Abstract
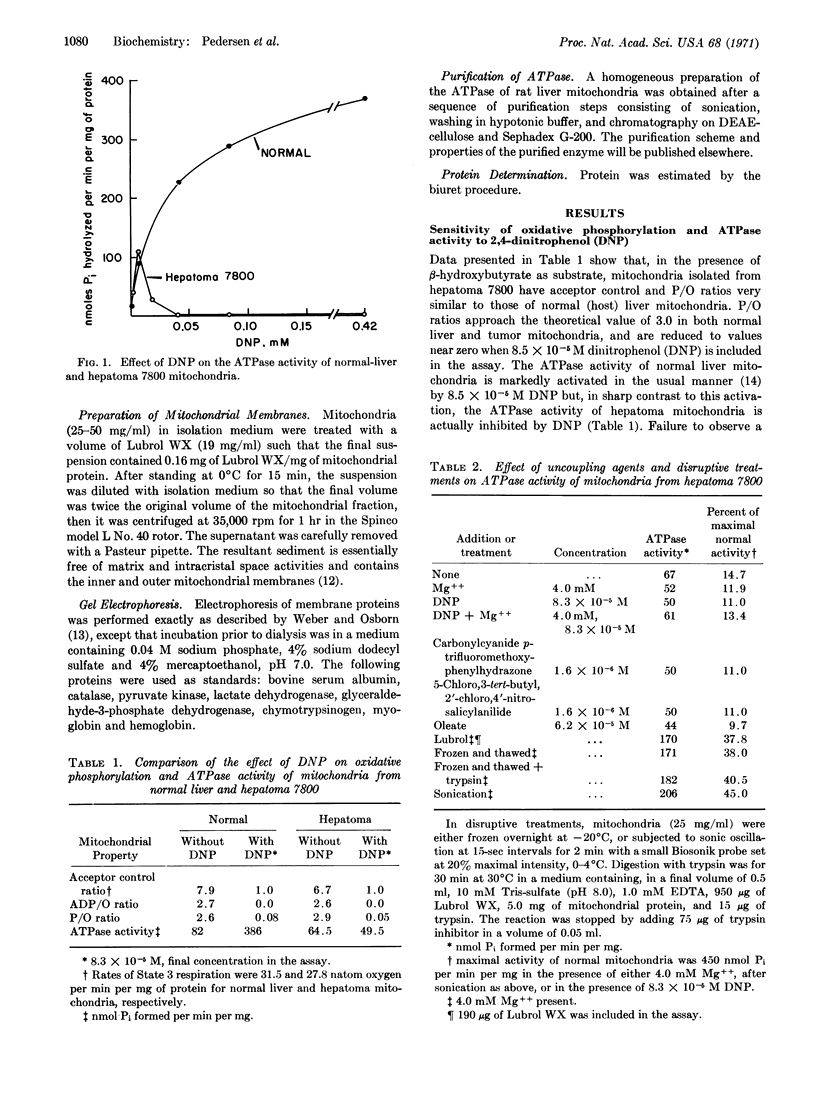
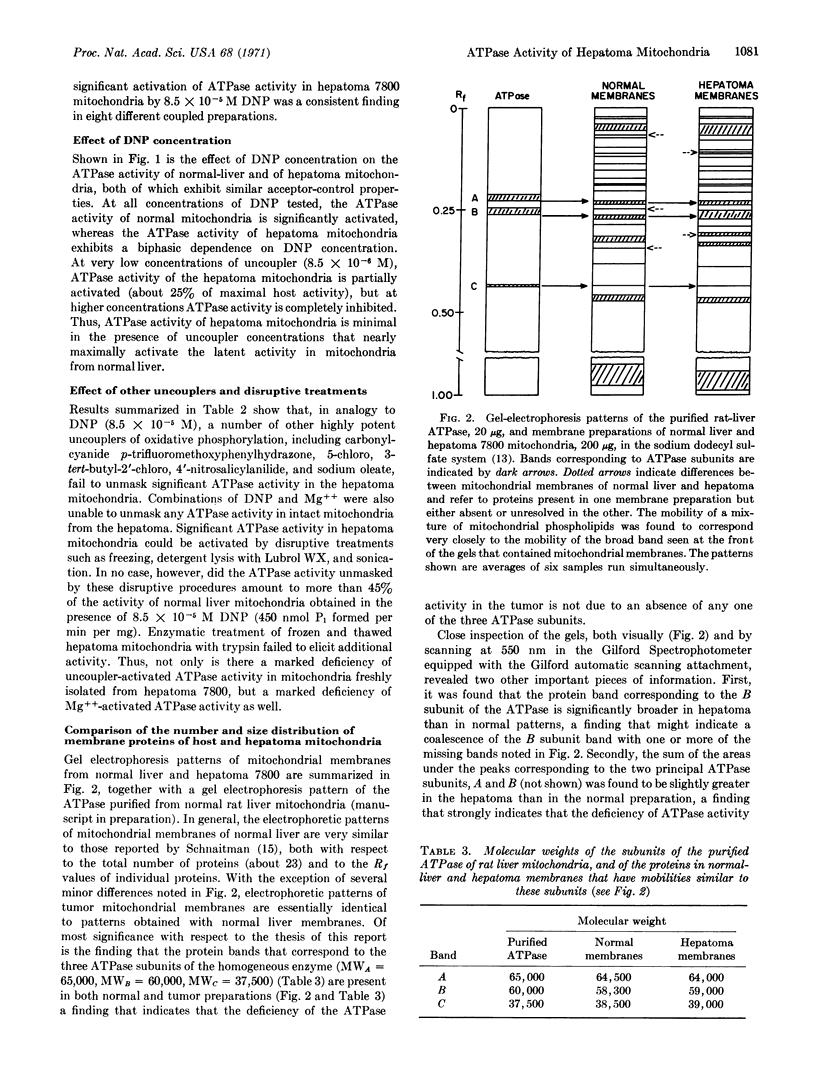
Tightly coupled mitochondria from the well-differentiated hepatoma 7800 failed to exhibit a significant 2,4-dinitrophenol-activated ATPase activity at concentrations of uncoupler sufficient to completely inhibit oxidative phosphorylation. ATPase activity could not be maximally activated by uncoupling agents more potent than 2,4-dinitrophenol, such as carbonylcyanide p-trifluoromethoxyphenylhydrazone and 5-chloro, 3-tert-butyl, 2′-chloro, 4′-nitrosalicylanilide, nor by Mg++ after the following treatments: sonication, freezing, detergent lysis, and digestion with trypsin. Gel electrophoresis patterns of the membrane proteins of the hepatome mitochondria revealed neither an absence of any one of the three different types of ATPase subunits characteristic of the homogeneous enzyme purified from normal liver mitochondria, nor a deficiency of the oligomeric molecule. Taken together, these data strongly suggest that the supramolecular structure of the membrane ATPase complex of mitochondria from hepatoma 7800 is altered in such a way that its capacity for ATP hydrolysis is severely diminished.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AISENBERG A. C., MORRIS H. P. Energy pathways of hepatoma No. 5123. Nature. 1961 Sep 23;191:1314–1315. doi: 10.1038/1911314b0. [DOI] [PubMed] [Google Scholar]
- Asami K., Juniti K., Ernster L. Possible regulatory function of a mitochondrial ATPase inhibitor in respiratory chain-linked energy transfer. Biochim Biophys Acta. 1970;205(2):307–311. doi: 10.1016/0005-2728(70)90261-6. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- EMMELOT P., BOS C. J., BROMBACHER P. J., HAMPE J. F. Studies on isolated tumour mitochondria: biochemical properties of mitochondria from hepatomas with special reference to a transplanted rat hepatoma of the solid type. Br J Cancer. 1959 Jun;13:348–379. doi: 10.1038/bjc.1959.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. Sulphydryl groups in haemoglobins. Biochem J. 1955 Apr;59(4):653–661. doi: 10.1042/bj0590653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. The catalytic effect of 2,4-dinitrophenol on adenosinetriphosphate hydrolysis by cell particles and soluble enzymes. J Biol Chem. 1953 Mar;201(1):357–370. [PubMed] [Google Scholar]
- LEHNINGER A. L. Oxidative phosphorylation. Harvey Lect. 1953;49:176–215. [PubMed] [Google Scholar]
- Morris H. P. Studies on the development, biochemistry, and biology of experimental hepatomas. Adv Cancer Res. 1965;9:227–302. doi: 10.1016/s0065-230x(08)60448-0. [DOI] [PubMed] [Google Scholar]
- Nowell P. C., Morris H. P. Chromosomes of "minimal deviation" hepatomas: a further report on diploid tumors. Cancer Res. 1969 Apr;29(4):969–970. [PubMed] [Google Scholar]
- Nowell P. C., Morris H. P., Potter V. R. Chromosomes of "minimal deviation" hepatomas and some other transplantable rat tumors. Cancer Res. 1967 Sep;27(9):1565–1579. [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- Pedersen P. L., Greenawalt J. W., Chan T. L., Morris H. P. A comparison of some ultrastructural and biochemical properties of mitochondria from Morris hepatomas 9618A, 7800, and 3924A. Cancer Res. 1970 Nov;30(11):2620–2626. [PubMed] [Google Scholar]
- Pedersen P. L., Schnaitman C. A. The oligomycin-sensitive adenosine diphosphate-adenosine triphosphate exchange in an inner membrane matrix fraction of rat liver mitochondria. J Biol Chem. 1969 Sep 25;244(18):5065–5074. [PubMed] [Google Scholar]
- SLATER E. C. Mechanism of phosphorylation in the respiratory chain. Nature. 1953 Nov 28;172(4387):975–978. doi: 10.1038/172975a0. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of rat liver mitochondrial and microsomal membrane proteins. Proc Natl Acad Sci U S A. 1969 Jun;63(2):412–419. doi: 10.1073/pnas.63.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Winkler H. H., Lehninger A. L. The atractyloside-sensitive nucleotide binding site in a membrane preparation from rat liver mitochondria. J Biol Chem. 1968 Jun 10;243(11):3000–3008. [PubMed] [Google Scholar]


