Abstract
Background: Ethylene glycol ingestion can lead to acute kidney injury from tubular deposition of oxalate crystals. The diagnosis of ethylene glycol intoxication is based on a history of ingestion, clinical examination, high anion gap metabolic acidosis, high osmolal gap, and a measured serum level of ethylene glycol. However, depending on the delay in time from ingestion to arrival to a hospital, the osmolal gap may become normal, thereby creating a confusing clinic picture for the treating clinician.
Case: A 71 year-old man with a history of alcohol abuse had been unconscious for an unknown period of time. Upon hospitalization, he was found to have a high anion gap metabolic acidosis but a normal serum osmolal gap and subsequently developed acute kidney injury. The serum lactic acid and glucose levels were unremarkable, and there were no ketones in the serum. Urine analysis showed numerous red blood cells and calcium oxalate crystals. The renal biopsy showed multiple oxalate crystals in the renal tubules demonstrating birefringence under polarized light. Given the history of alcohol abuse, the clinical presentation, the unexplained high anion gap metabolic acidosis, and the biopsy findings, ethylene glycol intoxication was deemed the most likely diagnosis.
Conclusions: In cases of ethylene glycol intoxication, a high serum osmolal gap is supportive of ethylene glycol intoxication, but a normal serum osmolal gap does not exclude the diagnosis, especially when the time of ingestion is unknown. Physicians should be aware of potentially normal serum osmolal gap values in cases of ethylene glycol intoxication.
Keywords: Acute Kidney Injury, Oxalate Nephropathy, Ethylene Glycol, Normal Serum Osmolal Gap
Introduction
Ethylene glycol is a common component of automotive radiator antifreeze solution. It is colorless, odorless, sweet tasting and is a known human toxin. Ethylene glycol is sometimes used as a substitute for ethanol by alcoholics; it can be ingested accidentally or taken in a suicidal or homicidal attempt.
Ethylene glycol reaches a peak serum level 2 to 4 hours after ingestion. It is oxidized by alcohol dehydrogenase to glycolaldehyde, which is then rapidly oxidized to glycolate (1). Its active metabolites (glycolate, oxalate, and other toxic acids) can cause acute renal injury, hypocalcemia, damage to the central nervous system and cranial nerves, and cardiovascular instability (2). The elimination half- life of ethylene glycol without treatment is 3 to 8 hours.
Case
A 71-year-old white male presented to the hospital after being found unconscious for an unknown period of time. The wife reported that the patient was in his usual state of health the night before without fever, nausea, vomiting or changes in his behavior. He had a history of diabetes mellitus type 2, hypertension, alcohol abuse, mild dementia, angioedema induced by lisinopril, and ischemic stroke diagnosed two months ago. The patient had a visit to the emergency department three weeks prior complaining of abdominal pain, and was discharged home without a definitive diagnosis.
On examination, the rectal temperature was 33°C, the pulse was 106 beats per minute, the blood pressure was 230/110 mm Hg, and the respiratory rate was 25 breaths per minute. The trachea was intubated and assisted ventilation in the intensive care unit was begun.
Laboratory values on the day of admission to the hospital: white blood cell count 15,600 per mm3 with 85% polymorphonuclear cells, hemoglobin 18.2 g/dl, sodium 141 mEq/L, potassium 4.9 mEq/L, chloride 108 mEq/L, bicarbonate 9 mEq/L, glucose 181 mg/dL, calcium 10 mg/dL, blood urea nitrogen 14 mg/dL. The serum creatinine on admission was 1.26 mg/dl and subsequently rose to 2.88 mg/dL and 5.34 mg/dL on the two subsequent hospital days. The baseline serum creatinine value was 1.05 mg/dL three weeks prior to the hospital admission. Upon admission, the serum anion gap was 24, measured osmolality 305 mOsm/Kg, calculated osmolarity 297 mOsm/L, and the serum osmolal gap was only 8. The arterial blood gas on admission showed: pH 7.185, pCO2 12.3, and pO2 187 with supplemental oxygen by face mask. The lactic acid, creatine kinase, C3, and C4 complement levels were within normal limits. The salicylate and ethanol levels were undetectable; the urine toxicology screen, Antinuclear Antibody Test (ANA), Human Immunodeficiency Virus (HIV), and the hepatitis B and C serologies were negative. The initial urinalysis showed no protein, no glucose, no blood by dipstick, and no cellular elements or crystals. The subsequent urine analyzed was red in color, turbid in appearance, had 3+ protein, 10 to 15 white cells per High Power Field (HPF), too numerous to count red cells, and calcium oxalate crystals were present. Cerebrospinal fluid analysis and renal ultrasonography were normal. In the following days, the patient developed worsening metabolic acidosis and azotemia with a gradual increase of creatinine up to 13.27 mg/dl as well as anuria.
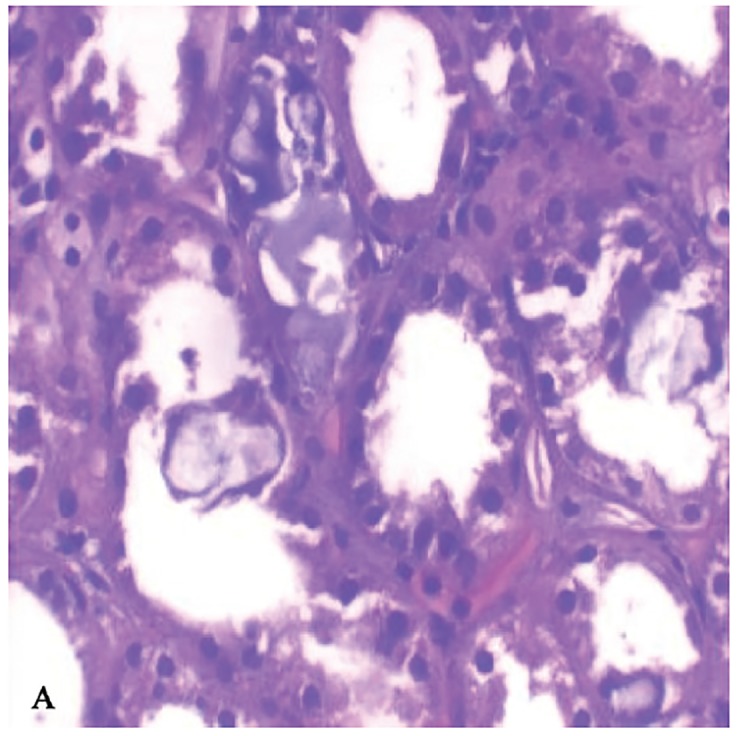
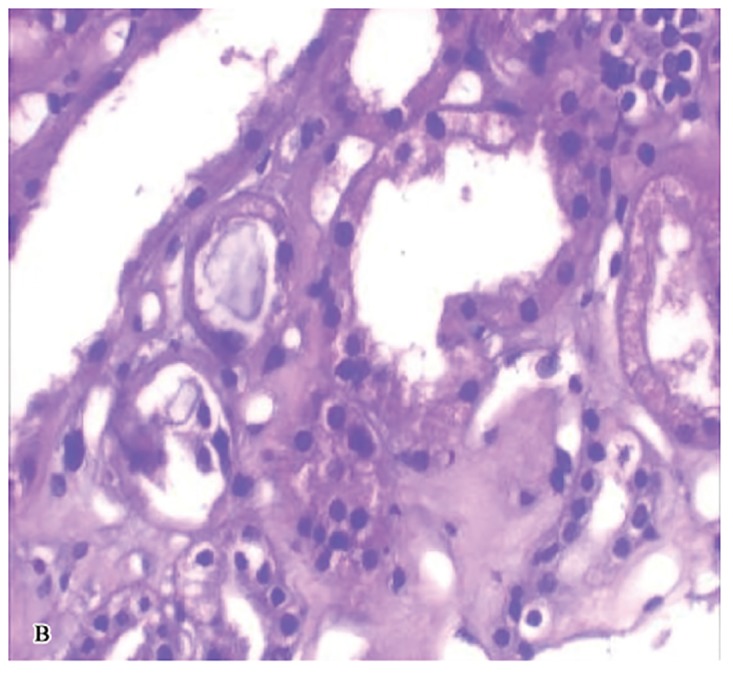
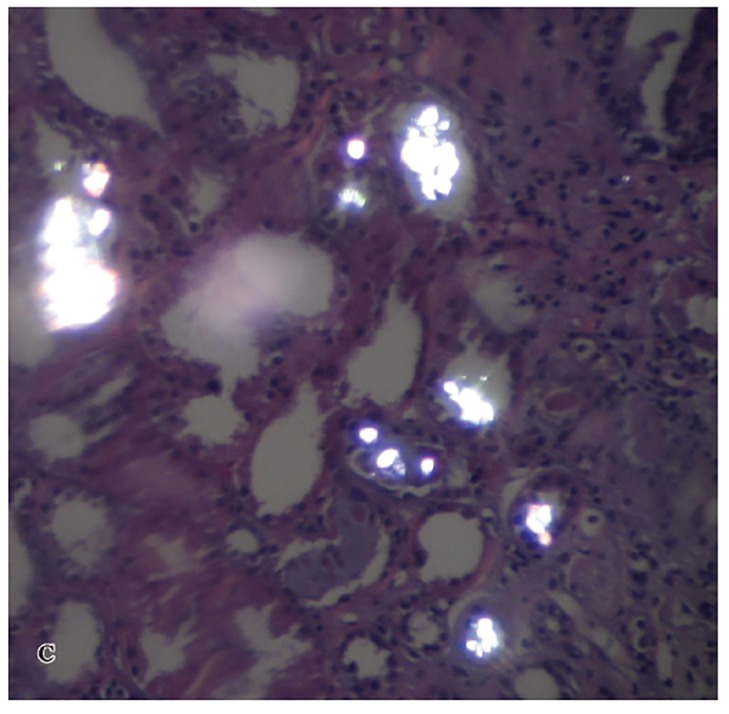
A kidney biopsy was performed on the fourth day of admission and showed multiple oxalate crystals in the renal tubules demonstrating birefringence under polarized light (Figures 1A, 1B, and 1C). The tubules containing the crystals showed degenerative changes and focal tubular necrosis; no oxalate crystals were identified in the interstitial compartments.
Figure 1A & 1B.
Renal biopsy light micrographs showing dilated tubules with reactive and degenerative changes and intraluminal oxalate crystals (H & E stain 40X).
Figure 1C.Intraluminal tubular oxalate crystals in polarized light (H & E, 40X).
A.
B.
C.
Given the history of alcohol abuse, the clinical presentation, the unexplained high anion gap metabolic acidosis, and the biopsy findings, ethylene glycol intoxication was deemed the most likely diagnosis, though, unfortunately, a serum ethylene glycol level was never obtained.
During the hospitalization, the patient required intermittent hemodialysis. Eventually, he was extubated and transferred to a skilled nursing facility for rehabilitative treatment.
Discussion
The diagnosis of ethylene glycol (EG) intoxication is based on a history of ingestion, clinical examination, high serum anion gap metabolic acidosis, high serum osmolal gap, hypocalcemia, and an elevated serum level of EG.
However, the diagnosis can be difficult to ascertain in some cases. The alcoholic or suicidal patient may be unable to provide a history of ingestion. The measurement of EG serum level is not available in many hospitals, and results may not be immediately available. Therefore, the use of the osmolal gap is important in this setting. The important question in the case presented is, does a normal osmolal gap rule out EG intoxication?
While the high anion gap metabolic acidosis is mainly due to glycolate and its metabolites, and some part to lactate production. The high serum osmolal gap is due to ethylene glycol itself (3). The osmolal gap is the difference between the measured osmolality and the calculated osmolarity. There are 15 different equations for calculating the serum osmolarity. The one that is most commonly used is: 2*[Na] + [glucose] /18 + [BUN] / 2.8 + [Ethanol] / 4.6. The osmolal gap is considered high if it is > 10 mOsm/Kg.
However, the high osmolal gap lacks the sensitivity to be an ideal test to rule out ethylene glycol intoxication. As ethylene glycol is osmotically active, it raises the measured osmolality. Thus, it forms the high osmolal gap, whereas, the glycolate does not contribute to the osmolal gap. Thus, as ethylene glycol is metabolized, the serum osmolality will begin to decrease, whereas the serum anion gap will rise further or remain elevated (4).
Therefore, patients who present late after the EG ingestion may have a normal osmolal gap. Another factor is the considerable variation in the normal osmolal gap in the general population (5). Assessing measured osmolality by vapor pressure instead of freezing point depression is another reason for an absent osmolal gap when volatile alcohols are ingested. Thus, a high osmolal gap is supportive of ethylene glycol intoxication, but a normal osmolal gap does not exclude the diagnosis.
Treatment of EG intoxication includes antidotes that inhibit the metabolism of ethylene glycol to its toxic metabolites (glycolate, oxalate, and other toxic acids). Ethanol as an antidote has been used historically, but only fomepizole has been approved by the US Food and Drug Administration (6). Fomepizole has few side effects, is generally very safe and more convenient to use than alcohol, but is also much more expensive (1).
Hemodialysis is very effective at clearing ethylene glycol and its metabolites and corrects the metabolic acidosis. In the absence of renal dysfunction and significant metabolic acidosis, the use of fomepizole may eliminate the need for hemodialysis (6).
Few case reports have described the finding of normal osmolal gap in ethylene glycol intoxication (7,8). However, and despite the suspicion of ethylene glycol poisoning (high anion gap metabolic acidosis, and the presence of oxalate crystal in the urine), physicians hesitate to use fomepizole when there is a normal osmolal gap.
Conclusions
Physicians should be aware of potentially normal serum osmolal gap values in cases of EG intoxication. In the appropriate clinical setting consistent with EG intoxication, a normal serum osmolal gap should not be relied upon to exclude the diagnosis and withhold checking the EG serum level and treatment.
Authors’ contributions
TA wrote the primary draft. JEB reported the pathology. JB and ATM provided extensive intellectual contribution. GTH provided extensive senior intellectual contribution and prepared the final manuscript.
Conflict of interest
The author declared no competing interests.
Funding/Support
None declared
Implication for health policy/practice/research/medical education:
Physicians should be aware of potentially normal serum osmolal gap values in cases of ethylene glycol intoxication. In the appropriate clinical setting consistent with ethylene glycol intoxication, a normal serum osmolal gap should not be relied upon to exclude the diagnosis and withhold checking the ethylene glycol serum level and treatment.
Please cite this paper as: Alhamad T, Blandon J, Meza AT, Bilbao JE, Hernandez GT. Acute kidney injury with oxalate deposition in a patient with a high anion gap metabolic acidosis and a normal osmolal gap. J Nephropathology. 2013; 2(2): 139-143. DOI: 10.5812/nephropathol.10657
References
- 1.Jacobsen D, McMartin KE. Antidotes for methanol and ethylene glycol poisoning. Clin Toxicol. 1997;35:127–43. doi: 10.3109/15563659709001182. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen D, McMartin KE. Methanol and ethylene glycol poisonings: mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol. 1986;1:309–34. doi: 10.1007/BF03259846. [DOI] [PubMed] [Google Scholar]
- 3.Abramson S, Singh A. Treatment of the alcohol intoxication: Ethylene glycol, methanol and isopropranolol. Current opinion in nephrology and hypertension. 2000; 9:695–701. doi: 10.1097/00041552-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Kraut JA, Kurtz I. Toxic Alcohol Ingestions: Clinical Features, Diagnosis, and Management. Clin J Am SocNephrol. 2008;3:208–225. doi: 10.2215/CJN.03220807. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman RS, Smilkstein MJ, Howland MA. Osmol gaps revisited: normal values and implications. Clin Toxico. 1993;31:81–93. doi: 10.3109/15563659309000375. [DOI] [PubMed] [Google Scholar]
- 6.Barceloux DG, Krenzelok EP, Olson K, Watson W. American Academy of Clinical Toxicology Practice Guidelines on the Treatment of Ethylene Glycol Poisoning. J Toxicol Clin Toxicol. 1999;37(5):537–60. doi: 10.1081/clt-100102445. [DOI] [PubMed] [Google Scholar]
- 7.Steinhart B. Severe ethylene glycol intoxication with normal osmolal gap: “A chilling thought”. J Emerg Med. 1990;8:583–585. doi: 10.1016/0736-4679(90)90454-4. [DOI] [PubMed] [Google Scholar]
- 8.Darchy B, Abruzzese L, Pitiot O, Figueredo B, Domart Y. Delayed admission for ethylene glycol poisoning: lack of elevated serum osmol gap. Intensive Care Med. 1999;25:859–861. doi: 10.1007/s001340050966. [DOI] [PubMed] [Google Scholar]


