R antibodies
The detection of circulating antibodies against PLA2R was performed using an indirect immunofluorescence test (IFA, EUROIMMUN AG, Lubeck, Germany) (6). The slide contained a mosaic of two different biochips in one incubation field. The first biochip is coated with HEK293 cells (a human embryonic kidney cell line) transfected with PLA2R. The second biochip contained non-transfected HEK293 cells as substrates. For measuring anti-PLA2R antibodies, serum samples were diluted 1/10 in phosphate-buffered saline containing 0.2% Tween and incubated for 30 min using the recombinant cell-based assay. A fluorescein isothiocyanate (FITC)-conjugated anti-human IgG antibody from goat (EUROIMMUN AG, Lubeck, Germany) was used to detect bound antibodies using an Immunofluorescence (IF) microscope. Two independent observers evaluated all immunostains. A specific cytoplasmic fluorescence of transfected cells at a dilution of 1:10 or higher was considered as positive. In those who were positive at 1:10 dilution, we continued dilution up to 1:100 and1:1000 and reexamined the positivity.
3.4. Measurement of anti-secreted PLA2 (anti-sPLA2) antibodies
As a complementary observation to our study, we also studied serum samples with strong positive results for anti-PLA2R antibodies for the presence of anti-sPLA2 antibodies using an ELISA kit (sPLA2, USCN Life Science Inc., china).The micro tubes were precoated with monoclonal antibodies directed against sPLA2. Samples were added to appropriate micro titer plate wells with biotin-conjugated polyclonal antibody preparation specific for sPLA2. Then Avidin conjugated to horseradish peroxidase (HRP) was added to each microplate well and incubated. Thereafter, a TBM substrate solution was added to each well. Only those wells that contain PLA2, Biotin conjugated antibody and enzyme-conjugated Avidin exhibit a change in color. The enzyme-substrate reaction was terminated by adding sulfuric acid. The color change was measured using a spectrophotometer at a wavelength of 450+/-10nm. The concentration of sPLA2 antibodies in the samples was then determined (pictogram/deciliter) by comparing the optical density (OD) with the standard curve.
3.5.Statistics analysis
Statistics analysis was performed using SPSS software version 16. For correlation analysis, Spearman analysis test was used. The differences were considered significant with a P value <0.05.
4. Results
Sera from 23 patients (12 men and 11 women; average age 34+/-9.8 years) with iMN were examined ( table 1). In addition, sera from two patients with secondary MN (one secondary to SLE and another due to HBV) and five patients with nephrotic syndrome due to other forms of primary glomerular diseases were examined ( table 1). Patients with iMN were on quite same treatment at the time of serum collection and received angiotensin converting enzyme inhibitor (ACE) and angiotensin receptor blockers (ARB) combined with corticosteroids and cyclosporine. None of them received Rituximab.
Table 1 . Baseline characteristics of all patients are given in Table 1 .
| Treatment |
Scr
(mg/dl) |
U/Pro
(g/d) |
sPLA2
(Pg/ml) |
aPLA2-R |
diag/dur
(months) |
Age/sex | Pts |
| Cyclo/P/ACE/ARB | 1.6 | 800 | nd | Neg | iMN/63 | 55/M | 1 |
| Cyclo/P/ACE/ARB | 1.2 | 700 | nd | Neg | iMN/71 | 48/M | 2 |
| Cyclo/P/ACE/ARB/MMF | 1.3 | 2700 | 248 | Neg | iMN/37 | 35/F | 3 |
| Cyclo/P/ACE/ARB | 1.7 | 1100 | nd | Neg | iMN/23 | 41/M | 4 |
| Cyclo/P/ACE/ARB | 2.2 | 3000 | 31 | Neg | iMN/16 | 38/F | 5 |
| Cyclo/P/ACE/ARB | 1.2 | 3200 | nd | Neg | iMN/11 | 27/F | 6 |
| Cyclo/P/ACE/ARB/MMF | 9.5 | 800 | nd | 1/10 | iMN/67 | 52/F | 7. Ƕ |
| Cyclo/P/ACE/ARB | 1.4 | 3600 | 2200 | 1/100 | iMN/15 | 38/M | 8 |
| Cyclo/P/ACE/ARB | 1.0 | 4300 | nd | 1/200 | iMN /10 | 25/M | 9 |
| Cyclo/P/ACE/ARB | 2.0 | 1900 | nd | 1/10 | iMN/8 | 32/F | 10. |
| Cyclo/P/ACE/ARB | 1.2 | 900 | nd | 1/10 | iMN/ 15 | 25/F | 11. |
| Cyclo/P/ACE/ARB | 1.3 | 1200 | nd | 1/10 | iMN/ 14 | 27/F | 12. |
| Cyclo/P/ACE/ARB | 2.0 | 3900 | nd | 1/10 | iMN / 7 | 30/F | 13. |
| Cyclo/P/ MMF/ACE | 2.0 | 770 | nd | 1/10 | iMN/ 8 | 28/M | 14. Ţ |
| Cyclo/P/ACE/ARB | 2.0 | 4900 | 2524 | 1/800 | iMN /11 | 31/M | 15. |
| Cyclo/P/ACE/ARB | 2.0 | 1770 | 2660 | 1/400 | iMN /16 | 25/F | 16. |
| Cyclo/P/ACE/ARB | 1.6 | 2400 | nd | 1/200 | iMN/ 12 | 34/M | 17. |
| Cyclo/P/ACE/ARB | 1.7 | 2200 | 2000 | 1/200 | iMN/ 9 | 28/M | 18. |
| Cyclo/P/ACE/ARB | 1.0 | 900 | nd | 1/10 - | iMN/ 10 | 18/M | 19. |
| Cyclo/P/ACE/ARB | 2.2 | 3800 | 1100 | 1/10 | iMN/ 56 | 50/F | 20. |
| Cyclo/P/ACE/ARB | 1.5 | 3100 | 7700 | 1/800 | iMN/17 | 29/M | 21. |
| Cyclo/P/ACE/ARB | 1.2 | 2700 | 10863 | 1/1000 | iMN/12 | 33/M | 22. |
| Cyclo/P/ACE/ARB | 1.4 | 4300 | 4381 | 1/800 | iMN/20 | 30/F | 23. |
| Cyclo/P/ACE/ARB | 1.7 | 2100 | nd | Neg | SLE(MN)/23 | 26/F | 24. |
| ACE/ARB | 2.1 | 2800 | nd | Neg | HBV(MN)/ 14 | 28/M | 25. |
| Cyclo/P/ACE/ARB | 1.1 | 4100 | nd | Neg | FSGS/16 | 22/M | 26. |
| Cyclo/P/ACE/ARB | 1.3 | 1100 | nd | Neg | FSGS/45 | 18/M | 27. |
| Cyclo/P/ACE/ARB | 3.1 | 3500 | nd | Neg | FSGS/38 | 21/F | 28. |
| Cyclo/P/ACE/ARB | 1.8 | 5500 | nd | Neg | MCD/24 | 24/M | 29. |
| Cyclo/P/ACE/ARB | 1.1 | 2800 | nd | Neg | MPGN/30 | 23/M | 30. |
Anti-PLA2R antibodies were only found in patients with iMN, but not in those with secondary MN or in those with nephrotic syndrome due to other glomerulopathies (Table 1). Anti-PLA2R antibodies were detected in74% (17/23) of patients with iMN. The titer of Anti-PLA2R antibodies, however, did not correlate with the degree of proteinuria (Figure 1). Anti-PLA2R antibodies were still detectable in a patient who was on hemodialysis and in another one who had been transplanted two years prior.
Figure 1.
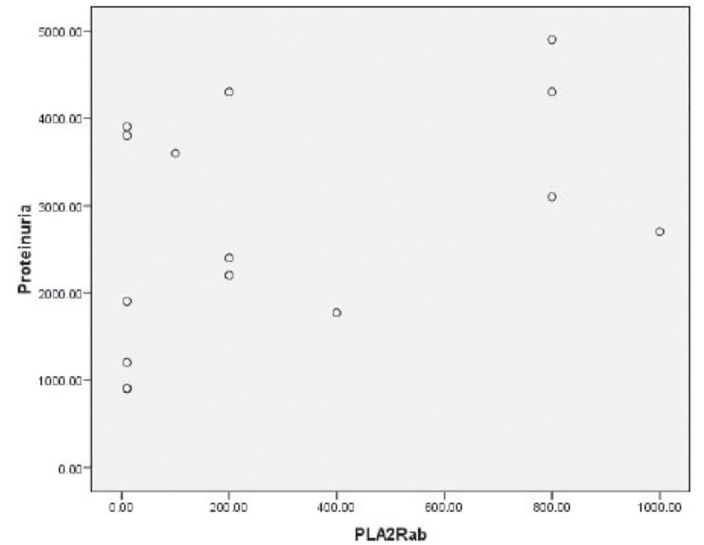
There was no correlation between anti-phospholipase A2 receptor antibody (PLA2R-Ab, florescence reaction of the serum at a dilution of 1:10 or higher) with the degree of proteinuria (mg/day). Pearson correlation analysis (p>0.05).
Patients with iMN and high titers of anti-PLA2R antibodies also had high titers of anti-sPLA2 antibodies ( Figure 2 ). There was a direct correlation between the levels of anti-PLA2R and anti-sPLA2 antibodies (p< 0.05) ( Figure 2). Furthermore, two patients with undetectableanti-PLA2R antibodies had low titers ofanti-sPLA2 antibodies.
Figure 2.

Anti-phospholipase A2 receptor antibody titer (PLA2R-Ab, florescence reaction of the serum at a dilution of 1:10 or higher) significantly correlated with the serum levels of anti-secreted PLA2 antibody (sPLA2, pictogram/dl), Kendall Tau-b analysis (p<0.05).
5. Discussion
In this study, we determined the presence of anti-PLA2R antibodies, using indirect IF method, in the serum of a cohort of patients with iMN from Iran.Anti-PLA2R antibodies were detected in 74% of patients with iMN. In our study, the titer of circulatinganti-PLA2R antibodies did not correlate with the degree of proteinuria as an index of disease activity. A group of patients who had high titers of anti-PLA2R antibodies also had high titers of anti-sPLA2 antibodies in their serum. There was a strong direct correlation between anti-PLA2R and anti-sPLA2 antibodies. Our results demonstrated the specificity of anti-PLA2R antibodies as a useful marker for the diagnosis of iMN. In addition, the presence of anti-PLA2R antibodies distinguished between iMN and secondary MN.
In the study by Hofstra et al., anti-PLA2R antibodies were assessed by a Western blot immunoassay in 54 serum samples from 18 patients with iMN, collected in various stages of their clinical disease. A direct correlation was found between the level of anti-PLA2R antibodies and the degree of proteinuria (4). Beck et al. found that serum levels of anti-PLA2R antibodies in patients with iMN decreased significantly after Rituximab therapy in the majority of responders (9). Data presented by Hoxha et al. indicated that a reduction in the levels of anti-PLA2R antibodies was followed by a decrease in proteinuria in patients treated with Rituximab (6). Higher titers of anti-PLA2R antibodies in iMN may lead to increased subepithelial immune complex formation resulting in more severe podocyte injury. Based on these observations, anti-PLA2R antibody titers may serve as a useful tool in monitoring the disease activity in iMN. Moreover, they might assist in selecting patients with more active disease that could benefit from immunosuppression and in excluding those with less active disease (4,6).
In our study, 17/23 (74%) patients with iMN had detectable anti-PLA2R antibodies. However, there was no correlation between the degree of proteinuria and anti-PLA2R antibody titers. This could be due to the small number of patients enrolled in the study. We also speculate that the high degree of proteinuria in some patients could have been secondary to structural glomerular damage rather than immunological activity of the disease.
Approximately one third of patients with iMN with nephrotic syndrome may develop spontaneous remission (SR)(10). Positive predictors of SR were age at presentation less than 50 years, female sex, baseline proteinuria less than 8 g/24 hand a decline in proteinuria greater than 50% during the first year of follow-up (10). In our study, none of patients received Rituximab and probably five patients(M/F 3/2) who were in remission during the sample collection probably were in their SR. Anti-PLA2R antibodies were undetectable in six patients with iMN: three patients had mild proteinuria and were probably in remission, while the other three had significant proteinuria. We should take into consideration that proteinuria does not necessarily imply immunological activity as it may reflect irreversible structural changes in the glomeruli or interstitium that can persist even after immunological remission and disappearance of anti-PLA2R antibodies (11). Alternatively, patients with negative results for anti-PLA2R antibodies might have been misclassified as iMN and actually they might have had secondary MN (4,11). The absence of circulating anti-PLA2R antibodies at the time of measurement does not exclude a role for anti-PLA2R antibodies in the pathogenesis of iMN, since rapid clearance from circulation and deposition of antibodies in glomeruli may occur (12,13). Therefore, measurement of both circulating and tissue levels ofanti-PLA2R antibodies might be necessary (12,13).
Despite recent developments, the triggers of anti-PLA2R antibody production and mechanisms of its action remain unknown. In Heymann nephritis, subepithelial immune complex formation results in complement activation and formation of membrane attack complex (C5b-9)(14,15). Complement activation causes podocyte injury through activation of cytoplasmic PLA2 (cPLA2) and actin cytoskeleton collapse (14,15).In humans, anti-PLA2R antibodies are mainly IgG4 that weakly activates the complement system (3,16). Whether IgG4 and PLA2R interaction in humans is similar to complement-mediated podocyte injury in Heymann nephritis remains to be seen. The antibody response in iMN may be directed against a “risk” PLA2-R epitope on podocytes in genetically susceptible individuals (13,17). Inflammatory cytokines such as IL-6, TNF-α, and IL-1ß stimulate synthesis and release of sPLA2 from different cells (18). In mammals, sPLA2 binding to PLA2R induce pro-inflammatory signaling. In humans, PLA2R is expressed not only on podocytes but also in the lungs, pancreas, placenta, and skeletal muscle cells (18,19). It is postulated that inflammatory cytokine-induced sPLA2 production could be a trigger for anti-PLA2R antibodies production (19). Alternatively, IgG4 anti-PLA2R antibodies could compete with sPLA2 for receptor binding (19).
6. Conclusions
That is the first report from Iran confirming that anti-PLA2R antibodies are specific for iMN as it has been shown in other studies. While we only measured anti-PLA2R antibodies at one time point, we cannot conclude that there is no correlation between antibody level and degree of proteinuria in our iMN patients. Measurement of anti-PLA2R antibodies activity in a selected group of patients with high serum levels of anti-sPLA2 antibodies was just a preliminary study. Because of the small number of patients in subgroups, a conclusion could not be reached. However, it provides a basis for in-depth studies in the future to examine whether there is a connection between anti-sPLA2 andanti-PLA2Rantibodies.
Authors’ contributions
MRA designed and managed the research. MRA, HN and AG gathered the specimens. JM analyzed the data and wrote some parts of paper. BN also provided extensive intellectual contribution. HN and BB reviewed the draft too. MRA prepared the final draft.
Funding/Support
This study was supported by a grant from Tabriz University of Medical Sciences.
Conflict of interest
The authors declare that there were no conflicts of interest throughout the study.
Implication for health policy/practice/research/medical education:
Idiopathic membranous nephropathy should no longer be considered “idiopathic”. Anti phospholipase A2-receptor (anti-PLA2R) antibodies are a useful method for its diagnosis. However, more studies are needed to confirm its presence in different population and exploring its mechanisms of action in the pathogenesis of idiopathic membranous nephropathy.
Please cite this paper as: Ardalan MR, Ghafari A, Hamzavi F, Nasri H, Baradaran B, Majidi J, Nikbin B.Antiphospholipase A2 receptor antibody in idiopathic membranous nephropathy: A report from Iranian population.J Nephropathology. 2013; 2(4): 241-248, DOI: 10.5812/nephropathol.11631
References
- 1.Beck LH Jr, Bonegio RG. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debiec H, Guigonis V, Mougenot B. et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346:2053–60. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 3.Qin W, Beck LH Jr, Zeng C. et al. Anti-Phospholipase A2 Receptor Antibody in Membranous Nephropathy. J Am Soc Nephrol. 2011;22:1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofstra JM, Beck LH Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–91. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debiec H, Martin L, Jouanneau C. et al. Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. Am J Transplant. 2011;11:2144–52. doi: 10.1111/j.1600-6143.2011.03643.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoxha E, Harendza S. et al. An immunofluorescence test for phospholipase-A2 -receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 7.Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochimica et Biophysica Acta. 2006;11:1246–59. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42:827–840. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Beck LH Jr, Fervenza FC, Beck DM. et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polanco N, Gutierrez E. et al. Spontaneous Remission of Nephrotic Syndrome in Idiopathic Membranous Nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cravedi P, Ruggenenti R, Remuzzi G. Circulating Anti-PLA2R Autoantibodies to Monitor Immunological Activity in Membranous Nephropathy. J Am Soc Nephrol. 2011;22:1391–1402. doi: 10.1681/ASN.2011060610. [DOI] [PubMed] [Google Scholar]
- 12.Debiec H, Ronco P. Nephrotic syndrome: A new specific test for idiopathic membranous nephropathy. Nat Rev Nephrol. 2011;7:496–8. doi: 10.1038/nrneph.2011.106. [DOI] [PubMed] [Google Scholar]
- 13.Debiec H, Ronco P. PLA R autoantibodiesand PLA R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 14.Cybulsky AV, Quigg RJ, Salant DJ. Experimental membranous nephropathy redux. Am J Physiol Renal Physiol. 2005;289(4):F660–71. doi: 10.1152/ajprenal.00437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murtas C, Ravani P, Ghiggeri GM. New insights into membranous glomerulonephritis: from bench to bedside. Nephrol Dial Transplant. 2011;26(8):2428–30. doi: 10.1093/ndt/gfr336. [DOI] [PubMed] [Google Scholar]
- 16.Hofstra JM, Debiec H. et al. Antiphospholipase A Receptor Antibody Titer and Subclass in Idiopathic Membranous Nephropathy. J Am Soc Nephrol. 2012;23:1735–1743. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanescu HC, Arcos-Burgos M. et al. Risk HLA-DQA1 and PLA R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616–626. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
- 18.Granata F, Petraroli A, Boilard E. et al. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the Mtype receptor. J Immunol. 2005;174(1):464–74. doi: 10.4049/jimmunol.174.1.464. [DOI] [PubMed] [Google Scholar]
- 19.Ardalan MR. Triggers, Bullets and Targets, Puzzle of Membranous Nephropathy. Nephro-Urology Monthly. 2012;4:599–602. doi: 10.5812/numonthly.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]


