Abstract
Background: Candiduria presents as an increasingly common nosocomial infection, which may involves urinary tract. Spectrum of disease is varying from asymptomatic candiduria to clinical sepsis. Disease is most commonly caused by Candida albicans.
Objectives: The aim of the present study was to determine the frequency of candiduria in children attending Abuzar Pediatrics Hospital.
Patients and Methods: Urine samples were collected from 402 patients attending to the Abuzar Pediatrics Hospital, Ahvaz. 10µl of each urine sample was cultured on CHROMagar Candida plates and incubated at 37°C. Ketoconazole, amphotericine B, clotrimazole, fluconazole, miconazole and nystatin disks were used for determination of susceptibility.
Results: In the present study, 402 patients with the age range <1-14 years were sampled (59.2% males and 40.8% females). Prevalence of Candida among enrolled patients was found to be 5.2% (71.4% males and 28.6% females). In our study C. albicans was identified in 19 cases as the most common yeast followed by nine C. glabrata and one C. krusei. Urine cultures were yielded more than 10000 CFU/ml in 14.3% of the cases followed by 600-10000 CFU/ml (28.5%) and 100-600 CFU/ml (57.2%). Antifungal susceptibility testing revealed that only one isolate of C. glabrata and seven isolates of C. albicans were resistant to nystatin and ketoconazole, respectively. However, all tested isolates were resistance to fluconazole.
Conclusion: Asymptomatic candiduria is relatively more prevalent among children in Ahvaz and the most common agent is C. albicans. In addition, isolated Candida species were sensitive to use antifungals, with exception to fluconazole.
Keywords: Candiduria, Candida albicans, Nosocomial infections, children
1. Background
Candiduria presents as an increasingly common nosocomial infection, which may involves urinary tract. Spectrum of disease is varying from asymptomatic candiduria to clinical sepsis. Several reports showed that the frequency of urinary tract infection (UTI) due to yeasts has increased during the last decades (1-4). Prolonged hospitalization, long stay in NICU, urinary tract abnormality, immunocompromised patients, antibacterial therapy with broad spectrum for long time and prophylaxis by antifungal agents are presented as more important risk factors for UTI (5-8). Many of researchers believed that candiduria is not a as marker for disseminated candidiasis (8,9). As a result, detection asymptomatic candiduria from bladder and renal infection is problematic. Nevertheless, candiduria in hospitalized patients in intensive care unit (ICU)/ NICU can be a relevant marker for systemic candidiasis (3,10).
UTIs are among the most important infections between children and are associated with high morbidity (11). Candida species accounted for 5.5% of UTIs infections in children less than 12 year. In another study Candida albicans were recovered from 97% of urine cultures in children (12). Disease is most commonly caused by C. albicans. However, during last decades an increase in non-albicans species was also observed (4,6,8,13). C. tropicalis, C. glabrata, C. parapsilosis, C. lusitaniae, C. guilliermondii and C. krusei are non-albicans species that cause candiduria (14-16).
The susceptibility range of Candida varies to antifungal drugs. C. albicans are usually sensitive to amphotericine B. However, several reports show that non-albicans are more resistant to antifungals, especially fluconazole (2,4,17,18). Yang et al. (17) believe that differences in sensitivity Candida species to antifungal are associated to geographical distributions.
2. Objectives
There is a limited information in the literature on the presentation of candiduria in children outpatients/inpatients. The aim of the present study was to determine the frequency of candiduria in children attending Abuzar Pediatrics Hospital, Ahvaz, Iran. In addition, susceptibility isolates was also considered against several antifungal drugs.
3. Patients and Methods
3.1. Patients and sampling
In the present study, during six months (from January 2010 to June 2011) urine samples were collected from 402 children attending (inpatients/outpatients) to the Abuzar Pediatrics Hospital, Ahvaz. Urine samples were collated at sterile urine bottles and urine bags (when necessary). 10 µl of each urine sample was cultured on CHROMagar Candida plates (CHROMagar Candida®, France) and transferred immediately to Medical Mycology Department, Ahvaz Jundishapur University of Medical Sciences. All plates were incubated at 37°C for maximum one week.
3.2. Yeasts identification
A direct smear from grown colony was prepared and confirmed as yeast by microscopic examinations. All yeast colonies were initially identified based on color colony production on CHROMagar Candida when compared with standard color photographs, supplied by the manufacturer as previously identified by authors (13,19). Colonies that were green in color were confirmed as C. albicans by the germ tube test, morphology on cornmeal agar (Difco, USA) and grow in 45-47°C. Pink colonies were confirmed based on their morphology on cornmeal agar as C. glabrata (small yeasts with budding cells without any pseudohyphae). C. krusei produced large pinkish colonies on CHROMagar Candida and its morphology on cornmeal agar was pseudohyphae with elongate blastoconidia similar to treelike dorm.
3.3. Susceptibility methods
In the present study disk diffusion methods was used for susceptibility test (19,20). Several antifungal, including ketoconazole (10 µg), amphotericine B (20 µg), clotrimazole (50 µg), fluconazole (100 µg), miconazole (10 µg) and nystatin (100 U) disks (Liofilchem Bacteriology Products, Italy) were used for determination of susceptibility.
4. Results
4.1. Patients’ results
In the present study 402 inpatients with the age range from less than one year old to 14 years were sampled. 238 (59.2%) of patients were male and 164 (40.8%) were female. In addition, 356 (88.6%) and 46 (11.4%) were inpatients and outpatients, respectively. The age range sampled patients was less than one year in 104 (25.9%), 1-5 years old in 193(48.0%), 6-10 years old in 87 (21.6%) and 11-14 years old in 18 (4.5%). In our study, 21 (5.2%) of the patient urines were yielded Candida species. 15 (71.4%) of positive patients were male and the rest of them were female 6 (28.6%). In addition 16 (76.2%) of them were inpatients and 5 (23.8%) were outpatients. The age range positive patients were less than one year in 9 (42.9%), 1-5 years old in 5 (23.8%), 6-10 years old in 3 (14.3%) and 11-14 years old in 4 (19.0%).
4.2. Culture results
In this study, C. albicans was confirmed in 19 (65.5%) cases as the most common Candida species, followed by 9 (31.0%) C. glabrata, and 1 (3.5%) C. krusei. In addition, the results of colony counts of urine cultures were more than 10000 CFU/ml in 3 (14.3%) of the cases followed by 600-10000 CFU/ml, (6, 28.5%) and 100-600 CFU/ml, 12 (57.2%). In the present, the mixture of C. albicans and C. glabrata were observed in eight urine samples.
4.3.Interpretive criteria for susceptibility of antifungals
Table 1, shows the interpretive criteria for the used antifungal drugs, including fluconazole, nystatin, amphotericine B, ketoconazole, itraconazole and econazole (19-21). Antifungal susceptibility testing revealed that none of the tested isolates were resistant to amphotericine B. In the present study, 61.9% and 38.1% of isolates were dose dependent and sensitive to amphotericine B, respectively (Table 2). In this work, 100% of isolates of C. albicans were sensitive to nystatin, whereas one isolate of C. glabrata was resistant to antifungal and the rest were dose dependent.
Table 1 . Interpretive criteria of susceptibility and resistance of used antifungal disks .
| Antifungal drugs | Zone diameter in mm | ||
| Sensitive | Dos dependent | Resistance | |
| Amphotericine B | >15 | 10-14 | <9 |
| Nystatin | ≥25 | 17-24 | <16 |
| Fluconazole | ≥19 | 15-18 | ≤14 |
| Ketoconazole | ≥30 | 29-23 | ≤22 |
| Clotrimazole | ≥20 | 19-12 | ≤11 |
| Miconazole | ≥20 | 19-12 | ≤11 |
Table 2 . Susceptibility of isolates of Candida to antifungal drugs .
| Susceptibility | C. albicans | C. glabrata | C. krusei | Total | |
| Amphotericine B | Resistance | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) |
| Dose dependent | 4(19.0%) | 8(38.1%) | 1(4.8%) | 13(61.9%) | |
| Sensitive | 7(33.3%) | 1(4.8%) | 0(0.0%) | 8(38.1%) | |
| Total | 11(52.3%) | 9(42.9%) | 1(4.8%) | 21(100%) | |
| Nystatin | Resistance | 0(0.0%) | 1(4.8%) | 0(0.0%) | 1(4.8%) |
| Dose dependent | 0(0.0%) | 8(38.1%) | 1(4.8%) | 9(42.9%) | |
| Sensitive | 11(52.3%) | 0(0.0%) | 0(0.0%) | 11(52.3%) | |
| Total | 11(52.3%) | 9(42.9%) | 1(4.8%) | 21(100%) | |
| Ketoconazole | Resistance | 7(33.3%) | 0(0.0%) | 0(0.0%) | 7(33.3%) |
| Dose dependent | 3(14.3%) | 3(14.3%) | 0(0.0%) | 6(28.6%) | |
| Sensitive | 1(4.8%) | 6(28.6%) | 1(4.8%) | 8(38.1%) | |
| Total | 11(52.3%) | 9(42.9%) | 1(4.8%) | 21(100%) | |
| Clotrimazole | Resistance | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) |
| Dose dependent | 5(23.8%) | 2(9.5%) | 0(0.0%) | 7(33.3%) | |
| Sensitive | 6(28.6%) | 7(33.3%) | 1(4.8%) | 14(66.7%) | |
| Total | 11(52.3%) | 9(42.9%) | 1(4.8%) | 21(100%) | |
| Miconazole | Resistance | 0(0.0%) | 0(0.0%) | 0(0.0%) | 0(0.0%) |
| Dose dependent | 3(14.3%) | 0(0.0%) | 0(0.0%) | 3(14.3%) | |
| Sensitive | 8(38.1%) | 9(42.9%) | 1(4.8%) | 18(85.7%) | |
| Total | 11(52.3%) | 9(42.9%) | 1(4.8%) | 21(100%) |
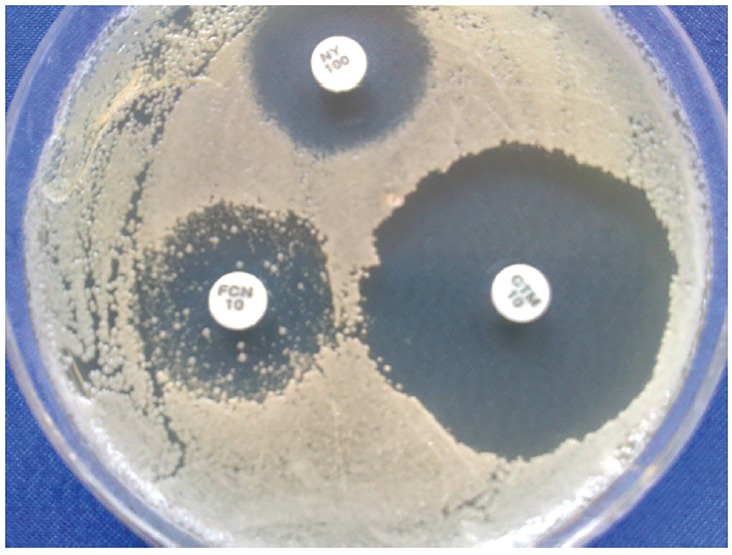
All tested isolates were sensitive or dose dependent to both clotrimazole (66.7% sensitivity) and miconazole (85.7% sensitivity) antifungals drugs. In our study, 33.3% isolate were resistant to ketoconazole and the rest were sensitive and dose dependent to drug. All resistant isolates were C. albicans (Table 2). Antifungal susceptibility testing revealed that all of the isolates of C. albicans and one isolate of C. krusei were resistant to fluconazole. In addition, only three of nine isolates of C. glabrata were sensitive to fluconazole. These three isolates were also presented several resistant colonies to fluconazole, inside inhibition zone (Figure1).
Figure 1.
Several resistant colonies inside inhibition zone in C. glabrata
5. Discussion
Fungal UTI is one of the important factors for mortality and morbidity in hospitals and it incidence is increasing in all hospitalized patients (all areas of medical and surgical practice). Several factors have contributed to this change including; broad spectrum antibiotics therapy, corticosteroids agents, immunosuppressive drugs, use indwelling catheters for urinary drainage, hematologic malignancies, urinary tract abnormalities and prematurity (8,12). Ahmadzadeh et al. (22) reported a case of fungal UTI in an infant with abnormality in urinary system. They have detected fungus ball of C. albicans in urine samples.
Talaat et al. (23) believed that long stay in ICUs is an important risk factor for catheter-associated urinary tract infections. In addition, in a study conducted by Robinson et al. (8), mortality rate due to candiduria in infants in the neonatal intensive care unit was significant (30%). In our study, 76.2% of patients with candiduria were hospitalized for several days. Many risk factors have been suggested to candiduria, such as indwelling urinary catheter, surgical procedures (5,15,16). However, we observed that antibiotic therapy and male sex were more common in our patients. In the present study the age range of the most of the patients with candiduria (42.9%) was less than 1 year.
Our results showed that 5.2% of specimens yielded several species of Candida. These results are compatible with other studies that have believed in typical general hospital 1-5% of all urinary isolates are fungi (11,12,24). However, this rate was reported to be 15.7% in Al Benwan et al. (25) report from Kuwait and 0.33% in Spahiu and Hasbahta (26) report from Kosovo. Hollenbach (27) believed that the rate of candiduria in healthy people is as low as 0–0.3%. Candiduria may be a result of contamination of the urine samples, urinary tract colonization or indicative of invasive UTI (8). Probably, for these reasons, physicians have several responses to the finding of yeast in the urine samples. However, several investigators believed that Candida infections are an emerging problem in pediatrics (24). Outcome of candiduria in patients with generally healthy is little. However, in high-risk patients, should be carefully evaluated for disseminated infection.
Several reports showed that the distribution of Candida species in candiduria varies. The main isolated species from candiduria in type II diabetes mellitus patients was C. glabrata (48%) followed by C. albicans (35%) (28). Whereas, C. albicans was the most common pathogen in neonate patients with candiduria followed by C. parapsilosis (10). In other study, C. albicans was responsible for 97% of positive cultures in children (12). Previously, we have reported the frequency of candiduria in 16.5% of hospitalized patients in two general hospitals in Ahvaz with the most common agent of C. albicans (29).
Management of candiduria remains controversial. Some of the clinicians have believed that the presence of Candida spp. in urine samples is marked as harmless colonization, or lower tract infection. On the other hand, candiduria is well known as an important risk factor for invasive candidiasis with considerable morbidity and mortality (28). Clinically two important antifungals, amphotericine B and fluconazole were used for the treatment candiduria in patients (27,30).
There are a few reports about in vitro assessment of antifungal activity candiduria agents. In a study conducted by Manzano-Gayosso et al. (28) several species of Candida, isolated from candiduria were tested against some antifungal drugs. They have shown that amphotericine B, and ketoconazole have less activity against C. glabrata isolates, whereas fluconazole presented higher activity (28). In addition, Chen et al. reported that all candiduria isolates were susceptible to fluconazole, and amphotericin (31). Our study shows that all isolates of C. albicans, C. krusei and C. glabrata were resistant to fluconazole, with the exception of three isolates of C. glabrata that were sensitive to fluconazole. These three isolates were also presented several resistant colonies inside inhibition zone (Figure 1). Although all isolates were sensitive to amphotericine B, clotrimazole and miconazole, however one isolate of C. glabrata and seven isolates of C. albicans were resistant to nystatin and ketoconazole, respectively. As shown in table 2 the frequency of sensitivity to clotrimazole and miconazole among tested isolates were 66.7% and 85.7%, respectively. Toll et al. (32) reported that intravesicular administration of clotrimazole solution treated fungal cystitis in a cat.
6. Conclusions
In conclusion, asymptomatic candiduria is relatively more prevalent among children in Ahvaz and the most common agent is C. albicans. In addition, isolated Candida species were sensitive to used antifungals with exception to fluconazole.
Authors’ contributions
AZM designed and managed the research. ZS, MA, and ZS collected urine samples, cultured and identified in laboratory. AS presented suitable patients for sampling in hospital. MA analyzed data and wrote some parts of paper. ZS also provided extensive intellectual contribution. ZS reviewed the draft too. AZM prepared the final draft.
Financial Disclosure
The study was entirely carried out at the Department of Medical Mycology and Pediatric Infectious Division, Abuzar Children’s Medical Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, and no funding was received.
Conflict of interest
The authors declare that there were no conflicts of interest throughout the study.
Implication for health policy/practice/research/medical education:
Detection of Candida species in urine cultures from critically ill patients may be an additional important sign for establishing diagnosis of invasive candidosis. In addition, evaluation of their antifungal susceptibility is useful for choosing suitable treatment.
Please cite this paper as: Candiduria in children and susceptibility patterns of recovered Candida species to antifungal drugs in Ahvaz. Seifi Z, Azish M, Salehi Z, ZareiMahmoudabadi A, Shamsizadeh A. J Nephropathology. 2013; 2(2): 122-128. DOI: 10.5812/nephropathol.10113
References
- 1.Laverdiere M, Labba AC, Restieri C, Rotstein C, Heyland D, Madger S. et al. Susceptibility patterns of Candida species recovered from Canadian intensive care units. J Crit Care. 2007;22(3):245–50. doi: 10.1016/j.jcrc.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Ozcelik B, Kaynak F, Cesur S, Sipahi B, Sultan N. In vitro activities of voriconazole as a triazole derivative and caspofungin as an echinocandin were compared with those of some antifungal agents against Candida species isolated from clinical specimens. J Infect Dis. 2007;60:302–4. [PubMed] [Google Scholar]
- 3.da Silva EH, da Silva Ruiz L, Matsumoto FE, Auler ME, Giudice MC, Moreira D. et al. Candiduria in a public hospital of São Paulo (1999-2004): characteristics of the yeast isolates. Rev Inst Med trop S Paulo. 2007;49(6):349–53. doi: 10.1590/s0036-46652007000600003. [DOI] [PubMed] [Google Scholar]
- 4.Saha R, Das Das S, Kumar A, Kaur IR. Pattern of Candida isolates in hospitalized children. Indian J Pediatr. 2008;75:858–60. doi: 10.1007/s12098-008-0159-6. [DOI] [PubMed] [Google Scholar]
- 5.NaymanAlpat S, Özguneş I, Ertem OT, Erben N, DoyukKartal E, Tözun M. et al. Evaluation of risk factors in patients with candiduria. Mikrobiyol Bul. 2011;45(2):318–24. [PubMed] [Google Scholar]
- 6.Weinberger M, Sweet S, Leiboviciy L, Pitlik SD, Samraz Z. Correlation between candiduria and departmental antibiotic use. J Hosp Infect. 2003;53:183–6. doi: 10.1053/jhin.2002.1354. [DOI] [PubMed] [Google Scholar]
- 7.Dalen DM, Zvonar RK, Jessamine PG. An evaluation of the management of asymptomatic catheter-associated bacteriuria and candiduria at The Ottawa Hospital. Can J Infect Dis Med Microbiol. 2005;16(3):166–70. doi: 10.1155/2005/868179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson JL, Davies HD, Barton M, O’Brien K, Simpson K, Asztalos E. Characteristics and outcome of infants with candiduria in neonatal intensive care - a Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect Dis. 2009;9: 183. doi: 10.1186/1471-2334-9-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukhary ZA. Candiduria: a review of clinical significance and management. Saudi J Kidney Dis Transpl. 2008;19(3):350–60. [PubMed] [Google Scholar]
- 10.Kristina B, Charles M, Gerard R. Renal candidiasis in neonates with candiduria. Pediatr Infect Dis J. 1999;18(11):959–63. doi: 10.1097/00006454-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Taneja N, Chatterjee SS, Singh M, Singh S, Sharma M. Pediatric urinary tract infections in a tertiary care center from north India. Indian J Med Res. 2010;131:101–5. [PubMed] [Google Scholar]
- 12.Carvalho M, Guimarães CM, Mayer JR Jr, Bordignon GP, Queiroz-Telles F. Hospital-associated funguria: analysis of risk factors, clinical presentation and outcome. Braz J Infect Dis. 2001;5(6):313–8. doi: 10.1590/s1413-86702001000600004. [DOI] [PubMed] [Google Scholar]
- 13.ZareiMahmoudabadi A, Keradmand AR, Enayatollahi N. Frequency of candiduria in inpatients and outpatients in department of urology, Golestan hospital, Ahvaz, Iran. IJKD. 2009;3:114–5. [PubMed] [Google Scholar]
- 14.Lagrotteria D, Rotstein C, Lee CH. Treatment of candiduria with micafungin: A case series. Can J Infect Dis Med Microbiol. 2007;18(2):149–50. doi: 10.1155/2007/768734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cl´audia Castelo Branco Artiaga Kobayashi, Orionalda de F´atima Lisboa Fernandes, Karla Carvalho Miranda, Efigênia Dantas de Sousa, Maria do Ros´ario Rodrigues Silva. Candiduria in hospital patients: A study prospective. Mycopathologia. 2004; 158: 49-52. [DOI] [PubMed]
- 16.Paul N, Mathai E, Abraham OCa, Michael JS, Mathai D. Factors associated with candiduria and related Mortality. J Infect. 2007;55:450–5. doi: 10.1016/j.jinf.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Yang YL, Cheng HH, Ho YA, Hsiao CF, Lo HJ. Fluconazole resistance rate of Candida species from different regions and hospital types in Taiwan. J MicrobiolImmunol Infect. 2003;36:187–91. [PubMed] [Google Scholar]
- 18.Yanga YL, Wangb AH, Wangb CW, Chengb WT, Lic SY, Lob HJ. Susceptibilities to amphotericin B and fluconazole of Candida species in Taiwan Surveillance of Antimicrobial Resistance of Yeasts 2006. Diagn Microbiol Infect Dis. 2008;61:175–80. doi: 10.1016/j.diagmicrobio.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 19.ZareiMahmoudabadi A, Najafyan M, Alidadi M. Clinical study of Candida vaginitis in Ahvaz, Iran and susceptibility of agents to topical antifungal. Pak J Med Sci. 2010;26(3):607–10. [Google Scholar]
- 20.Pfaller MA, Diekema DJ, Colombo AL, Kibbler C, Ng KP, Gibbs DL. Newell VL, and the Global Antifungal Surveillance Group. Candida rugosa, an emerging fungal pathogen with resistance to azoles: geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program. J Clin Microbiol. 2006;44(10):3578–82. doi: 10.1128/JCM.00863-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pakshir K, Bahaedinie L, Rezaei Z, Sodaifi M, Zomorodian K. In vitro activity of six antifungal drugs against clinically important dermatophytes. Jundishapur J Microbiol. 2009;2(4):158–63. [Google Scholar]
- 22.Ahmadzadeh AR, Valavi E, Shamsizadeh A, Zarei Mahmoudabadi A, Hydari M, Ahmadzadeh A. Fungal urinary tract infection in an infant with posterior urethral valves. Jundishapur J Microbiol. 2011;4(Suppl 1):S71–6. [Google Scholar]
- 23.Talaat M, Hafez S, Saied T, Elfeky R, El-Shoubary W, Pimentel G. Surveillance of catheter-associated urinary tract infection in 4 intensive care units at Alexandria university hospitals in Egypt. Am J Infect Control. 2010;38:222–8. doi: 10.1016/j.ajic.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Raymond J. Epidemiology of nosocomial infections in pediatrics. PatholBiol (Paris) 2000;48(10):879–84. [PubMed] [Google Scholar]
- 25.Al Benwan K, Al Sweih N, Rotimi VO. Etiology and antibiotic susceptibility patterns of community- and hospital-acquired urinary tract infections in a general hospital in Kuwait. Med PrincPract. 2010;19(6):440–6. doi: 10.1159/000320301. [DOI] [PubMed] [Google Scholar]
- 26.Spahiu L, Hasbahta V. Most frequent causes of urinary tract infections in children. Med Arh. 2010;64(2):88–90. [PubMed] [Google Scholar]
- 27.Hollenbach E. To treat or not to treat – critically ill patients with candiduria. Mycoses. 2008;51(Suppl 2):12–24. doi: 10.1111/j.1439-0507.2008.01570.x. [DOI] [PubMed] [Google Scholar]
- 28.Manzano-Gayosso P, Hernández-Hernández F, Zavala-Velásquez N, Méndez-Tovar LJ, Naquid-Narváez JM, Torres-Rodríguez JM. et al. Candiduria in type 2 diabetes mellitus patients and its clinical significanceCandida sppantifungal susceptibility. Rev Med InstMexSeguro Soc. 2008;46(6):603–10. [PubMed] [Google Scholar]
- 29.Zarei Mahmoudabadi A, Zarrin M, Ghanatir F, Vazirianzadeh B. Candiduria in hospitalized patients in educational hospitals of Ahvaz. Iran J Microbiol. 2012;4(4):15–24. [PMC free article] [PubMed] [Google Scholar]
- 30.Kauffman CA, Vazquez JA, Sobel JD, Gallis HA, McKinsey DS, Karchmer AW. et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000;30(1):14–18. doi: 10.1086/313583. [DOI] [PubMed] [Google Scholar]
- 31.Chen SC, Tong ZS, Lee OC, Halliday C, Playford EG, Widmer F. et al. Clinician response to Candida organisms in the urine of patients attending hospital. Eur J Clin Microbiol Infect Dis. 2008;27(3):201–8. doi: 10.1007/s10096-007-0427-9. [DOI] [PubMed] [Google Scholar]
- 32.Toll J, Ashe CM, Trepanier LA. Intravesicular administration of clotrimazole for treatment of candiduria in a cat with diabetes mellitus. J Am Vet Med Assoc. 2003;223(8):1156–8. doi: 10.2460/javma.2003.223.1156. [DOI] [PubMed] [Google Scholar]