Abstract
Background:
The genus Amaranthus has potential activity as a hepatoprotective agent.
Objective:
The present pharmacological investigation focuses on evaluation of the efficacy of aqueous extract of roots of Amaranthus tricolor Linn. for their protection against paracetamol (PCM) overdose induced hepatotoxicity.
Materials and Methods:
The aqueous extract of roots of A. tricolor Linn. was prepared and phytochemical screening was done. The biochemical investigation viz. serum glutamic oxaloacetate transaminase (SGOT), serum glutamic pyruvate transaminase (SGPT), alkaline phosphatase (ALP) and total Bilirubin (TB) was done against PCM-induced hepatotoxicity in wistar albino rats. The histopathological studies of liver were also done.
Results:
The phytochemical screening of the aqueous extract showed the presence of alkaloids, carbohydrates, flavanoids, amino acids, proteins, fixed oil, saponins and tannins, and phenolic compounds. Pretreatment with the aqueous extract of root significantly prevented the physical, biochemical, histological, and functional changes induced by paracetamol in the liver. The extract showed significant hepatoprotective effects as evidenced by decreased serum enzyme activities like SGPT, SGOT, ALP, and TB, which was supported by histopathological studies of liver. The aqueous extract showed significant hepatoprotective activity comparable with standard drug silymarin as well as hepatotoxin drug PCM.
Conclusion:
From these results, it is concluded that the A. tricolor has potential effectiveness in treating liver damage in a dose dependent manner.
Keywords: Amaranthaceae, Amaranthus tricolor Linn., aqueous extract, hepatoprotective activity, paracetamol
INTRODUCTION
Liver regulates many important metabolic functions, and any injury causes distortion of these metabolic functions. As per an estimate, about 20,000 deaths occur every year in United States due to liver disorders. Hepatocellular carcinoma is one of the 10 most common tumors in the world, with over 2,50,000 new cases registered each year.[1] Liver-protective herbal drugs contain a variety of chemical constituents like phenols, coumarins, lignans, essential oil, monoterpenes, carotenoids, glycosides, flavanoids, organic acids, lipids, alkaloids, and xanthones derivatives. Extracts of about 25 different plants have been reported to cure liver disorders.[2] In spite of tremendous efforts made in the field of modern medicine, there is hardly any drug that stimulates liver function, offer protection to the liver from damage or help regeneration of hepatic cell.[3] Many Indian ethnobotanic traditions propose a rich repertory of medicinal plants used by the population for treatment of liver diseases. However, there were not enough scientific investigations on the hepatoprotective activities conferred to these plants.[4]
Amaranthus tricolor Linn. commonly known as Lal Chaulai or Joseph's coat is an important medicinal plant belonging to the family Amaranthaceae.[5] This weed is cultivated throughout India and found in West Bengal, Bihar, and Uttar-Pradesh. In Africa, it originated in Central America and Mexico. In Asia, it originated in Southeast region and then spread through the tropics and the temperate zones, primarily in southern China.[6] The plant has taproot, which are cylindrical in shape, yellowish in color and 0.3-0.5 cm. Roots are usually thick with a few secondary roots, numerous rootlets and rooting at nodules.[5,7,8] Traditionally root-paste mixed with warm water, when given internally, induces vomiting and, thereby, purges out toxic matters from bowel. Powdered root is effective in onychia. Root-paste in combination with honey and rice water is useful in leucorrhea.[9] The plant possesses hepatoprotective activity,[10] antiviral activity,[11] and antiproliferative activity.[12]
MATERIALS AND METHODS
Collection and authentication
Roots of A. tricolor Linn. were collected from the Agricultural land of Sector-12, Sonipat district, Haryana. The plant was authenticated by Dr. H. B. Singh, Scientist F and Head, Raw Materials Herbarium and Museum, NISCAIR, New Delhi. A Voucher Specimen No.-NISCAIR/RHMD/Consult/2010-11/1528/126 was submitted to the Department of Pharmacognosy and Phytochemistry, Hindu College of Pharmacy, Sonipat, Haryana (India).
Preparation of extracts
The roots of A. tricolor Linn. were collected, washed and cut into small pieces, then shade dried. The dried roots were crushed to coarse powder. The powdered sample was successively extracted with different solvents viz. petroleum ether, chloroform, ethyl acetate, ethanol and water to get the respective extracts. All the extracts were filtered individually, evaporated to dryness using the rotary evaporator.[13]
Preliminary phytochemical screening
The chemical tests were carried out to identify the various phytoconstituents present in the aqueous extract of roots of A. tricolor Linn. Aqueous extract of roots of A. tricolor Linn. were subjected to qualitative chemical tests for the detection of various phytoconstituents such as alkaloids, carbohydrates, proteins and amino acids, anthraquinone glycosides, steroids, flavanoids, tannins and phenolic compounds, saponins, fats and fixed oil, and gums and mucilages.[14]
Animals
Wistar albino rats of either sex (200-250 g) were selected and housed in cages at a temperature of 22-25°C and provided with food (pellet diet) and water ad libitum. An alternating 12 h light-dark cycle at relative humidity of 55-65% was maintained throughout the experimental period. Ethical committee clearance was obtained from the Institutional Animal Ethics Committee of Hindu College of Pharmacy, Sonipat held on 18th Dec, 2010 with Regn. No.-585/02/c/CPCSEA and Protocol No.– I (5).
Acute toxicity studies
Two groups of five animals each were selected and were fasted overnight with free access to water prior to study. Group I i.e., Control group received single oral dose of 1% Sodium carboxymethyl cellulose (SCMC). Group II i.e., Extract group received single oral dose of (2,000 mg/kg body weight) aqueous extract of roots of A. tricolor Linn. Major changes and mortality were examined individually during first 30 min. after dosing, periodically during first 24 h (with special attention during first 4 h) and, thereafter, once daily for a period of 14 days.[15]
Hepatoprotective activity
Hepatoprotective activity of aqueous extracts of roots of A. tricolor Linn. was evaluated by using paracetamol-induced hepatotoxic model. The animals were fasted overnight with water ad libitum before experimentation. The animals were divided into five groups of six animals (n = 6) each.
Group I: Control group, received 1% SCMC at 0, 24 and 48 hrs orally
Group II: Hepatotoxin group received 1% SCMC at 0, 24 and 48 hrs followed by paracetamol at a dose of 3 g/kg orally
Group III: Positive control group, received the first dose of silymarin (200 mg/kg, orally) at 0 hr, second dose at 24 hr, and at 48 hr third dose of silymarin followed by dose of paracetamol
Group IV, V: Test groups, received aqueous extract of roots of A. tricolor Linn. at a dose of 200 and 400 mg/kg body weight, respectively. They received first dose of extract at 0 hr, second dose at 24 hr, and at 48 hr the third dose of extract followed by a dose of paracetamol.
Collection of blood samples
All the animals were sacrificed on 7th day under light ether anesthesia. The blood samples were collected separately in sterilized dry centrifuge tubes by puncture in retro-orbital plexus and allowed to coagulate for 30 min at 37°C. The clear serum was separated at 2,500 rpm (Micro centrifuge) for 10 min and subjected to biochemical investigation viz. serum glutamic oxaloacetate transaminase (SGOT), serum glutamic pyruvate transaminase (SGPT), alkaline phosphatase (ALP), and total Bilirubin (TB).[16,17]
Biochemical analysis
Liver function tests were determined by liver function test kits and the readings were observed in auto analyzer.
Histopathological study
After removal of blood, animal were sacrificed using ether anesthesia and liver was removed and immersed in 10% formalin solution. Later histopathological examinations were carried out.
Data analysis
Results of biochemical estimation were reported as mean ± SEM (Standard Error of the Mean) for determination of significant mean values. Inter group difference analyzed separately using one-way analysis of variance (ANOVA) followed by Dunnet's test using Graph Prism 5 software. The level of significance was P < 0.05 [Table 1].
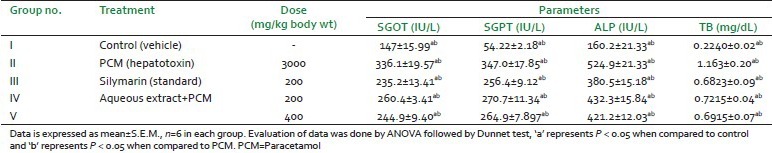
Table 1.
Hepatoprotective activity in paracetamol induced hepatotoxic model
RESULTS
Preliminary phytochemical screening
Preliminary phytochemical screening of aqueous extract of A. tricolor roots showed the presence of alkaloids, carbohydrates, flavanoids, tannins and phenolic compounds, amino acids, proteins, mucilages, steroids, glycosides, saponins, fats, and fixed oils. Gums were absent in the extract.
Acute toxicity studies
No lethality at a single oral dose of 2000 mg/kg body weight of aqueous extract of A. tricolor roots was observed. Thus, 1/10th (200 mg/kg) and 1/5th (400 mg/kg) of the safe dose were selected for experimental purpose.
Hepatoprotective activity
Aqueous extract of A. tricolor Linn. showed the hepatoprotective activity against paracetamol (PCM)-induced hepatotoxicity. The study was carried out at the two doses and maximum hepatoprotection was at 400 mg/kg b.wt. The results were comparable to the standard and found statistically significant with control and PCM group.
Biochemical analysis
The aqueous extract lowered the levels of biochemical parameters i.e,. SGOT (AST), SGPT (ALT), TB, and ALP significantly and dose-dependently. These reductions were comparable to the control as well as the hepatotoxin (PCM) group [Table 1].
Histopathological studies
The histoarchitecture of control group animals showed normal cells with distinct hepatic cells and sinusoidal spaces [Figure 1a]. The histoarchitecture of paracetamol treated rat liver sections showed disarrangement and degeneration of hepatocytes with intense centrilobular necrosis showing damage of approximately 95% cells. Necrosis of cells was also observed with complete degeneration of liver cells and cell death of approximately 95% cells [Figure 1b]. In the group of animals treated with aqueous extract of A. tricolor Linn. roots showed less disarrangement and degeneration of hepatocytes, indicating marked hepatoprotection [Figure 1d, e]. The liver sections of the rats treated with Silymarin showed a cell damage protection of nearly 85% [Figure 1c] while aqueous extract of doses of 200 and 400 mg/kg b.wt. shows a protection of approximately 35 and 40%, respectively. The above percentage has been arrived at by keeping in mind the cell permeability and the intensity of centrilobular necrosis as shown in Figure 1. These results provide evidence of aqueous extract of A. tricolor Linn. roots showed visible changes confirming the hepatoprotective activity.
Figure 1.
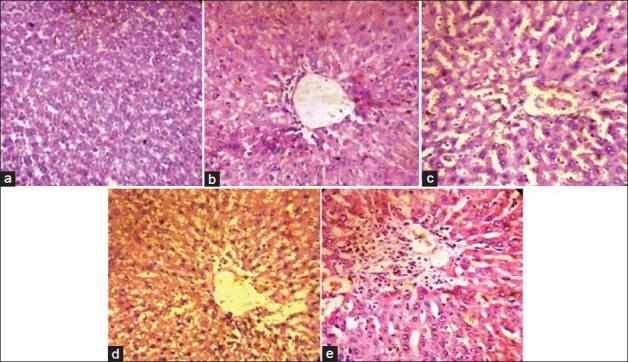
Liver section showing histopathology (a) Control group, (b) Paracetamol (Hepatotoxin), (c) Silymarin (Standard), (d,e) Aqueous extract (200 and 400 mg/kg b.wt., respectively) of Amaranthus tricolor Linn. roots
DISCUSSION
It is common to evaluate the hepatoprotective activity of plants using several methods to measure various biochemical parameters. There is no simple universal model by which hepatoprotective activity can be measured accurately and qualitatively.
PCM is a commonly used as analgesic and antipyretic drug and is safe in therapeutic doses but produces fatal hepatic necrosis with toxic doses. At lower doses, about 80% of ingested paracetamol is eliminated mainly as sulfate and glucoronide conjugates before oxidation and only 5% of the paracetamol is converted into N-acetyl-p-benzoquineimine. However, upon administration of toxic doses of paracetamol the sulfation and glucoronidation routes become saturated and hence, higher percentage of paracetamol molecules are oxidized to highly reactive N-acetyl-p-benzoquineimine (NAPQI) by cytochrome-450 enzymes. Semiquinone radicals, obtained by one electron reduction of NAPQI, can covalently binds to macromolecules of cellular membrane and increase the lipid peroxidation resulting in the tissue damage. Higher dose of paracetamol and NAPQI can alkylate and oxidize intracellular glutathione (GSH) and protein thiol group, which results in the depletion of liver GSH pool subsequently leading to increased lipid peroxidation and liver damage.[18]
In the present study, the damage of liver due to paracetamol over dosage was confirmed by elevated levels of biochemical parameters like SGPT, SGOT, ALP, and TB. This is due to the fact that hepatic cells possess a variety of metabolic activities and contain a host of enzymes. SGPT, SGOT found in higher concentration in cytoplasm and SGPT, particularly in mitochondria. In liver injury the transport function of hepatocytes is disturbed, resulting in the leakage of plasma membrane,[19] thereby, causing leakage of such enzymes leading to the increased serum levels of them. The elevated activities of SGPT, SGOT in PCM-induced liver injury in serum indicative of cellular leakage and loss of functional integrity of cell membrane in liver.[20] SGPT is the best parameter than SGOT to justify the liver damage, since SGOT also present in kidney and cardiac muscle. Paracetamol is nephrotoxic, so damages kidney cell and releasing SGOT to serum. In PCM-induced liver damage, the level of SGOT is more than SGPT.[21,22]
Aqueous extract of A. tricolor roots possess flavanoids, saponins, tannins, and phenolic compounds, which are natural antioxidants. They can scavenge off free radicals. So, the anti-oxidant principles may be involved in the hepatoprotective activity. In the experiment, paracetamol has enhanced the levels of SGPT, SGOT, TB, and ALP, significantly. Treatment with silymarin and 200 mg/kg and 400 mg/kg of aqueous extracts of Amaranthus tricolor roots has significantly brought down the elevated levels of SGPT, SGOT, TB, and ALP. These reductions were comparable to the control and the hepatotoxin (PCM) used. The treatment has also demonstrated the reduced hepatic damage or improvement in the hepatic architecture.
All the histological changes observed were in correlation with the physical, biochemical, and functional parameters of the liver. Examination of slides of liver of only PCM-treated rats showed more extensive cell damage as compared to control, though slides of the extract showed presence of mild necrosis and fibrosis in liver cells of rats and were comparable with reference drug slide.
CONCLUSION
In conclusion, the results of study demonstrate that aqueous extract of A. tricolor roots possess hepatoprotective property. This property may be attributed to the antioxidant principles of the extract. Further studies are required to identify, isolate, characterize, and evaluate the active principal responsible for hepatoprotective activity of plant. The toxicological aspect of plant is not studied in this project, so toxicological assessment could be carried out.
Footnotes
Source of Support: Hindu College of Pharmacy, Sonipat,
Conflict of Interest: None declared.
REFERENCES
- 1.Wolf PL. Biochemical diagnosis of liver diseases. Indian J Clin Biochem. 1999;14:59–90. doi: 10.1007/BF02869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma SK, Ali M, Gupta J. Recent Progress in Medicinal Plants (Phytochemistry and Pharmacology) Vol. 2. Houston: Research Periodicals and Book Publishing House; 2002. Evaluation of Indian Herbal Hepatoprotective Drugs; pp. 253–70. [Google Scholar]
- 3.Chatterjee TK. Herbal Options. 3rd ed. Calcutta: Books and Allied Private Limited; 2000. Medicinal plants with hepatoprotective properties; pp. 135–7. [Google Scholar]
- 4.Chattopadhyay RR. Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part II. J Ethnopharmacol. 2003;89:217–9. doi: 10.1016/j.jep.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Vardharajan S. Raw Materials. Revised edition. 1A. New Delhi: NISCAIR, CSIR; 1985. The Wealth of India- A Dictionary of India Raw Materials and Industrial Products; pp. 213–21. [Google Scholar]
- 6.Chatterjee A, Prakash SC. 1st ed. Vol. 1. New Delhi: National Institute of Science Communication 1992, revised; 2005. The Treatise of Indian Medicinal Plants; p. 90. [Google Scholar]
- 7.1st ed. Varanasi: Banaras Hindu University; 2004. Amaranthus Linn. Flora of BHU Campus; p. 122. [Google Scholar]
- 8.Kirtikar KR, Basu BD. 2nd ed. Vol. 9. Dehradun: International Book Distributors; 2003. Indian Medicinal Plants; pp. 2832–40. [Google Scholar]
- 9.Prajapati ND, Kumar U. India: Agrobios; 2003. Agro's Dictionary of Medicinal Plants; p. 22. [Google Scholar]
- 10.Al-Dosari MS. The effectiveness of ethanolic extracts of Amaranthus tricolor Linn.: A natural hepatoprotective agent. Am J Chin Med. 2010;38:1051–64. doi: 10.1142/S0192415X10008469. [DOI] [PubMed] [Google Scholar]
- 11.Roy S, Sadhana P, Begum M, Kumar S, Lodha ML, Kapoor HC. Purification, characterization and cloning of antiviral/ribosomes inactivating protein from Amaranthus tricolor leaves. Phytochemistry. 2009;67:1865–73. doi: 10.1016/j.phytochem.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Jayaprakashan B, Zhang Y, Nair MG. Tumour cell proliferation and cyclooxygenase enzyme inhibitory compounds in Amaranthus tricolor. J Agric Food Chem. 2004;52:6939–43. doi: 10.1021/jf048836z. [DOI] [PubMed] [Google Scholar]
- 13.Geneva, New-Delhi: APTBS Publisher and Distributor; 1998. WHO. Quality Control Methods for Medicinal Plant Materials; pp. 22–34. [Google Scholar]
- 14.Brain KR, Turner TD. Bristol: Wright-Scientechnica; 1975b. The Practical Evaluation of Phyto pharmaceuticals; pp. 36–45. [Google Scholar]
- 15.OECD Guidelines for the testing of chemicals. Acute oral toxicity-Acute toxic class method, guidelines 423. [Last cited on 2002 Dec 17].
- 16.Patel BA, Patel JD, Raval BP. Hepatoprotective activity of Sachharum officinarum against paracetamol induced hepatotoxicity in rats. Int J Pharm Sci Res. 2010;1:102–8. [Google Scholar]
- 17.Manokaran S, Jaswanth A, Sengottuvelu S, Nandhakumar J, Duraisamy R, Karthikeyan D, et al. Hepatoprotective activity of Aerva lanata Linn. against paracetamol induced hepatotoxicity in rats. Res J Pharm Tech. 2008;1:398–400. [Google Scholar]
- 18.Tripathi KD. Essentials of Medical Pharmacology. 5th ed. New-Delhi: Jaypee Brothers Medical Publishers Private Limited; 2004. Non-steroidal anti-inflammatory drugs and antipyretic-analgesics; pp. 181–3. [Google Scholar]
- 19.Rajesh MG, Latha MS. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol. 2004;91:99–104. doi: 10.1016/j.jep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Dortman RB, Lawhorn GT. Serum enzymes as indicators of chemical induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 21.Abraham P. Oxidative stress in paracetamol induced pathogenesis: (I) Renal damage. Indian J Biochem Biophys. 2005;42:59–62. [PubMed] [Google Scholar]
- 22.Ahmad A, Pillai KK, Najmi AK, Ahmad SJ, Pal SN, Balani DK. Evaluation of hepatoprotective potential of jigrine post treatment against thioacetamide induced hepatic damage. J Ethnopharmacol. 2002;70:35–41. doi: 10.1016/s0378-8741(01)00349-x. [DOI] [PubMed] [Google Scholar]


