Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a hematopoietic growth factor with immunostimulatory effects that include the activation and priming of neutrophils. Neutrophils are an important part of the human immune system, yet they have been implicated in the pathogenesis of acute lung injury (ALI). GM-CSF has been found to increase the amount of activated neutrophils recruited to the lung tissue as well as to increase the life span of neutrophils leading to substantial lung tissue injury and the development of ALI. While, there have been few cases reported of ALI following GM-CSF, the experience reported here is the first of ALI subsequent to local administration of GM-CSF in a patient with significant pulmonary comorbidities.
Keywords: Acute lung injury, adverse reaction, dyspnea, granulocyte-macrophage colony-stimulating factor
INTRODUCTION
The association of acute lung injury (ALI) after intravenous administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) has been rarely described.[1,2] GM-CSF is a cytokine produced by many different cell lines. It has immunostimulatory effects[3] and is commonly prescribed for the treatment of neutropenic patients after bone marrow suppressive therapy.[1] One of the effects seen with the use of GM-CSF is an increased neutrophil count.[4] Neutrophils are known to mediate tissue injury through interleukin-8 (IL-8) and epithelial neutrophil activating peptide-78,[5] and are involved in the pathogenesis of ALI.[6] GM-CSF has been reported to increase lung inflammation and injury by increasing neutrophil infiltration into lung tissue,[1] resulting in neutrophil dependent lung injury.[7]
CASE REPORT
A 77-year-old Black female with a past medical history significant for hypertension, peripheral vascular disease, hypercholesterolemia, chronic obstructive pulmonary disease (COPD) without recent exacerbation and gastroesophageal reflux disease. Her history is negative for coronary disease, stroke or diabetes. She presented with an acute osteomyelitis of the left foot, which required debridement of the 2nd and 3rd metatarsal bones. The social history revealed a former smoker, with a 70-pack-year smoking history who quit 1 year ago and denied alcohol use. Her surgical history was significant for a left femoral-popliteal bypass followed by a transmetatarsal amputation for gangrenous toes.
Pre-operative evaluation showed a chest X-ray significant for cardiomegaly [Figure 1]. A transthoracic echocardiogram revealed an ejection fraction of 55%, with moderate concentric left ventricular hypertrophy, Grade I diastolic dysfunction, trace mitral regurgitation, trace tricuspid regurgitation and no evidence of aortic regurgitation. Laboratory data showed a white blood cell count of 12.1 × 103/μL and a normal albumin of 3.6 g/dL.
Figure 1.
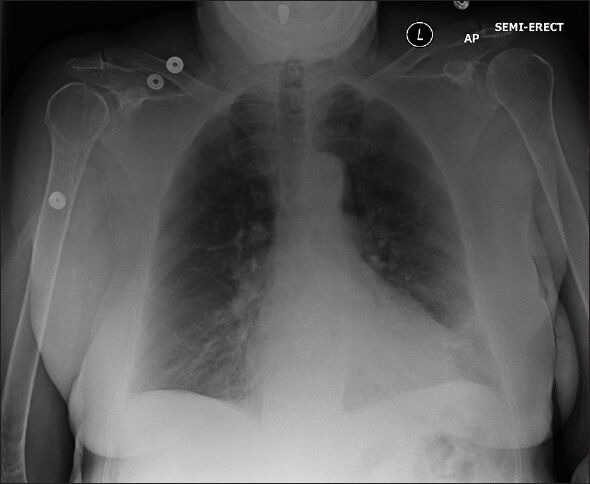
Pre-operative chest X-ray reveals an enlarged cardiac silhouette, slight increased markings emanating from a slightly lobulated enlarged right hilum, consistent with enlarged right pulmonary artery and possible pulmonary hypertension. There is also increased lower lobe stranding bilaterally suggestive of chronic pulmonary disease
She underwent a 17 min operative procedure under local anesthesia with midazolam and ketamine for sedation. This consisted of a debridement of the osteomyelitis of the left foot at the previous amputation site without any blood loss. At the conclusion of the debridement, 500 μg recombinant GM-CSF (sargramostim [Leukine, Genzyme]), which was injected locally into the operative site to stimulate wound healing (off label use). Intraoperatively, the patient was hemodynamically stable without hypotension and received minimal intravenous fluid.
After a brief period in the post-anesthesia care unit, where the patient was asymptomatic and hemodynamically stable with oxygen saturation of 95% by pulse oximetry via 3 L nasal cannula, patient was transferred to floor care. Twenty-two hours post-operatively, she developed acute onset of severe dyspnea with respiratory distress manifested by hypertension (blood pressure 180/95 mmHg), tachycardia (heart rate of 122), diaphoresis and tachypnea (respiratory rate of 26). Auscultation revealed diffuse rales without inspiratory or expiratory wheeze or stridor. Cardiovascular examination was negative for jugular venous distension and the absence of an S3 gallop. Oxygen saturation was 90% on a 50% FiO2 via an oxygen venturi mask and an arterial blood gas performed revealed a pH 7.35, PaO2 76 mmHg and PCO2 56 mmHg and the PaO2/FIO2 ratio was 152 with a calculated A-a gradient of 217.5 mmHg (expected 18.7 mmHg adjusted for age). Repeat laboratory studies showed a leukocytosis, with the white blood cell count of 23.6. A chest X-ray revealed an interval development of bilateral fluffy infiltrates consistent with acute pulmonary edema [Figure 2]. A 12 lead electrocardiogram showed no evidence of ischemia and serial cardiac biomarkers were negative. A repeat echo showed no changes from the prior study, with a preserved ejection fraction of 55%. Patient was placed on non-invasive ventilation (bilevel positive airway pressure [BIPAP] mode, inspiratory positive airway pressure = 12 cm H2O, expiratory positive airway pressure = 5 cm H2O, FiO2= 50%), given furosemide 40 mg intravenously and promptly transferred to the surgical intensive care unit.
Figure 2.

Chest X-ray reveals acute onset of new pulmonary vascular congestion consistent with acute lung injury. There are no effusions
The following day, despite negative fluid balance and BIPAP ventilation, the PaO2/FIO2 ratio remained low at 132, without any evidence of radiographic improvement. Given the absence of fluid overload, a lack of response to diuresis, no cardiogenic dysfunction and no apparent clinical exacerbation of COPD and given the temporal sequence of the event after the GM-CSF injection, a presumptive diagnosis of ALI was suspected and high dose methylprednisolone 500 mg once daily for 5 days was initiated. Over the next 5 days, the patient's symptoms improved and she was gradually weaned off BIPAP to nasal cannula, with daily chest X-rays confirming radiographic clearing of the ALI with resolution of the bilateral pulmonary infiltrates [Figure 3].
Figure 3.

Chest X-ray on the post-operative day #6 shows a resolution of the acute lung injury and a return to baseline
DISCUSSION
ALI is a severe pulmonary disease that can result in respiratory failure.[8] The definition of ALI includes an acute onset, PaO2/FIO2 ratio of less than 300, with diffuse bilateral infiltrates on X-ray and the absence of left atrial hypertension.[9] One of the factors involved in the pathogenesis of ALI are activated neutrophils, which accumulate in lung tissue causing inflammation and injury.[6] Activated neutrophils cause endothelial cell damage leading to an increased permeability and damage to the pulmonary alveolar capillary membrane leading to the acute respiratory failure.[2,10]
GM-CSF is currently approved for use in neutropenic states following myelotoxic chemotherapy and autologous bone marrow transplantation for lymphoid tumors.[11,12] Its use has also been described in congenital neutropenic states, the myelodysplatic syndrome, aplastic anemia and acquired immunodeficiency syndrome related neutropenia.[13] It is a proinflammatory cytokine that is directly involved in the activation of neutrophils, eosinophils and monocytes.[4] GM-CSF exerts many effects on neutrophil function including enhanced neutrophil survival, increasing synthesis of platelet-activating factor, gene up-regulation encoding for IL-1 and IL-6, increasing arachidonate acid, increasing synthesis of 5-lipoxygenase products, enhancing phagocytic and bactericidal function, altering expression of cell surface receptors as well as generating oxygen-derived free radicals. Neutrophil mobilization from the bone marrow to the periphery is further enhanced by GM-CSF.[6] It has also been shown not only to increase the amount of neutrophils, but also is involved in the priming of neutrophils.[14] GM-CSF also plays a role in neutrophil degranulation as well as release of another proinflammatory cytokine, IL-8, which has been implicated in ALI.[4]
GM-CSF is used clinically for its immunostimulating effects in neutropenic patients, most commonly after chemotherapy.[3] The clinical use of GM-CSF has allowed for high-dose chemotherapy to be utilized. The side-effects observed from the use of GM-CSF include bone pain, headache and fatigue. Secondary pulmonary complications include dyspnea, cough, pulmonary edema, with ALI/acute respiratory distress syndrome being quite rare. Locally injected GM-CSF for wound healing is an off label indication, with unpublished, anecdotal experience by one of the authors (HB) to stimulate a local inflammatory response and in turn, to prime for tissue healing. In his experience, the GM-CSF has been locally injected into over two thousand wounds and this is the first observed complication of the therapy.
CONCLUSION
To the best of our knowledge, this is the first case report of locally injected GM-CSF causing ALI. Although, causality between our patient's arterial desaturation and GM-CSF administration cannot be proven definitively, given the temporal sequence of the respiratory distress after exposure to the GM-CSF, the lack of myocardial dysfunction, the ineffectiveness of diuresis, with gradual improvement after the institution of corticosteroids a temporal relationship is strongly suggested. Given the mechanism of action of GM-CSF on neutrophils, steroids are the most effective treatment to reverse the proinflammatory state and this is supported by research on the use of early steroids in ARDS.[15] Although our patient is a unique case due to the local injection for wound healing, clinicians should be aware of this complication of locally injected GM-CSF and the need for supportive care combined with the effectiveness of high dose steroids for this complication.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Arimura K, Inoue H, Kukita T, Matsushita K, Akimot M, Kawamata N, et al. Acute lung Injury in a healthy donor during mobilization of peripheral blood stem cells using granulocyte-colony stimulating factor alone. Haematologica. 2005;90:ECR10. [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Choi JC, Jung JW, Kwak HW, Song JH, Jeon EJ, Shin JW, et al. Granulocyte macrophage-colony stimulating factor (GM-CSF) augments acute lung injury via its neutrophil priming effects. J Korean Med Sci. 2008;23:288–95. doi: 10.3346/jkms.2008.23.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frossard JL, Saluja AK, Mach N, Lee HS, Bhagat L, Hadenque A, et al. In vivo evidence for the role of GM-CSF as a mediator in acute pancreatitis-associated lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L541–8. doi: 10.1152/ajplung.00413.2001. [DOI] [PubMed] [Google Scholar]
- 5.Wiedermann FJ, Mayr AJ, Kaneider NC, Fuchs D, Mutz NJ, Schobersberger W. Alveolar granulocyte colony-stimulating factor and alpha-chemokines in relation to serum levels, pulmonary neutrophilia, and severity of lung injury in ARDS. Chest. 2004;125:212–9. doi: 10.1378/chest.125.1.212. [DOI] [PubMed] [Google Scholar]
- 6.Adachi K, Suzuki M, Sugimoto T, Uetsuka K, Nakayama H, Doi K. Effects of granulocyte colony-stimulating factor on the development of inflammation in bleomycin-induced lung injury. J Toxicol Pathol. 2001;14:289–97. doi: 10.1080/01926230390244924. [DOI] [PubMed] [Google Scholar]
- 7.Verhoef G, Boogaerts M. Treatment with granulocyte-macrophage colony stimulating factor and the adult respiratory distress syndrome. Am J Hematol. 1991;36:285–7. doi: 10.1002/ajh.2830360413. [DOI] [PubMed] [Google Scholar]
- 8.Fudala R, Krupa A, Stankowska D, Allen TC, Kurdowska AK. Anti-interleukin-8 autoantibody: Interleukin-8 immune complexes in acute lung injury/acute respiratory distress syndrome. Clin Sci (Lond) 2008;114:403–12. doi: 10.1042/CS20070272. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet. 2007;369:1553–64. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 10.Hierholzer C, Kelly E, Lyons V, Roedling E, Davies P, Billiar TR, et al. G-CSF instillation into rat lungs mediates neutrophil recruitment, pulmonary edema, and hypoxia. J Leukoc Biol. 1998;63:169–74. doi: 10.1002/jlb.63.2.169. [DOI] [PubMed] [Google Scholar]
- 11.Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1) N Engl J Med. 1992;327:28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- 12.Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2) N Engl J Med. 1992;327:99–106. doi: 10.1056/NEJM199207093270207. [DOI] [PubMed] [Google Scholar]
- 13.Scarffe JH, Kamthan A. Clinical studies of granulocyte colony stimulating factor (G-CSF) Cancer Surv. 1990;9:115–30. [PubMed] [Google Scholar]
- 14.Rapoport AP, Abboud CN, DiPersio JF. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF): Receptor biology, signal transduction, and neutrophil activation. Blood Rev. 1992;6:43–57. doi: 10.1016/0268-960x(92)90007-d. [DOI] [PubMed] [Google Scholar]
- 15.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest. 2007;131:954–63. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]


