Abstract
Psychostimulant effects are enhanced by ovarian hormones in women and female rodents. Estradiol increases behavioral responses to psychostimulants in women and female rats, although the underlying mechanism is unknown. This study utilized mice to investigate the time frame and receptor mediation of estradiol’s enhancement of cocaine-induced behavior as mice enable parallel use of genetic, surgical and pharmacological methods. The spontaneous behavior of Sham and Ovariectomized (Ovx) female wild-type (WT) mice was determined during habituation to a novel environment and after cocaine administration. Ovx mice were replaced with vehicle (sesame oil) or 17β-estradiol (E2) for 2 days or 30 min prior to a cocaine challenge to investigate the time course of E2’s effects. To examine receptor mediation of estradiol effects, Ovx mice replaced for 2 days with either the ERα-selective agonist PPT or the ERβ-selective agonist DPN were compared to Sham mice, and mice lacking either ERα (αERKO) or ERβ (βERKO) were compared to WT littermates. Ovx mice exhibited fewer ambulations during habituation than Sham females. Cocaine-induced increases in behavioral ratings were greater in Sham than in Ovx mice. Two days but not 30 min of E2 replacement in Ovx mice increased cocaine responses to Sham levels. PPT replacement also increased the cocaine response relative to vehicle- or DPN- treated Ovx mice. αERKO mice displayed modestly attenuated behavioral responses to novelty and cocaine compared to αWT littermates, but no behavioral differences were found between βERKO and βWT mice. These results suggest that E2 enhances cocaine-stimulated locomotion in mice predominantly through ERα.
Keywords: Behavior, Cocaine, Dopamine, Estradiol, Mice, Ovariectomy
1. Introduction
Psychostimulant effects are influenced by ovarian hormones in women and female rodents (reviewed in Anker and Carroll (2011); Becker and Hu (2008); Lynch et al. (2002)). Female addicts report more positive subjective effects of amphetamine (AMPH) during the follicular phase when estradiol (E2) levels are rising than during the luteal phase when E2 levels are low (Anker and Carroll, 2011; Becker and Hu, 2008; Lynch et al., 2002). Women given exogenous E2 report greater pleasant feelings after AMPH than women treated with a placebo (Justice and de Wit, 2000). Preclinical studies demonstrate similar interactions between E2 and psychostimulant effects in animal models. Behavioral responses to psychostimulants fluctuate over the rodent estrous cycle and are maximal on proestrus and estrus, during and after the peak in circulating E2 levels (Becker and Cha, 1989; Quinones-Jenab et al., 1999; Sell et al., 2000; Zhang et al., 2008). Ovariectomy (Ovx), which removes the main endogenous source of E2 in female rats, attenuates behavioral responses to psychostimulants and decreases drug-seeking behavior (Becker and Hu, 2008; Becker et al., 2012; Parylak et al., 2008; Walker et al., 2001, 2012). E2 replacement increases conditioned place preference for AMPH (Silverman and Koenig, 2007) and enhances acquisition and reinstatement of cocaine (COC) self-administration in Ovx female rats (Hu et al., 2004; Jackson et al., 2006; Larson and Carroll, 2007; Larson et al., 2005; Lynch et al., 2001) but not in male rats (Jackson et al., 2006).
The reinforcing and locomotor-stimulating effects of psychostimulants like COC and AMPH are mediated by increased extracellular dopamine (DA) levels in the nucleus accumbens (NuAcc) and caudate putamen (CP) of the striatum ((Creese and Iversen, 1975; Kelly and Iversen, 1976; Kelly et al., 1975), reviewed in (Koob and Volkow (2010)). These forebrain regions are innervated by DA neurons from the ventral tegmental area (VTA) and substantia nigra pars compacta (SNpc) in the midbrain (reviewed in Bjorklund and Dunnett (2007)). E2’s enhancement of psychostimulant-induced behaviors is mediated by several activational effects within this DA system. E2 replacement in Ovx rats increases DA neuron activity and DA release (Becker, 1990a, 1990b; Becker and Beer, 1986; Becker and Ramirez, 1981; Thompson and Moss, 1994; Zhang et al., 2008). Ovx induces a loss of DA neurons and a decrease in DA D2 receptor levels, while E2 replacement increases both measures (Bazzett and Becker, 1994; Hruska and Silbergeld, 1980; Johnson et al., 2010; Le Saux et al., 2006; Leranth et al., 2000; Morissette et al., 2008; Walker et al., 2012).
Estradiol can exert its activational effects through multiple estrogen receptors (ERs), including the two classical receptors known as estrogen receptors α and β (ERα and ERβ, respectively). These receptors are ligand-activated nuclear hormone receptors that can interact with each other or with other transcription factors to regulate the transcription of target genes in the brain (reviewed in Toran-Allerand (2004)). Both ERα and ERβ are expressed in distinct, species-specific regional patterns within the brain, and these receptors are generally expressed in separate cell types within a given region (Creutz and Kritzer, 2002; Gundlah et al., 2000; Kritzer, 1997; Mitra et al., 2003; Pau et al., 1998; Shughrue et al., 1997; Vanderhorst et al., 2005). The rodent ventral midbrain contains more ERβ than ERα, and ERβ has been detected within a small population of DA neurons in the SNpc and VTA of rats (Creutz and Kritzer, 2002, 2004; Kritzer, 1997; Mitra et al., 2003). The existing literature provides evidence that both receptors influence the DA system and psychostimulant-induced behaviors in female rats, though the functional role of each receptor may differ (Larson and Carroll, 2007; Le Saux and Di Paolo, 2006; Le Saux et al., 2006; Morissette et al., 2008).
We currently do not know which receptor mediates E2’s enhancement of the behavioral effects of psychostimulants, particularly in mice and non-human primates, as previous animal studies investigating sex and ovarian hormone effects on psychostimulant-induced behavior have employed rats almost exclusively. Our laboratory has recently shown that mice exhibit a sex difference in the response to psychostimulants like that seen in rats, with females demonstrating more locomotion after COC and AMPH than males (Van Swearingen et al., 2013). Mice are therefore a valuable but underutilized model for studying the biological basis of observed sex differences in the responses to psychostimulants. The effects of ERα and ERβ on DA-mediated behaviors may vary with species, and mice may better model E2 effects on DA-mediated behaviors in the human brain than rats. Mouse models provide the additional advantage of genetic tractability as well as classical pharmacological and surgical methods in studies of E2 effects on the ascending DA system. Our primary aim in the present study was to elucidate the receptor mediating E2’s enhancement of COC-induced behavior in female mice using surgical, pharmacological, and genetic methods. We hypothesized that estradiol enhances psychostimulant-induced behavior in female mice via an ERα-mediated mechanism. This study demonstrates that activational effects of E2 over 2 days of replacement increase COC-stimulated behavior in Ovx adult female mice primarily through ERα. The present experiments also highlight how future work in mouse models can clarify previous work in rats to define the relative roles of ERα and ERβ in psychostimulant effects by enabling genetic manipulation as well as surgical and pharmacological approaches.
2. Methods and materials
2.1. Animals and hormone manipulations
Drug-naïve female C57BL/6 mice (55–65 days old, Charles River, Raleigh, NC) received sham (Sham) or active bilateral ovariectomy (Ovx) at the supplier and were shipped the following morning. Sham surgery included anesthesia, cutting of the abdominal muscles and overlying skin, and closing of the wound with clips. Mice were allowed to recover for 4 weeks before behavioral testing when they were 85–95 days old. Sham and Ovx mice were administered the vehicle or hormone replacements outlined in Table 1. For the knockout experiments, intact adult female ERα (αERKO) and ERβ (βERKO) knockout mice and their wildtype (WT) littermate controls were compared (Hewitt and Korach, 2003). Due to the limited availability of these mice, knockout mice in the present study were tested as intact animals to focus specifically on the involvement of ERα and ERβ in cocaine-induced behavior in the presence of circulating ovarian hormones. Sham and intact females were not selected based on estrous cycle stage as chronic vaginal lavaging attentuates the COC-stimulated behavior of female rats (Walker et al., 2002). All mice were housed by group/genotype in ventilated plastic cages with ad libitum food and water on a 12-h light/dark cycle (lights on at 0600 h). All studies were approved by Duke’s Institutional Animal Care and Use Committee. All efforts were made to minimize animal discomfort and to reduce the number of animals used.
Table 1.
The hormone replacements administered to Sham and Ovx mice prior to a cocaine challenge. Shams received vehicle for the same time-frame as hormone-replaced Ovx mice. All injections were given in the morning. All mice received a final injection of vehicle or hormone before being placed into locomotor boxes for habituation. For the 30 min versus 2 day replacement study, mice instead received their final injection after the saline treatment during testing, 30 min prior to the COC injection. E2 = 17β-estradiol; PPT = 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol; DPN = 2,3-bis(4-Hydroxyphenyl)-propionitrile; N/A = not applicable.
| Hormone treatment | Length of replacement | Dose | Injected volume | Activated ER(s) |
|---|---|---|---|---|
| Vehicle | 2 days | N/A | 100 μL | N/A |
| 30 min E2 | 30 min | 3 μg once | 100 μL | α, β |
| 2 Day/High E2 | 2 days | 3 μg/day | 100 μL | α, β |
| Medium E2 | 2 days | 0.3 μg/day | 100 μL | α, β |
| Low E2 | 2 days | 0.03 μg/day | 100 μL | α, β |
| PPT | 2 days | 2 mg/kg/day | 4 mL/kg | α |
| DPN | 2 days | 8 mg/kg/day | 4 mL/kg | β |
2.2. Behavioral measurements
All experiments were conducted during the light cycle. Mice were placed into 40 × 40 cm open field locomotor boxes (Kinder Scientific, Inc., Poway, CA, USA) for 2.5 h of habituation starting at 0900 h to control for novelty-induced locomotion before injections. Photobeam interruptions were automatically recorded as horizontal (e.g. ambulations, fine movements) or vertical (e.g. rearing) movements by the Motor Monitor software (Kinder Scientific, Inc., Poway, CA, USA). Mice were then injected with saline and cocaine (see below), and behavior was recorded for 1 h after each injection.
2.3. Hormone and drug treatments
17β-estradiol (E2) was purchased from Sigma Aldrich (St. Louis, MO, USA). The ERα-selective agonist 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) and the ERβ-selective agonist 2,3-bis(4-Hydroxyphenyl)-propionitrile (DPN) were purchased from Tocris (Minneapolis, MN, USA). PPT has 410-fold selectivity for ERα over ERβ (Stauffer et al., 2000), and DPN is 70-fold ERβ selective (Meyers et al., 2001). All hormones were dissolved in sesame oil for subcutaneous injection. The PPT and DPN doses were comparable to the 3 μg/day E2 dose based on their relative binding affinities for their respective ERs and have previously been shown to maintain DA neuron number in Ovx mice with chronic replacement (Johnson et al., 2010).
Cocaine HCl (COC) was provided by the National Institute of Drug Abuse through the Research Triangle Institute (Research Triangle Park, NC, USA). COC was given as intraperitoneal injections in sterile saline (10 mL/kg). Following habituation, mice were administered saline and then either a single dose of 30 mg/kg COC or sequential ascending doses of 5, 15, and 30 mg/kg COC on the same day at 75 min intervals.
2.4. Behavior scoring
After all injections, a trained observer blind to animal group/treatment monitored the behavior of each mouse. Each mouse was observed for two consecutive 15-s bins every 5 min, and each observed behavior was recorded. Behaviors were later assigned a post-hoc score (Table 2) (Van Swearingen et al., 2013) and the relative frequency of each score out of the total number of observations was calculated using Prism 5.0 (GraphPad Software Inc, La Jolla, CA, USA).
Table 2.
The rating scale used to score observed behaviors in mice. A higher score on this non-linear rating scale indicates a greater response. Scores 1–3 are normal behaviors seen in mice after saline injections, while scores 4–9 are induced by psychostimulants.
| Score | Behavior Category | Included behaviors |
|---|---|---|
| 1 | Asleep/Inactive | Asleep; Awake but still |
| 2 | Light in-place directed activity | Normal grooming; Light sniffing |
| 3 | Normal exploratory behavior | Slow intermittent locomotion; Occasional rearing; Sniffing or gnawing bedding |
| 4 | Fast exploratory behavior | Continuous, fast exploratory locomotion; Increased rearing; Fast sniffing |
| 5 | Intense, in-place stereotyped behaviors | Continuous sniffing; Head bobbing/weaving; Circling/pivoting; Intense grooming/ self-gnawing |
| 6 | Patterned locomotion | Continuous running in a pattern (i.e. around periphery of box) with head up and erect tail |
| 7 | Continuous, patterned rearing | Continuous “up-and-down” motion along wall or in corner; Often licking wall |
| 8 | Maintained rear | In a full rear for entire 15-s observation bin |
| 9 | Dyskinetic/Sick | Splayed hindlimbs; Laying on side; Seizures |
2.5. Brain and serum COC and metabolite levels
To assess COC metabolism, Ovx mice (32 total, n = 8 per group) administered vehicle, E2 (3 μg/day), PPT or DPN for 2 days as above were given a second dose of 30 mg/kg COC at least 1.5 h after the first COC injection. We have previously shown that COC does not accumulate in the brains of mice after a similar schedule of repeated COC dosing (Van Swearingen et al., 2013). Brains and serawere collected and analyzed for COC levels according to previously published methods (Van Swearingen et al., 2013) courtesy of Dr. David Moody, Dr. David Andrenyak, and Dr. Wenfang Fang at the University of Utah (Lin et al., 2001, 2003; Slawson et al., 2002).
2.6. Confirmation of surgery and replacement
Visual inspection of vaginal appearance (Byers et al., 2012; Champlin et al., 1973) and uterine wet weight confirmed the success of surgery and hormone replacements in vehicle and hormone treated Ovx mice. The wet weight of both uterine horns was collected after removal of all connective tissue (and ovaries in Sham, WT, αERKO and βERKO mice) and is an indirect marker of ERα action (O’Brien et al., 2006). One vehicle-treated and 5 PPT-replaced Ovx mice failed these confirmations and were excluded from all analyses. The excluded vehicle-treated mouse exhibited an intact-like vaginal appearance, evidence of incomplete Ovx surgery. The excluded PPT-replaced mice had Ovx-like vaginal appearances indicative of poor replacement. The difficulties with PPT replacement may be due to high protein binding and/or high hepatic clearance of PPT in vivo (Harris et al., 2002; Vijaykumar et al., 2003).
2.7. Data analysis
The numbers of ambulations, fine movements, and rears were determined by the Motor Monitor software and were divided into 10- and 60-min intervals (mean ± SEM). Habituation behavior was analyzed separately from behavior after saline and psychostimulant injections. Habituation results were analyzed by 2-way repeated-measures ANOVA (RM-ANOVA) (NCSS, Kaysville, UT, USA) with group as the between factor and time as the repeated measure. After injections, automatically recorded behaviors were analyzed in NCSS for main effects of time, treatment (saline versus COC) and/or group using 2- and 3-way repeated-measures ANOVA (RM-ANOVA) with time and/or treatment as the repeated measures. A second global 3-way RM-ANOVA was conducted on the automatically recorded behaviors in the PPT versus DPN study for main effects of ERα activity, ERβ activity, and time, with time as the repeated measure. ERα was active in the Sham, E2- and PPT-treated Ovx groups and inactive in vehicle- and DPN-treated Ovx mice. For ERβ, the active group contained Sham, E2- and DPN-replaced mice while vehicle- and PPT-treated mice were in the inactive group. Significant effects (p < 0.05) were followed by post-hoc Fisher’s LSD tests to compare treatments and groups.
The relative frequencies of observed behavior scores were analyzed according to previously published methods (Van Swearingen et al., 2013). Animals were classified as high responders (HR) or low responders (LR) to simplify presentation of the behavior score data. Mice with at least 25% of their bins scoring a 6 (for patterned locomotion) during the first 30 min after 30 mg/kg COC (i.e. during maximal behavioral activation) were considered high responders. One Sham mouse that received scores of 9 (for dyskinesia) was excluded as an outlier as the doses of COC used in the present study do not normally induce dyskinesia in mice. A global chi square test determined whether there was a main effect of group on the frequency distribution of behavior scores or the percentage of high versus low responders. Significant effects were followed by post-hoc pair wise comparisons on each score’s relative frequency using Prism 5.0.
The concentration of COC is reported as ng/g brain tissue or as ng/mL serum (mean ± SEM). The effects of group or ERα/β status on COC levels were determined by 1-way ANOVA. Significant effects were followed by post-hoc Fisher’s LSD tests. Statistical outliers were determined using the Grubbs test.
3. Results
3.1. Ovariectomy reduces novelty-induced behavior in mice
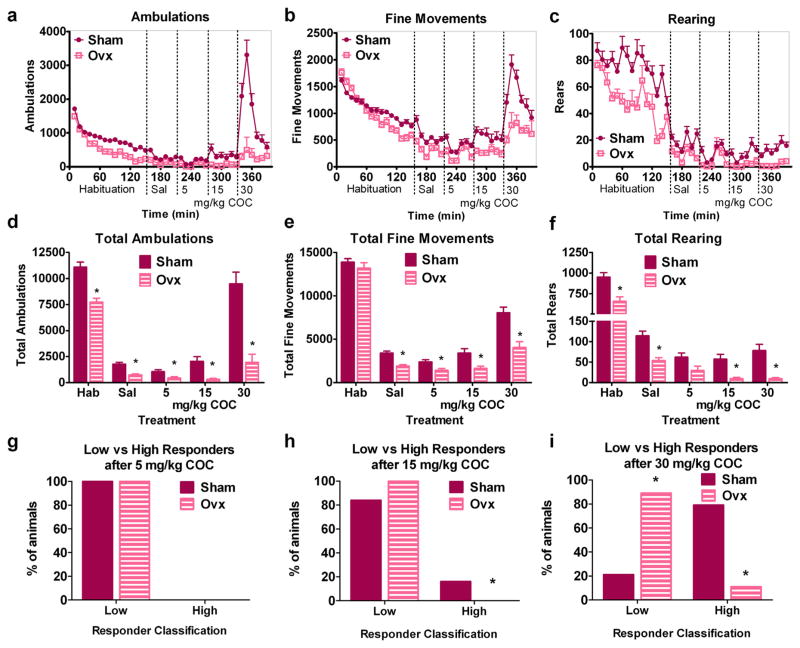
Mice ovariectomized one month prior to behavioral testing exhibited decreased responses to novelty relative to Sham animals. Photobeam interruptions recorded fewer ambulations and rears in Ovx females compared to Sham mice during habituation to the novel open field chambers (Fig. 1a–f). A main effect of group was found on ambulations [F(1,29) = 25.70, p < 0.0005] and rearing [F(1,29) = 13.90, p < 0.001] but not on fine movements. Post-hoc tests confirmed that Ovx mice exhibited fewer ambulations and rears during habituation relative to Sham females.
Fig. 1.
Ovariectomy reduces the response to novelty and to cocaine in adult female mice. Time courses of automatically recorded ambulations (a), fine movements (b), and rearing (c) in Sham and Ovx mice during habituation and after injections of saline and 5, 15, and 30 mg/kg COC. Total ambulations (d), fine movements (e), and rears (f) for each treatment from a–c above. Percent of animals classified as low or high responders based on their observed behavior scores after 5 (g), 15 (h), and 30 (i) mg/kg COC in Sham and Ovx mice. *denotes Ovx significantly different from Sham. n = 19 Shams, 9 Ovx. X-axis labels: Hab = habituation, Sal = saline injection.
3.2. Ovariectomy reduces psychostimulant-induced behavior in mice
Ovx mice also demonstrated attenuated behavioral responses after increasing doses of COC relative to Shams (Fig. 1a–f). Global ANOVA demonstrated a main effect of COC dose on the automatically recorded ambulations [F(3,111) = 30.70, p < 0.0001], fine movements [F(3,111) = 43.23, p < 0.0001], and rearing [F(3,111) = 7.95, p < 0.0005]. Post-hoc analyses showed that mice exhibited more fine movements after saline than after 5 mg/kg COC and more rears after saline than after any dose of COC. Post-hoc tests confirmed that administration of 30 mg/kg COC induced more ambulations and fine movements than saline and the lower COC doses. Main effects of group on ambulations [F(1,111) = 19.43, p < 0.0005], fine movements [F(1,111) = 17.84, p < 0.0005], and rearing [F(1,111) = 18.40, p < 0.0005] were also found. Ovx mice exhibited fewer ambulations, fine movements, and rears relative to Shams.
Lower-level ANOVAs were conducted within treatments to compare Sham and Ovx mice after saline and each COC dose. An effect of group after saline was found for ambulations [F(1,27) = 15.74, p < 0.001], fine movements [F(1,27) = 20.72, p < 0.0005], and rears [F(1,27) = 12.35, p < 0.005], with Ovx mice demonstrating reduced levels of each behavior compared to Shams. After 5 mg/kg COC, Ovx mice exhibited fewer ambulations and fine movements than Sham females (effect of group on ambulations, [F(1,27) = 5.81, p < 0.05], and fine movements, [F(1,27) = 5.98, p < 0.05]). An effect of group on ambulations [F(1,27) = 6.34, p < 0.05], fine movements [F(1,27) = 5.71, p < 0.05], and rearing [F(1,27) = 7.60, p < 0.05] after 15 mg/kg COC confirmed that Ovx mice showed reduced behavioral responses relative to Sham females after this intermediate COC dose. Thirty mg/kg COC also induced more of these behaviors in Shams than in Ovx mice. This difference was confirmed by a main effect of group on ambulations [F(1,27) = 18.57, p < 0.0005], fine movements [F(1,27) = 14.37, p < 0.001], and rearing [F(1,27) = 9.63, p < 0.01]. Post-hoc analyses confirmed that Ovx females exhibited reduced levels of these behaviors after each COC dose relative to Shams.
Behavioral observations confirmed that Ovx mice were less behaviorally activated by COC than Sham mice (Fig. 1g–i). Both Sham and Ovx mice after 5 mg/kg COC exhibited predominantly normal behaviors and were low responders at this dose (Fig. 1g.). A few individual Sham mice were slightly behaviorally activated by this low COC dose as these mice exhibited in-place stereotyped behaviors (predominantly as intense sniffing with head bobbing/weaving) and fast exploratory behavior. After 15 mg/kg COC, Ovx mice were less activated than were Shams. This intermediate COC dose induced similar in-place stereotyped behaviors in both Sham and Ovx mice, but a few Sham mice were high responders that progressed into patterned locomotion and patterned rearing (effect of group on responder distribution, p < 0.0001) (Fig. 1h). The highest COC dose tested (30 mg/kg) increased behavior more in Sham than in Ovx mice (effect of group, p < 0.0001) (Fig. 1i). After 30 mg/kg COC, most Sham mice were high responders that demonstrated patterned locomotion with some patterned rearing. Most Ovx mice were low responders exhibiting in-place stereotyped behavior (i.e. intense sniffing and head bobbing/weaving). Both automated measures and behavioral observations determined that COC stimulated fewer ambulations and less behavioral activation in Ovx mice relative to Sham females.
3.3. E2 replacement for 2 days restores behavioral response to cocaine but not to novelty
For the hormone replacement studies, we utilized a protocol with a single dose of COC to enable better resolution of the timing of E2’s effects on behavior. With this protocol, the total elapsed time between the first E2 exposure and the end of COC-stimulated behavioral testing would be approximately 1.5 h in 30-min E2-replaced mice.
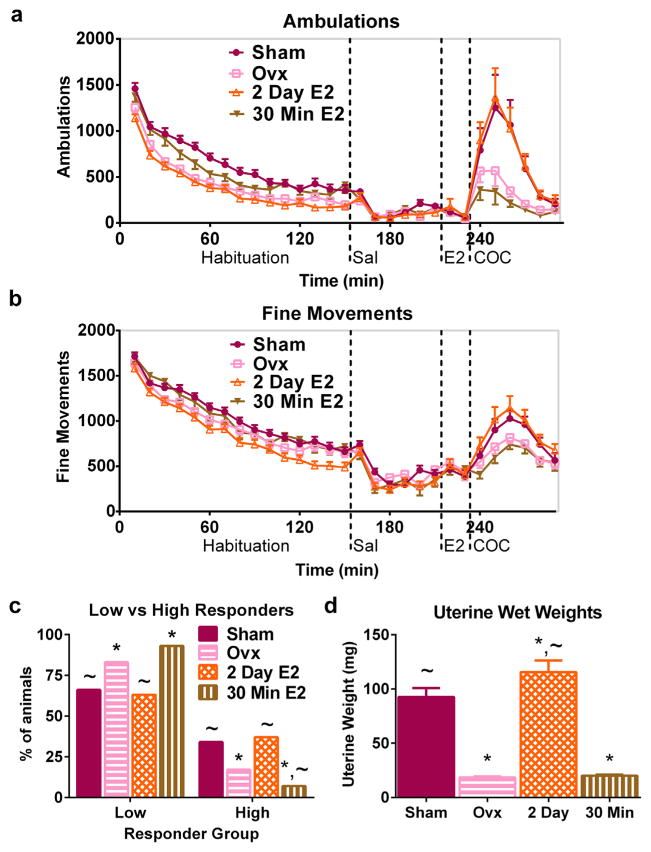
Automatically recorded ambulations showed that Ovx females given vehicle or E2 for 2 days had fewer ambulations than Shams during novelty-induced behavior (Fig. 2a). Two day E2-replaced mice also exhibited fewer fine movements than Shams during habituation (Fig. 2b). The 30-min replacement group had fewer ambulations than Shams as expected. These group differences were confirmed by post-hoc tests after a main effect of group was found on ambulations [F(3,1574) = 16.66, p < 0.0001] and fine movements [F(3,1574) = 4.49, p < 0.01] during habituation.
Fig. 2.
Two days but not 30 min of estradiol replacement restores the response to cocaine in ovariectomized mice. Time course of automatically recorded ambulations (a) and fine movements (b) during habituation and after injections of saline, vehicle/E2, and 30 mg/kg COC in Sham and Ovx mice administered vehicle or E2 starting 2 days or 30 min prior to COC. (c) Percentage of high and low responders in Sham, Ovx, and E2-replaced mice after 30 mg/kg COC based on behavioral observations. (d) Uterine wet weights after behavioral testing. *denotes different from Sham, ~different from Ovx. n = 29–30 per group for Sham, Ovx, and 2 day; n = 15 for 30 min group in (a–c). n = 22–30 per group for Sham, Ovx, and 2 day; n = 8 for 30 min group in (d). X-axis labels: Sal = saline injection, E2 = vehicle or E2 injection, COC = 30 mg/kg COC injection.
Lower-level analyses were completed within-treatment (saline, vehicle/E2, or COC) to compare animal groups after each injection. Analysis did not detect an effect of group on ambulations or fine movements after saline or after the injection with oil vehicle or E2 (Fig. 2a,b). COC induced more ambulations and fine movements in all groups relative to saline treatment as ANOVA reported a main effect of treatment on ambulations [F(3,1235) = 28.45, p < 0.0001] and fine movements [F(3,1235) = 80.42, p < 0.0001] after injections.
A single dose of 30 mg/kg COC induced more ambulations in Sham and 2-day E2-replaced females than in Ovx and 30-min E2-replaced animals (Fig. 2a). ANOVA confirmed a main effect of group on ambulations [F(3,617) = 3.59, p < 0.05] and fine movements [F(3,617) = 2.99, p < 0.05] after COC. A group × time interaction was also found on ambulations [F(15,617) = 2.04, p < 0.05]. Post-hoc analyses demonstrated that Sham and 2-day E2-replaced mice had more ambulations and fine movements (2-day only) than Ovx and 30-min replaced animals.
Behavioral observations confirmed the automatically recorded data by demonstrating a main effect of group on the distribution of behavior scores (p < 0.0001). Relative to Ovx mice, more Sham mice were categorized as high responders that exhibited sustained patterned locomotion after 30 mg/kg COC (Fig. 2c). There were more high responders and fewer low responders in the 2-day E2-replaced mice after COC than in Ovx mice administered only vehicle. Thirty-minute replaced animals were similar to vehicle-treated Ovx animals as they had fewer high responders and more low responders after COC relative to Sham or 2 day-replaced mice. After 30 mg/kg COC, low responders in all groups exhibited similar in-place stereotyped behaviors, namely intense sniffing, head bobbing/weaving, and circling/pivoting.
Uterine wet weights confirmed the efficacy of the 2-day E2 replacement protocol, as shown by a one-way ANOVA effect of group on uterine weight (p < 0.0001). Ovx decreased uterine wet weight, and 2 days of replacement with E2 increased uterine weight (Fig. 2d). Uterine weights in the 30-min replacement group were lower than those from Sham mice and were similar to those from vehicle-treated Ovx animals. These results demonstrate that 2 days but not 30 min of E2 replacement restores COC-stimulated behavior and uterine wet weight in Ovx female mice.
3.4. E2 doses spanning physiological levels increase cocaine-stimulated behavior
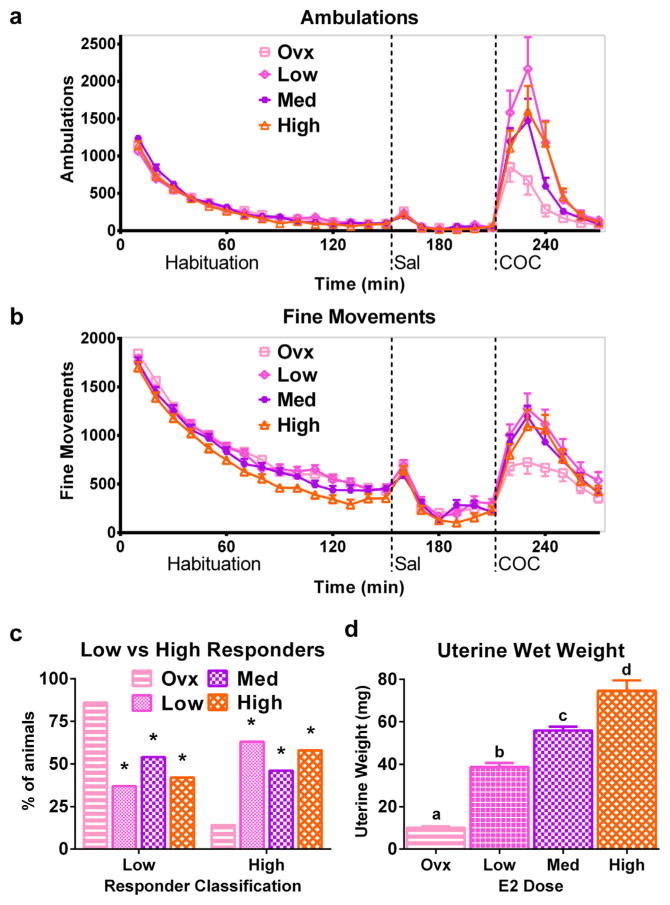
We tested three doses of E2 over a 100-fold range in Ovx mice to generate circulating E2 levels spanning from low or sub-physiological to supraphysiological levels (Ingberg et al., 2012). During habituation, Ovx mice administered any of the three doses of E2 exhibited similar numbers of ambulations as Ovx mice given vehicle (Fig. 3a). Ovx mice administered the high E2 dose (3 μg/animal/day) had fewer fine movements than Ovx mice given vehicle or either of the lower E2 doses (Fig. 3b). A main effect of group on fine movements [F(3,1439) = 6.16, p < 0.001] was found, and post-hoc analyses confirmed that high-E2 animals had fewer fine movements during habituation than the other groups.
Fig. 3.
A wide range of estradiol doses can increase the response to cocaine in ovariectomized mice. Time course of automatically recorded ambulations (a) and fine movements (b) during habituation and after injections of saline and 30 mg/kg COC in Ovx mice administered 0.03 μg/day (Low), 0.3 μg/day (Med), or 3 μg/day (High) E2 for 2 days prior to COC challenge. (c) Percentage of high and low responders in Ovx, Low, Med, and High E2-replaced mice after 30 mg/kg COC based on behavioral observations. (d) Uterine wet weights following behavioral testing. *denotes different from Ovx. Letters above bars in (d) indicate statistically different groups. n = 22–24 per group in (a–c). n = 16 per group in (d). X-axis labels: Sal = saline injection, COC = 30 mg/kg COC injection.
After an injection of saline, Ovx mice receiving any dose of E2 exhibited similar numbers of ambulations and fine movements as vehicle-treated Ovx mice (Fig. 3a,b). No main effect of group was found on ambulations or fine movements after saline. COC increased behaviors in all groups relative to saline (effect of treatment on ambulations [F(1,1127) = 3.04, p < 0.01] and fine movements [F(1,1127) = 79.12, p < 0.0001]).
COC induced more ambulations in Ovx mice administered any of the three tested E2 doses than in vehicle-treated Ovx mice (main effect of group, F(3,563) = 3.14, p < 0.05) (Fig. 3a,b). Post-hoc tests confirmed that low- and high-dose E2 replaced animals exhibited more ambulations than vehicle-treated Ovx females. Analysis did not yield an effect of group on fine movements or effect of E2 dose (i.e. 0.03, 0.3, versus 3 μg/day) on ambulations or fine movements. Thus E2 restored the behavioral response to COC even at low, subphysiological doses of hormone.
Classification of animals as high or low responders based on the behaviors observed after 30 mg/kg COC showed an effect of E2 administration (Fig. 3c). Mice given the low, medium, or high dose of E2 exhibited more patterned locomotion than vehicle-treated Ovx mice. Thus these groups had more high responders and fewer low responders after 30 mg/kg COC (effect of group, p < 0.0001). All three E2-replaced groups, including the low-dose group exposed to subphysiological E2 levels, showed similar percentages of high and low responders.
Uterine wet weights exhibited a dose-dependent increase with increasing E2 replacement dose (Fig. 3d). A main effect of group (p < 0.0001) on uterine wet weight was found. All E2-replaced mice had uteri that were larger than those from vehicle-treated Ovx mice. The uterine wet weight increased with increasing dose of E2 (Low < Medium < High). These results indicate that 2 days of E2 replacement over a 100-fold range spanning from sub- to supra-physiological levels enhances COC-stimulated locomotion in Ovx mice, and that uterine wet weight is E2-dose dependent.
3.5. Activation of ERα but not ERβ enhances cocaine-stimulated behavior
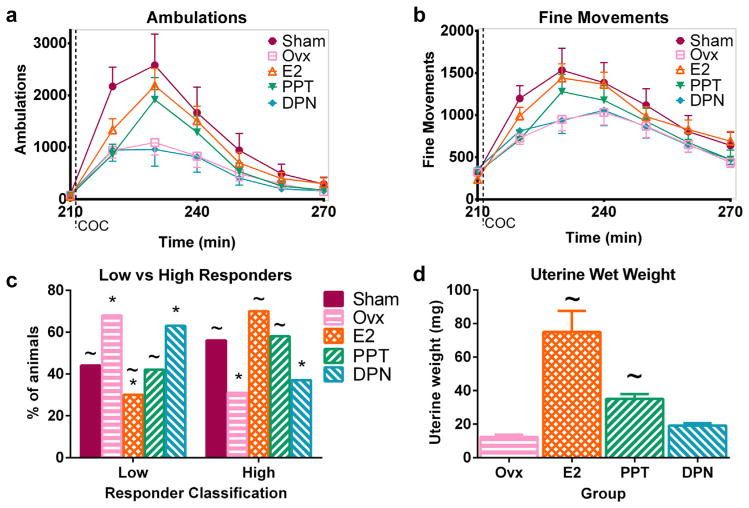
Utilization of selective estrogen receptor modulators (SERMs) to activate either ERα (with PPT) or ERβ (with DPN) in Ovx mice demonstrated that activation of ERα but not ERβ enhanced the response to 30 mg/kg COC (Fig. 4). Automatically recorded data showed a main effect of group [F(4,575) = 2.51, p < 0.05] and a group × time interaction [F(20,575) = 2.76, p < 0.0001] on ambulations but not on fine movements (Fig. 4a,b). Post-hoc tests confirmed that vehicle- and DPN-treated Ovx mice exhibited fewer ambulations and fine movements during the peak of behavioral stimulation after 30 mg/kg COC relative to Sham and E2-replaced females.
Fig. 4.
The ERα-selective agonist PPT but not the ERβ-selective agonist DPN increases the response to cocaine in ovariectomized mice. Time course of automatically recorded ambulations (a) and fine movements (b) after 30 mg/kg COC in Sham and Ovx mice administered vehicle, E2, PPT, or DPN for 2 days prior to a COC challenge. (c) Percentage of high and low responders in Sham, Ovx, E2, PPT, and DPN mice after 30 mg/kg COC based on behavioral observations. (d) Uterine wet weights of mice after behavioral testing. *denotes different from Sham, ~different from Ovx. n = 16–23 per group in (a–c). n = 8 per group in (d). X-axis labels: COC = 30 mg/kg COC injection.
Analysis of the automatically recorded behavior from combined groups for main effects of ERα or ERβ activity yielded a main effect of ERα [F(1,575) = 4.89, p < 0.05] on ambulations and an ERα × time interaction on ambulations [F(5,575) = 6.63, p < 0.0001] and fine movements [F(5,575) = 2.71, p < 0.05]. ERα-active mice had more ambulations and fine movements than ERα-inactive mice during the peak of the behavioral response to 30 mg/kg COC. The analysis did not demonstrate a main effect of ERβ activity or an ERα × ERβ interaction on COC-stimulated ambulations or fine movements.
Behavioral observations yielded similar results to the automatically recorded data (Fig. 4c). A main effect of group (p < 0.0001) was found on the percentage of high and low responders after 30 mg/kg COC. Sham females exhibited more sustained patterned locomotion after 30 mg/kg COC and thus had more high responders than vehicle-treated Ovx females which exhibited the in-place stereotyped behaviors typical of low responders. Treatment with E2 or PPT in Ovx mice induced more patterned locomotion than vehicle, and the E2 and PPT groups had more high responders after COC relative to vehicle-treated Ovx mice. The percentages of high and low responders in DPN-treated animals were similar to those of Ovx animals, and DPN mice had fewer high responders and more low responders than Shams.
Uterine wet weights measured after behavior confirmed that E2 and PPT were uterotrophic (Fig. 4d). One-way ANOVA on uteri from Ovx and hormone-replaced animals revealed a main effect of group [F(3,31) = 18.43, p < 0.0001] on uterine weight. E2- and PPT-replaced animals had larger uteri than vehicle-treated Ovx females, while DPN-treated mice had uterine weights similar to those of vehicle-treated Ovx mice. Uteri from PPT-treated mice were smaller than those from the E2-replaced females in this experiment and were more similar to the uterine weights of Low-E2 treated mice in the previous experiment (see Fig. 3d, Section 3.4). This partial replacement by PPT may explain the lack of complete restoration of COC-stimulated ambulations in PPT-treated Ovx females. These results demonstrate that activation of ERα but not ERβ in Ovx mice increases both the locomotor response to COC and uterine wet weight.
3.6. Hormone replacement in Ovx mice does not alter brain levels of cocaine
The observed differences in COC-stimulated behavior were not due to differences in brain levels of COC. Ovx mice administered vehicle, E2, PPT, or DPN for 2 days were injected with 30 mg/kg COC and sacrificed at the time of maximal behavioral activation after COC. Hormone replacement did not alter the amount of COC in the brain at this timepoint as there was no significant effect of group (Table 3). Reclassifying animals into groups based on the status of ERα or ERβ signaling (i.e. active or inactive for either receptor) did not reveal a main effect of ERα or ERβ activation or their interaction on brain COC levels. Similar results were obtained in serum as hormones did not alter serum concentrations of COC (Table 3). ANOVA did not show an effect of treatment group, ERα activation, ERβ activation, or an ERα × ERβ interaction on COC levels in serum.
Table 3.
Hormone replacement in ovariectomized mice did not alter the concentration of cocaine in the brain or serum. Concentrations of brain and serum COC in Ovx mice treated with vehicle, E2, PPT, or DPN for 2 days. Brains and sera were collected 15 min after an injection of 30 mg/kg COC. Data shown as mean ± SEM. n = 7–8 animals per group.
| Group
|
||||
|---|---|---|---|---|
| Ovx | E2 | PPT | DPN | |
| Brain COC (ng/g tissue) | 10916 ± 646 | 12202 ± 367 | 12862 ± 2437 | 11174 ± 483 |
| Serum COC (ng/mL serum) | 1393 ± 324 | 1534 ± 339 | 1315 ± 252 | 857 ± 119 |
3.7. Genetic ablation of ERα reduces the behavioral response to novelty and to cocaine
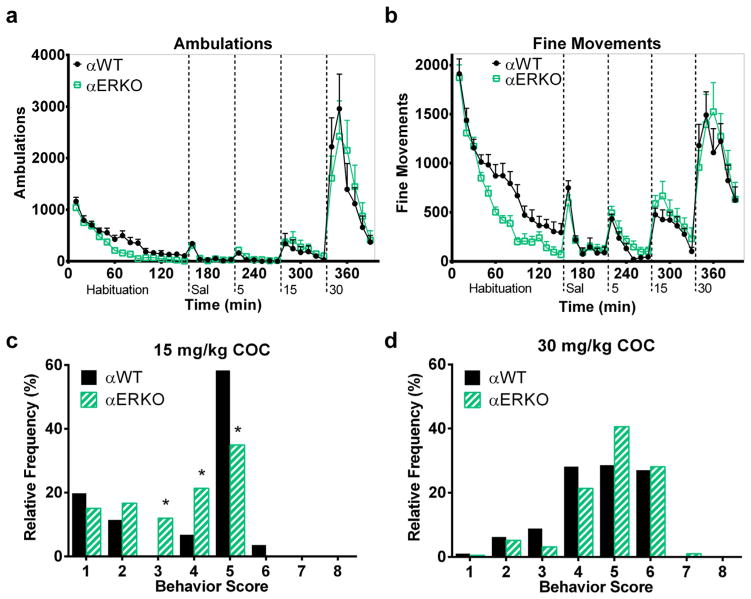
We investigated whether constitutive genetic ablation of either estrogen receptor alters COC-stimulated behavior in female mice. Due to the limited availability of these KO mice, an ascending doses paradigm was used to enable detection of either decreased or increased sensitivity to COC relative to WT littermates. Automatically recorded behaviors showed that female αERKO mice responded less to a novel environment than their αWT littermates. A main effect of genotype [F(1,239) = 6.79, p < 0.05] and a genotype × time interaction [F(14,239) = 2.27, p < 0.01] were found on ambulations during habituation (Fig. 5a). ANOVA also reported a main effect of genotype [F(1,239) = 6.95, p < 0.05] and a genotype × time interaction [F(14,239) = 2.28, p < 0.01] in fine movements (Fig. 5b). Post-hoc tests confirmed that αERKO mice demonstrated fewer ambulations and fine movements after exposure to novelty than WT mice.
Fig. 5.
Genetic ablation of ERα modestly decreases the responses to novelty and cocaine in female mice. Time courses of automatically recorded ambulations (a) and fine movements (b) in αERKO and αWT mice during habituation and after injections of saline and 5, 15, and 30 mg/kg COC. Relative frequencies of each category of observed behaviors after 15 (c) and 30 (d) mg/kg COC in αERKO and αWT mice. *denotes αERKO significantly different from αWT. n = 8 per genotype. X-axis labels: Sal = saline injection; 5, 15, and 30 = mg/kg COC injection.
Both groups responded to the increasing doses of COC with increasing levels of ambulations and fine movements, as shown by a main effect of COC dose (0 [saline], 5, 15, and 30) on ambulations [F(3,383) = 34.32, p < 0.0001] and fine movements [F(3,383) = 53.51, p < 0.0001]. The response of αERKO mice to COC did not differ from that of αWT mice. Analysis did not report an effect of genotype or genotype by dose interaction in the automatically recorded ambulations (Fig. 5a) or fine movements after injections (Fig. 5b). Lower-level analyses within saline or COC doses did not yield an effect of genotype on ambulations or fine movements after any treatment.
In contrast with the automatically recorded data, behavioral observations did detect a difference between αERKO and αWT mice behavior after COC. αWT and αERKO mice after 5 mg/kg COC predominantly responded with normal behaviors (scores of 1–3) like grooming and exploratory behavior, resulting in a similar behavior distribution for both genotypes (data not shown). After 15 mg/kg COC the predominant behavior displayed by αWT mice was in-place stereotyped behaviors (e.g. intense sniffing with head bobbing/weaving, score of 5) with some patterned locomotion (score of 6) in a few individuals. The behavior distribution of αERKO mice after 15 mg/kg COC demonstrated a shift toward less behavioral activation relative to αWTs (effect of genotype, p < 0.0001) (Fig. 5c). αERKO mice after 15 mg/kg COC exhibited fewer in-place stereotyped behaviors and more fast exploratory (score of 4) and normal exploratory (score of 3) behaviors relative to αWTs. No effect of genotype was detected in the behavior distribution of αERKO and αWT mice after 30 mg/kg COC (Fig. 5d). After 30 mg/kg COC both αERKO and αWT mice displayed patterned locomotion (score of 6) as well as in-place stereotyped behaviors and fast exploratory behaviors. These behavioral observations indicate that novelty- and COC-stimulated behaviors are attenuated in female αERKO mice relative to αWT littermates.
Uterine wet weights showed that loss of ERα in mice reduces uterine size. Uteri from αERKO females were smaller than those from αWT littermates (Table 4). A two-tailed t-test comparing αERKO and αWT uterine weights yielded a difference between these two genotypes [t(14) = 5.24, p < 0.0005], with αERKO uteri weighing less than those from αWT females. These results confirm that ERα signaling is uterotrophic. Genetic ablation of ERα and one month of estradiol deficiency due to Ovx both yield similar reductions in uterine size in female mice.
Table 4.
Uteri were smaller in αERKO but not βERKO mice relative to wildtype females. Uterine wet weights from αWT, αERKO, βWT, and βERKO female mice. Data shown as mean ± SEM. n = 7–8 animals per group.
| Genotype
|
||||
|---|---|---|---|---|
| αWT | αERKO | βWT | βERKO | |
| Uterine wet weight (mg) | 112 ± 17 | 21 ± 3a | 127 ± 17 | 98 ± 13 |
Denotes different from WT littermates.
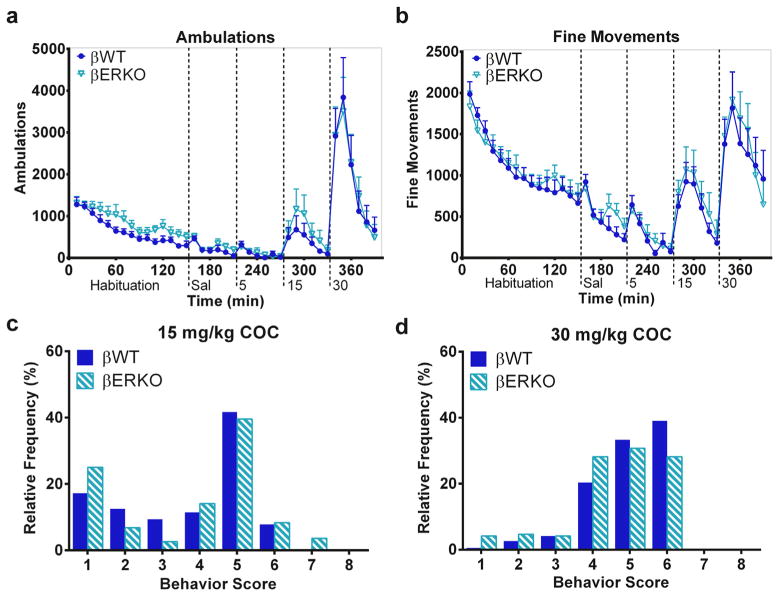
3.8. Genetic ablation of ERβ does not alter novelty- or cocaine-stimulated behaviors
Assessment of behavior in βERKO mice showed that these KOs had a similar behavioral response to novelty during habituation as their βWT littermates (Fig. 6a,b). No effect of genotype or genotype × time interaction was reported for ambulations or fine movements during habituation. After injections, βERKO and βWT mice responded similarly to injections of saline and COC based on automatically recorded methods (Fig. 6a,b). Both groups exhibited increased ambulations and fine movements with increasing doses of COC (effect of dose on ambulations, [F(3,383) = 27.10, p < 0.0001], and fine movements, [F(3,383) = 21.87, p < 0.0001]). Analysis did not detect an effect of genotype or genotype × dose interaction with automatically recorded ambulations or fine movements. Analyses within COC doses (0 [saline], 5, 15, or 30 mg/kg) did not detect an effect of genotype in either automatically recorded behavior.
Fig. 6.
Genetic ablation of ERβ does not alter the responses to novelty or to cocaine in female mice. Time courses of automatically recorded ambulations (a) and fine movements (b) in βERKO and βWT mice during habituation and after injections of saline and 5, 15, and 30 mg/kg COC. Relative frequencies of each category of observed behaviors after 15 (c) and 30 (d) mg/kg COC in βERKO and βWT mice. n = 8 per genotype. X-axis labels: Sal = saline injection; 5, 15, and 30 = mg/kg COC injection.
Behavioral observations supported the findings of the automatically recorded data. Five mg/kg COC induced predominantly normal behaviors (i.e. sniffing and grooming, scores of 1–3) in both genotypes (data not shown). Fifteen mg/kg COC induced more behavioral activation as both genotypes exhibited increased frequencies of in-place stereotyped behaviors and some patterned locomotion (score of 6) relative to 5 mg/kg COC (Fig. 6c). Thirty mg/kg COC further increased behavioral activation in both βERKO and βWT mice as both genotypes displayed more patterned locomotion (Fig. 6d). Analyses did not report an effect of genotype on the behavior distribution after any dose of COC. Overall, βERKO and βWT mice exhibited similar behavioral responses to novelty and COC.
Genetic ablation of ERβ did not alter uterine wet weights in female mice. βERKO mice had similarly sized uteri as βWT littermates (Table 4). A two-tailed t-test comparing the uterine wet weights of βWT and βERKO mice did not detect a significant difference between the two groups. Our data demonstrate that the uterotrophic effects of estradiol are primarily mediated by ERα and not by ERβ in female mice.
4. Discussion
4.1. Two days of estradiol replacement restores cocaine-stimulated behavior but not novelty-induced locomotion in ovariectomized C57BL/6 mice through ERα
The current study shows that ovariectomy decreases and E2 replacement over 2 days enhances COC-stimulated locomotion in female C57BL/6 mice. Our pharmacological replacement studies, and to a lesser degree the genetic ablation experiments, indicated a clear role for ERα in the enhancement of novelty- and COC-stimulated locomotion. These findings agree with an existing body of literature on psychostimulant-induced behavior that has almost exclusively been conducted in rats. Our laboratory and others have previously shown that Ovx in rats attenuates and E2 increases the response to psychostimulants (Becker and Hu, 2008; Kuhn et al., 2010; Lynch et al., 2002; Parylak et al., 2008; Russo et al., 2003; Segarra et al., 2010; Sell et al., 2000; van Haaren and Meyer, 1991; Walker et al., 2001, 2012). Activation of ERα has been shown to enhance AMPH-induced rotational behavior and COC-stimulated locomotion in female rats (Larson and Carroll, 2007; Schultz et al., 2009; Zhang et al., 2008). Our results extend previous findings in rats to mice and show that the alterations in psychostimulant-induced behavior with E2 and ERα replacement are not due to differences in the brain COC levels (Bowman et al., 1999; Niyomchai et al., 2006a).
This report also demonstrates that ovariectomy in C57BL/6 mice reduces novelty-induced behavior, which E2 replacement over 2 days does not restore. Previous work with open field behavior in mice did not demonstrate an effect of ovariectomy, perhaps due to the short duration (<5 min) of the behavioral testing (da Rocha et al., 2012; Xu and Zhang, 2006). However, αERKO female mice have previously been shown to exhibit reduced open field activity relative to αWT females (Ogawa et al., 2003). The utilized doses of E2 replacement in the present study do not increase novelty-induced locomotion, and our results agree with previous studies showing that E2 administration in Ovx rodents does not increase activity in the open field during anxiety testing (Walf and Frye, 2010; Walf et al., 2008) or during novelty-induced behavior (Eikelis and Van Den Buuse, 2000).
ERα’s ability to enhance the response to an initial COC exposure may increase addiction vulnerability in females. Some models suggest that the initial response to psychostimulants predicts addiction vulnerability and later drug intake (Davidson et al., 1993; Koob and Le Moal, 2001). A previous study in rats found that a high initial locomotor response to COC correlates with high COC-seeking behaviors in self-administration protocols (Schramm-Sapyta et al., 2011), though evidence for an opposite correlation have also been shown (Mandt et al., 2008). E2’s enhancement of psychostimulant-induced locomotion may also indicate increased addiction susceptibility as E2 may alter the ascending dopaminergic innervation to the nucleus accumbens that mediates both the locomotor and reinforcing effects of psychostimulants.
The enhancement of COC-induced behavior required 2 days of replacement with E2 or an ERα-selective agonist. This finding agrees with a previous report showing that E2 replacement starting 2 days prior to training enhances methamphetamine conditioned place preference in Ovx mice (Chen et al., 2003). In Ovx rats, E2 replacement over several days increases sensitization as well as the intake and reinstatement of COC during self-administration (Larson and Carroll, 2007; Larson et al., 2005; Yang et al., 2007). Two previous studies in Ovx rats reported either no change in COC-stimulated behavior (Niyomchai et al., 2006b) or attenuated COC self-administration (Grimm and See, 1997) with E2 replacement when tested 2–3 days after a single E2 injection. Taken together, these results suggest that repeated pulses of E2 over several days and/or the presence of E2 at the time of behavioral testing may be required to enhance psychostimulant-induced motor behavior.
One of the modes of action for estrogen responsiveness involves rapid non-genomic mechanisms, in addition to the genomic activities (reviewed in Al Sweidi et al. (2012); Hall et al. (2001)). E2 did not restore COC-stimulated behavior in mice through a rapid, non-genomic mechanism in the present study. Thirty minutes of E2 replacement did not increase COC-induced behavior in drug-naïve Ovx mice. A similar study in Ovx rats found that a 30 min E2 exposure attenuated COC-induced locomotion (Niyomchai et al., 2006b). However, extensive work in Ovx rats has demonstrated rapid enhancements of AMPH-stimulated striatal DA release and behavior 30 min after E2 injection (Bazzett and Becker, 1994; Becker, 1990a, 1990b; Castner and Becker, 1996). Other studies in Ovx rats reported enhanced psychostimulant-induced responses and self-administration after 30 min of E2 replacement, but the E2-induced increases in these studies emerged after repeated E2 administration across multiple days and not after the initial 30 min E2 exposure (Becker and Hu, 2008; Hu and Becker, 2008; Schultz et al., 2009; Zhao and Becker, 2010). The present study is consistent with previous findings that E2 replacement over several days enhances the response to psychostimulants.
The activational effects of 2-day E2 and ERα replacement on COC-stimulated behavior could occur through a transcriptional mechanism. In the uterus, E2 induces a biphasic pattern of transcriptional events through ERα that first increases water uptake and hyperemia a few hours after E2 administration and then stimulates epithelial cell proliferation 24–72 h after exposure (O’Brien et al., 2006). E2’s effects on other behaviors are mediated by regulation of transcription within the brain. E2 facilitates reproductive and maternal behaviors in part by stimulating expression of the progesterone receptor, the neuropeptide oxytocin and the oxytocin receptor in the brain (reviewed in Pfaff et al. (2011); Rissman (2008)). E2 and ERα signaling may similarly increase the behavioral response to psychostimulants through regulation of DA-related or DA-modulating genes.
Our results do not exclude a possible additional influence of ERβ on psychostimulant effects. Indeed, activation of either ERα or ERβ has been shown to enhance COC reinstatement in self-administering Ovx rats (Larson and Carroll, 2007). ERβ may mediate E2’s enhancement of AMPH conditioned place preference in Ovx rats (Silverman and Koenig, 2007). These studies suggest that the classical estrogen receptors may have two different but complementary roles in behavior, with ERα enhancing the locomotor response to psychostimulants and ERβ increasing the motivation to take psychostimulants (Larson and Carroll, 2007).
The effects of E2 on psychostimulant-induced behavior may be mediated by presynaptic alterations in DA neurons or their afferents. Ovariectomy decreases and E2 administration increases DA release in the striatum, which may be due in part to E2-induced alterations in gene expression (Becker, 1990a, 1990b; Becker and Beer, 1986; Becker and Hu, 2008; Thompson and Moss, 1994; Walker et al., 2012). E2’s regulation of DA-related genes varies by region, likely due to the regional- and cell-type specific expression patterns of ERα and ERβ (Kritzer, 1997; Shughrue et al., 1997; Zhou et al., 2002). E2 through ERα also alters the firing rate of DA neurons in the rat VTA after COC exposure (Zhang et al., 2008). This effect of ERα is likely due to changes in afferent cells that regulate DA neuron activity, as very few if any DA neurons in the ventral midbrain express this receptor in rats (Kritzer, 1997; Kritzer and Creutz, 2008). E2 decreases the extracellular concentration of GABA in the striatum and may thereby disinhibit DA release through ERα (Hu et al., 2006; Schultz et al., 2009). E2 may similarly enhance DA neuron activity through attenuation of tonic GABA-mediated inhibition in the ventral midbrain. Future studies in our laboratory will investigate whether E2’s enhancement of psychostimulant-induced locomotion through ERα is mediated by a presynaptic alteration of DA firing and/or release.
The separate effects of ERα and ERβ agonists on psychostimulant-induced behavior may also be due to differential modulation of postsynaptic signaling pathways (reviewed in Al Sweidi et al. (2012)). In the striatum, E2 and ERα-selective PPT induce phosphorylation of multiple components of the same PI3K pathway that that lies downstream of D2 receptors and regulates DA-mediated behaviors (Beaulieu et al., 2004, 2007; D’Astous et al., 2006). These ERα-induced changes in the PI3K pathway oppose the effects of D2 receptor signaling. In contrast, previous studies in female rats have suggested that ERβ enhances D2 signaling. ERβ increases striatal levels of D2 receptors and DAT and also decreases the expression of RGS proteins that regulate D2 signaling (Le Saux and Di Paolo, 2006; Le Saux et al., 2006; Morissette et al., 2008; Silverman and Koenig, 2007). ERα and to a lesser degree ERβ modulate the phosphorylation of CREB, a downstream target of D1 receptors in the striatum (Grove-Strawser et al., 2010). These effects of ERα and ERβ on DA-responsive signaling pathways in post-synaptic striatal neurons may be secondary to presynaptic changes in DA release but potentially explain how these receptors differentially regulate DA-mediated behaviors.
Our laboratory and others have previously shown that ovariectomy decreases the number of DA neurons in the SNpc and VTA of rats, mice and non-human primates (Johnson et al., 2010; Leranth et al., 2000; Walker et al., 2012). The data presented here and previously from our laboratory (Walker et al., 2012) suggest that the effects of gonadal hormones on DA system anatomy are just one of several possible mechanisms through which hormones alter DA-mediated behaviors. Two days of E2 replacement likely does not provide sufficient time to stimulate neurogenesis of midbrain DA neurons and thereby enhance behavioral responses to COC. Indeed, it is unclear whether midbrain DA neurogenesis occurs at all in the adult midbrain (reviewed in Borta and Hoglinger (2007)). The present results further suggest that the trophic effects of E2 on DA anatomy are distinct from E2’s short-term enhancement of psychostimulant-induced locomotion.
One complication in the present and previous studies investigating the roles of estrogen receptors in behavior is the lack of a good biomarker for ERβ activation. Uterine weight can be used to confirm activation of ERα signaling due to its uterotrophic effects ((O’Brien et al., 2006), reviewed in Couse and Korach (1999)). Indeed, in the current study the modest effect of our PPT replacement on uterine weight suggests that ERα was less active in these animals than in our E2-replaced females. The small, Ovx-like uteri of the αERKO females observed in the present study resemble previous reports of reduced uterine sizes in these mice and indirectly confirms their lack of ERα signaling ((O’Brien et al., 2006), reviewed in Couse and Korach (1999)). In contrast, the present and previous studies (reviewed in Couse and Korach (1999)) demonstrate that the uteri of βERKO mice are normally sized. Confirmation of ERβ activity is difficult without a similar indirect measure. However, doses of DPN similar to or lower than the 8 mg/kg/day dose used in the present study have been shown to maintain normal levels of midbrain DA neurons, DA transporters, and D2 receptors in Ovx mice and rats (Johnson et al., 2010; Le Saux and Di Paolo, 2006; Le Saux et al., 2006). Lower doses of DPN enhance COC-induced reinstatement (Larson and Carroll, 2007) and also increase memory recognition and alter DA metabolism in the hippocampus and prefrontal cortex (Jacome et al., 2010) of Ovx rats. Thus, the dose of DPN utilized in the current study was likely sufficient to activate ERβ in the brains of Ovx mice yet did not enhance the behavioral response to COC in our experimental paradigm.
The present study demonstrates that E2 enhances COC-stimulated behavior in female mice but does not address whether the other main ovarian hormone progesterone, either alone or in combination with E2, also alters psychostimulant-induced behavioral responses in female mice. Previous work indicates that progesterone suppresses psychostimulant effects in women and female non-human primates and rodents (reviewed in Anker and Carroll (2010)). Progesterone attenuates the reported subjective effects of COC in women (reviewed in Anker and Carroll (2010); Kuhn et al. (2010)) and reduces COC self-administration and seeking behaviors in female non-human primates (Feltenstein et al., 2009; Feltenstein and See, 2007; Mello et al., 2011, 2007). Thus, progesterone in female mice could attenuate the behavioral response to COC. Co-administration of progesterone and E2 in Ovx females would determine whether progesterone opposes the enhancing effects of E2 on psychostimulant-induced behaviors in mice, as has been previously demonstrated in rats ((Jackson et al., 2006; Perrotti et al., 2001), reviewed in Anker and Carroll (2010)). Future studies in our laboratory will investigate the effects of progesterone alone or in combination with E2 on psychostimulant-induced behavior in female mice.
4.2. Behavioral observation of psychostimulant-induced behavior in mice is a valuable supplement to automatically-recorded methods
The results in the ERKO mice highlight an advantage of using behavioral observation methods to supplement automatic recording. Female αERKO mice had similar ambulation counts as αWTs after 15 mg/kg COC. However, observations showed that the αERKOs exhibited fast exploratory locomotion whereas the αWTs exhibited in-place stereotyped behaviors with some patterned locomotion. Fast exploratory locomotion represents a lower level of behavioral activation than in-place stereotyped behaviors or patterned locomotion in mice, thus αERKO mice were less behaviorally activated by this dose of COC than αWT littermates. Observation of behaviors enabled the detection of these qualitatively different behaviors which yielded similar ambulation counts by automatic methods. Observational methods also enable the detection of subtle differences between small experimental groups, which can be an obstacle in any study utilizing valuable transgenic or knockout mice.
Genetically manipulated mice often develop compensatory mechanisms to adapt to genetic changes. The ERKO mice used in this study lacked either ERα or ERβ respectively, but still express the other ER form ((Couse et al., 1997), reviewed in Couse and Korach (1999)) and may have developed mechanisms to adapt to the lack of signaling by either estrogen receptor. These compensatory mechanisms may influence both the novelty- and the psychostimulant-induced behaviors of ERKO mice. Our laboratory has previously reported that female αERKO, but not βERKO, mice have fewer midbrain DA neurons relative to WT mice (Johnson et al., 2010) yet did not exhibit robust behavioral differences after COC exposure in the present study. These results suggest that the brain does not adapt to the loss of ERα’s neurotrophic effects within the DA system but can largely compensate for ERα’s influence on psychostimulant-induced behavior.
The ERKO mice provide the opportunity to investigate the relative roles of ERα and ERβ in behavior without the need for surgical manipulations that also alter the levels of other hormone levels. Circulating levels of E2 are elevated in αERKO females relative to WT and βERKO mice, while progesterone levels are normal in both ERKOs (reviewed in Couse and Korach (1999)). The altered hormonal milieu of ERKO female mice, particularly in the αERKOs, may have altered their behavioral responses to COC and confounded our comparison between ERKO and WT mice. As discussed above, progesterone attenuates COC-induced behavioral responses in females, and the loss of ER in ERKOs may lead to decreased progesterone signaling. In contrast, the endogenous circulating E2 would activate signaling through the remaining ER in the ERKOs. In the αERKOs, E2 levels are elevated beyond normal levels and may induce greater activation of the intact ERβ, which may compensate for the constitutive lack of ERα and thereby increase COC-induced behavior.
These potential compensatory mechanisms could be elucidated in future studies. To investigate whether ERβ can compensate for loss of ERα in COC-stimulated behavior, an ERβ antagonist could be administered to WT and αERKO mice, though no such compound is currently available. Alternatively, existing double ER knockout (αβERKO) mice could be used to determine whether the loss of both receptors yields different behavioral effects after COC than those shown here in mice lacking just one estrogen receptor. Ovariectomy in adult ERKO females may also alter COC-stimulated behavior if the ERKO mice have developed compensatory E2 or progesterone mechanisms. Future studies will elucidate the contribution of the endogenous E2 and progesterone in the αERKO and βERKO females through Ovx of ERKO mice and pharmacological replacement with ER-selective agonists and/or progesterone.
4.3. Conclusions
Ovariectomy attenuated novelty- and COC-stimulated behavior in C57BL/6 female mice, and E2 replacement for 2 days but not 30 min restored the behavioral response to COC. E2’s enhancement of COC-induced behavior may be mediated by ERα as an ERα-selective agonist enhanced and genetic ablation of ERα modestly decreased the behavioral response to COC. Whether these effects of E2 and ERα signaling are pre- or post-synaptic and whether they are due to transcriptional regulation or some other mechanism is unclear. Mice can provide a useful complementary model to rats in future studies that address these questions by enabling the use of genetic manipulation in parallel with surgical and pharmacological methods.
Acknowledgments
The authors thank the laboratory of Dr. David Moody at the Center for Human Toxicology, University of Utah for the assessment of COC concentrations through NIDA Contract N01DA-9-7767. This work was supported by NIDA grant #DA009079 to CMK. Support was provided by the Division of Intramural Research of the NIEHS to KSK Z01ES70065. All experiments in this study comply with all applicable current laws in the United States.
Footnotes
Conflict of interest
The authors have no conflicts to declare.
Contributor Information
Amanda E.D. Van Swearingen, Email: aedvs@email.unc.edu.
Cristina L. Sanchez, Email: cristina.sanchez@duke.edu.
Suzanne M. Frisbee, Email: suzannefrisbee@alumni.ecu.edu.
Antonia Williams, Email: antonia.williams@yahoo.com.
Q. David Walker, Email: qdwalker@duke.edu.
Kenneth S. Korach, Email: korach@niehs.nih.gov.
Cynthia M. Kuhn, Email: ckuhn@duke.edu.
References
- Al Sweidi S, Sanchez MG, Bourque M, Morissette M, Dluzen D, Di Paolo T. Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson’s disease. J Neuroendocrinol. 2012;24:48–61. doi: 10.1111/j.1365-2826.2011.02193.x. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. The role of progestins in the behavioral effects of cocaine and other drugs of abuse: human and animal research. Neurosci Biobehav Rev. 2010;35:315–333. doi: 10.1016/j.neubiorev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990a;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990b;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Cha JH. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204:361–372. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Borta A, Hoglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther. 1999;290:1316–1323. [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One. 2012;7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castner SA, Becker JB. Sex differences in the effect of amphetamine on immediate early gene expression in the rat dorsal striatum. Brain Res. 1996;712:245–257. doi: 10.1016/0006-8993(95)01429-2. [DOI] [PubMed] [Google Scholar]
- Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8:491–494. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- Chen HH, Yang YK, Yeh TL, Cherng CF, Hsu HC, Hsiao SY, Yu L. Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. Chin J Physiol. 2003;46:169–174. [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The pharmacological and anatomical substrates of the amphetamine response in the rat. Brain Res. 1975;83:419–436. doi: 10.1016/0006-8993(75)90834-3. [DOI] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol. 2004;476:348–362. doi: 10.1002/cne.20229. [DOI] [PubMed] [Google Scholar]
- D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol. 2006;69:1492–1498. doi: 10.1124/mol.105.018671. [DOI] [PubMed] [Google Scholar]
- da Rocha JT, Pinton S, Mazzanti A, Mazzanti CM, Beckemann DV, Nogueira CW, Zeni G. Effects of diphenyl diselenide on lipid profile and hepatic oxidative stress parameters in ovariectomized female rats. J Pharm Pharmacol. 2012;63:663–669. doi: 10.1111/j.2042-7158.2011.01255.x. [DOI] [PubMed] [Google Scholar]
- Davidson ES, Finch JF, Schenk S. Variability in subjective responses to cocaine: initial experiences of college students. Addict Behav. 1993;18:445–453. doi: 10.1016/0306-4603(93)90062-e. [DOI] [PubMed] [Google Scholar]
- Eikelis N, Van Den Buuse M. Cardiovascular responses to open-field stress in rats: sex differences and effects of gonadal hormones. Stress. 2000;3:319–334. doi: 10.3109/10253890009001137. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–352. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE. Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav. 1997;61:755–761. doi: 10.1016/s0031-9384(96)00532-x. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. Characterization of the biological roles of the estrogen receptors, ERalpha and ERbeta, in estrogen target tissues in vivo through the use of an ERalpha-selective ligand. Endocrinology. 2002;143:4172–4177. doi: 10.1210/en.2002-220403. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003;125:143–149. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Silbergeld EK. Increased dopamine receptor sensitivity after estrogen treatment using the rat rotation model. Science. 1980;208:1466–1468. doi: 10.1126/science.7189902. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend. 2008;94:56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis ofsex differences in the propensityto self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- Ingberg E, Theodorsson A, Theodorsson E, Strom JO. Methods for long-term 17beta-estradiol administration to mice. Gen Comp Endocrinol. 2012;175:188–193. doi: 10.1016/j.ygcen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94:488–498. doi: 10.1016/j.nlm.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22:226–237. doi: 10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000;71:51–59. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF. Selective colocalization of immunoreactivity for intracellular gonadal hormone receptors and tyrosine hydroxylase in the ventral tegmental area, substantia nigra, and retrorubral fields in the rat. J Comp Neurol. 1997;379:247–260. doi: 10.1002/(sici)1096-9861(19970310)379:2<247::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Creutz LM. Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. J Neurosci. 2008;28:9525–9535. doi: 10.1523/JNEUROSCI.2637-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, Johnson M, Thomae A, Luo B, Simon SA, Zhou G, Walker QD. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58:122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Carroll ME. Estrogen receptor beta, but not alpha, mediates estrogen’s effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32:1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Di Paolo T. Influence of oestrogenic compounds on monoamine transporters in rat striatum. J Neuroendocrinol. 2006;18:25–32. doi: 10.1111/j.1365-2826.2005.01380.x. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, Di Paolo T. ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50:451–457. doi: 10.1016/j.neuropharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SN, Moody DE, Bigelow GE, Foltz RL. A validated liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for quantitation of cocaine and benzoylecgonine in human plasma. J Anal Toxicol. 2001;25:497–503. doi: 10.1093/jat/25.7.497. [DOI] [PubMed] [Google Scholar]
- Lin SN, Walsh SL, Moody DE, Foltz RL. Detection and time course of cocaine N-oxide and other cocaine metabolites in human plasma by liquid chromatography/tandem mass spectrometry. Anal Chem. 2003;75:4335–4340. doi: 10.1021/ac030037c. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- Mandt BH, Schenk S, Zahniser NR, Allen RM. Individual differences in cocaine-induced locomotor activity in male Sprague–Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology (Berl) 2008;201:195–202. doi: 10.1007/s00213-008-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Kelly M, Fivel PA, Mendelson JH. Effects of progesterone and testosterone on cocaine self-administration and cocaine discrimination by female rhesus monkeys. Neuropsychopharmacology. 2011;36:2187–2199. doi: 10.1038/npp.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Knudson IM, Mendelson JH. Sex and menstrual cycle effects on progressive ratio measures of cocaine self-administration in cynomolgus monkeys. Neuropsychopharmacology. 2007;32:1956–1966. doi: 10.1038/sj.npp.1301314. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Niyomchai T, Akhavan A, Festa ED, Lin SN, Lamm L, Foltz R, Quinones-Jenab V. Estrogen and progesterone affect cocaine pharmacokinetics in female rats. Brain Res Bull. 2006a;68:310–314. doi: 10.1016/j.brainresbull.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Niyomchai T, Jenab S, Festa ED, Akhavan A, Quinones-Jenab V. Effects of short- and long-term estrogen and progesterone replacement on behavioral responses and c-fos mRNA levels in female rats after acute cocaine administration. Brain Res. 2006b;1126:193–199. doi: 10.1016/j.brainres.2006.07.099. [DOI] [PubMed] [Google Scholar]
- O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau CY, Pau KY, Spies HG. Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Mol Cell Endocrinol. 1998;146:59–68. doi: 10.1016/s0303-7207(98)00197-x. [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Russo SJ, Fletcher H, Chin J, Webb T, Jenab S, Quinones-Jenab V. Ovarian hormones modulate cocaine-induced locomotor and stereotypic activity. Ann N Y Acad Sci. 2001;937:202–216. doi: 10.1111/j.1749-6632.2001.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Waters E, Khan Q, Zhang X, Numan M. Minireview: estrogen receptor-initiated mechanisms causal to mammalian reproductive behaviors. Endocrinology. 2011;152:1209–1217. doi: 10.1210/en.2010-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J Neuroendocrinol. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cauley MC, Stangl DK, Glowacz S, Stepp KA, Levin ED, Kuhn CM. Role of individual and developmental differences in voluntary cocaine intake in rats. Psychopharmacology (Berl) 2011;215:493–504. doi: 10.1007/s00213-011-2216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Agosto-Rivera JL, Febo M, Lugo-Escobar N, Menendez-Delmestre R, Puig-Ramos A, Torres-Diaz YM. Estradiol: a key biological substrate mediating the response to cocaine in female rats. Horm Behav. 2010;58:33–43. doi: 10.1016/j.yhbeh.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Koenig JI. Evidence for the involvement of ERbeta and RGS9-2 in 17-beta estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav. 2007;52:146–155. doi: 10.1016/j.yhbeh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawson MH, Taccogno JL, Foltz RL, Moody DE. Quantitative analysis of selegiline and three metabolites (N-desmethylselegiline, methamphetamine, and amphetamine) in human plasma by high-performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J Anal Toxicol. 2002;26:430–437. doi: 10.1093/jat/26.7.430. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha and ER-beta. Exp Gerontol. 2004;39:1579–1586. doi: 10.1016/j.exger.2004.05.006. [DOI] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Van Swearingen AE, Walker QD, Kuhn CM. Sex differences in novelty-and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology (Berl) 2013;225:707–718. doi: 10.1007/s00213-012-2860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Vijaykumar D, Al-Qahtani MH, Welch MJ, Katzenellenbogen JA. Synthesis and biological evaluation of a fluorine-18 labeled estrogen receptor-alpha selective ligand: [18F] propyl pyrazole triol. Nucl Med Biol. 2003;30:397–404. doi: 10.1016/s0969-8051(02)00446-8. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol Behav. 2010;99:169–174. doi: 10.1016/j.physbeh.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Walker QD, Johnson ML, Van Swearingen AE, Arrant AE, Caster JM, Kuhn CM. Individual differences in psychostimulant responses of female rats are associated with ovarian hormones and dopamine neuroanatomy. Neuropharmacology. 2012;62:2266–2276. doi: 10.1016/j.neuropharm.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci. 2006;79:1553–1560. doi: 10.1016/j.lfs.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184:174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yang S, Yang C, Jin G, Zhen X. Estrogen regulates responses of dopamine neurons in the ventral tegmental area to cocaine. Psychopharmacology (Berl) 2008;199:625–635. doi: 10.1007/s00213-008-1188-6. [DOI] [PubMed] [Google Scholar]
- Zhao W, Becker JB. Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm Behav. 2010;58:8–12. doi: 10.1016/j.yhbeh.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Cunningham KA, Thomas ML. Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats. Brain Res Mol Brain Res. 2002;100:75–83. doi: 10.1016/s0169-328x(02)00134-1. [DOI] [PubMed] [Google Scholar]