Abstract
Controlling chronic immunoinflammatory diseases such as lesions in eye caused by infection with herpes simplex virus (HSV) represents therapeutic challenge. Since CD4+ T cells are the primary orchestrators of lesions, targeting activated CD4+ T cell subsets and increasing the representation of cells that express regulatory function would be a logical therapeutic approach. We show that this outcome can be achieved by therapy, systemic or local, with the lectin-family member galectin-9. This molecule, which is a natural product of many cell types, acts as a ligand to the inhibitory molecule TIM-3 that is expressed by activated but not naïve T cells. We show that 50% or more of T cells in ocular lesions caused by HSV in mice express TIM-3 and that blocking signals from its natural ligand with a monoclonal antibody results in more severe lesions. More importantly the provision of additional galectin-9, either systemically or more effectively by local subconjuctival administration, diminished the severity of SK lesions as well as the extent of corneal neovascularization. Multiple mechanisms were involved in inhibitory effects. These included apoptosis of the orchestrating effector T cells with consequent reduction of proinflammatory cytokines, an increase in the representation of two separate subtypes of regulatory cells as well as inhibitory effects on the production of molecules involved in neovascularization, an essential component of SK pathogenesis. Our results indicate that galectin-9 therapy may represent a useful approach to control HSV induced lesions, the commonest cause of infectious blindness in the Western World.
Introduction
One of the dire consequences of herpes simplex virus (HSV) infection is blindness resulting from infection in the eye and a subsequent chronic inflammatory reaction in the corneal stroma. This lesion is considered to be immunopathological orchestrated by T lymphocytes that recognize peptides derived from viral proteins or perhaps from altered self proteins of the damaged cornea (1, 2). Currently, herpetic stromal keratitis (HSK) is mainly controlled by combinations of drugs that include antivirals and steroids with the latter being administered for prolonged periods of time (3). Future therapies are expected to emerge from a better understanding of the disease pathogenesis so that critical steps can be counteracted more precisely. Identifying such steps has come mainly from studies in animal models, especially the mouse, where lesions that closely resemble those in humans can routinely be induced following primary infection with appropriate strains of virus (4). Such studies have revealed a critical role of CD4+ T cells of the Th1 subset as mediators of lesions (5, 6). In consequence, either preventing the access of Th1 cells to the eye or blunting their activity once at ocular sites represents potentially a valuable form of therapy. Recent studies on some autoimmune lesions caused by pathogenic T cells have indicated that one means of terminating the activity of such T cells is to engage receptors expressed by activated cells that deliver an inhibitory or lethal signal to the cell (7–11). This effect was achieved in some situations by engaging the TIM-3 (T cell immunoglobulin and mucin-3) receptor, a member of the T cell immunoglobulin and mucin family of proteins, with its recently identified ligand galectin-9 (8). Accordingly, the resolution of autoimmune lesions in collagen arthritis (a CD4+ Th1 subset mediated autoimmune lesion) occurred following treatment with galectin-9 (12). Some measure of control was also achieved with galectin-9 treatment in other immunoinflammatory lesions such as experimental autoimmune encephalomyelitis and graft versus host disease (8, 13, 14). To our knowledge, a role for TIM-3 galectin-9 interaction in controlling inflammatory lesions caused by microbial agents has yet to be explored. The present studies were designed, therefore, to evaluate if lesions in the eye caused by HSV were subject to control by manipulating the TIM-3/galectin-9 system on one or more cell types involved in causing HSK.
Our studies demonstrate that galectin-9 and TIM-3 interaction does influence the expression of lesions in the eye following ocular infection with HSV. Accordingly, lesions were significantly more severe if the signals delivered to TIM-3 were interrupted using anti-TIM-3 antibody. Moreover if galectin-9 was supplied in excess, either by systemic or local administration, lesion severity, which included particularly the extent of ocular neovascularization, was diminished. The mechanisms by which galectin-9 acted in vivo were likely multiple. These included induction of apoptosis of pathogenic effector Th1 cells, induction or the expansion of two types of regulatory cells as well as the diminished production of some factors involved in corneal neovascularization. Influencing the function of the TIM-3/galectin-9 pathway holds promise as a means to control the severity of HSK lesions.
Materials and Methods
Mice, Virus, cell lines
Female 6- to 8-wk-old C57B/6 mice were purchased from Harlane Sprague-Dawley (Indianapolis, IN). GFP-Foxp3 Knock-in mice were a kind gift from Dr. M. Oukka of Brigham and Women Hospital, Harvard Medical School. All animals were housed in the animal facilities at the University of Tennessee. BALB/c DO11.10 RAG2−/− mice were purchased from Taconic Farm and kept in our specific pathogen-free facility where food, water, bedding, and instruments were autoclaved. All manipulations were done in a laminar flow hood. All experimental procedures were in complete agreement with the Association for Research in Vision and Ophthalmology resolution on the use of animals in research. HSV-I RE Tumpey and HSV-I RE Hendricks was propagated and titrated on Vero cells (ATCC CCL81) using standard protocols. The virus was stored in aliquots at −80°C until use.
Antibodies and reagents
CD4-APC (RM4–5), DO11.10-PE (KJ1.26), CD25-FITC (7D4), CD103-FITC (M290), CD62L-APC (MEL-14), CD44-FITC (1M7), Foxp3-PE(FJK-16s), Foxp3-FITC (FJK-16s), CD69-FITC (H1.2F3), CD11c-PE (HL3), CD11c-APC (HL3), anti-IFN-γ-FITC, anti-IL-17-PE, CD11b-PerCP ( M1/70), Gr1-PE (RB6–8C5), Gr1-FITC (RB6–8C5), F4/80-FITC (BM8), Ly6C-FITC (AL-21 ), anti-CD3 (145.2C11), and anti-CD28 mAb (37.51) were purchased from BD PharMingen (San Diego, CA). Anti-TIM3-APC (cat.# FAB1529A), anti-TIM-3-PE (cat.# FAB1529P), recombinant IL-6 and recombinant human TGF-β1 were obtained from R & D. Anti-TIM-3 monoclonal antibody (RMT2–23) and rat IgG2a isotype control antibody (2A3) was obtained from Bio-X-cell, West Lebanon, NH. Mouse and human recombinant galectin-9 was provided by Gal Pharma, Japan. Galectin-3 was obtained from Sigma Chemical Co. Recombinant human IL-2 and OVA323–339 peptide were obtained from Hemagen and Genscript respectively. CFSE was obtained from Molecular Probe and used at a final concentration of 0.5 μM for 15 min. at 37°C in PBS. Complete RPMI-1640 was used for in vitro cultures.
Corneal HSV-1 infection and clinical observations
Corneal infections of C57B/6 mice were conducted under deep anesthesia. Mice were scarified on their corneas with a 27-gauge needle, and a 3-μl drop containing the required viral dose was applied to the eye. The eyes were examined on different days postinfection (p.i.) with a slit-lamp biomicroscope (Kowa, Nagoya, Japan), and the clinical severity of keratitis and angiogenesis of individually scored mice was recorded as described elsewhere (15, 16).
Treatment of animals with anti-TIM-3 antibody and galectin-9
Six- to 8-wk-old C57B/6 mice were ocularly infected under deep anesthesia with either 5×105 PFU HSV RE Hendricks or 5×103 PFU of HSV I RE Tumpey and divided randomly into groups. One group of animals infected with HSV I RE Hendricks was administered with 100μg of anti-TIM-3 antibody intraperitoneally (i.p.) every alternate day starting from day 3 until day 13 p.i. Animals in control group were given isotype control antibody following same regimen. Animals infected with HSV I RE Tumpey were given galectin-9 (10μg, 50μg or 100μg) ip starting from day 3 until day 13 daily while control animals received diluent. In some experiments, 6–8μl of galectin-9 (1, 5 or 10μg) was injected sub conjuctivally into eye daily starting either from day 4 or from day 8 until day 13. Galectin-9 was concentrated using Amicon ultracentriguge devices (Millipore). Tubes were treated with N/10 NaOH and washed with sterile PBS before using. Mice were observed for the development and progression of herpetic stromal keratitis (SK) lesions and angiogenesis from day 5 until day 14, as described elsewhere (15, 16). Most of the experiments were repeated at least three times unless stated
In vitro differentiation of CD4+CD25+Foxp3+ regulatory T cells and Th17 cells
Splenocytes isolated from DO11.10Rag2−/− mice were used as the precursor population for induction of Foxp3 in CD4+ T cells as described elsewhere (17). Briefly, 2×106 of total splenocytes after RBC lysis and several washings were cultured in 1 ml volume with previously optimized doses of plate-bound anti-CD3 Ab (0.125 μg/ml in 200 μl volume), rIL-2 (25–100 U/ml), and TGF-β (2.5 to 10 ng/ml) for 5 days at 37°C in a 5% CO2 incubator in 48-well plates. In some cultures in addition to IL-2, TGF-β either alone or in combination with various concentrations of Galectin-9 was added. After 5 days, cells were characterized phenotypically by flow cytometry.
For the differentiation of CD4+ T cells into Th17 cells, splenocytes isolated from DO11.10 Rag2−/− mice were cultured with 10 μg/ml of OVA323–339 peptide, TGF-β (2.5 to 10 ng/ml), IL-2 (25U) and IL-6 (30–60 ng/ml) for five days. To look for the effect of galectin-9 on Th17 cells, various concentration of galectin-9 or galectin-3 were added into cultures at the beginning. After 5 days, cells were analyzed by intracellular cytokine staining for the production of IL-17 and IFN-γ using BD bioscience kit. Foxp3 intranuclear staining was done using a kit from eBioscience.
Proliferation assays
Splenocytes and DLN cells isolated from control and galectin-9 treated HSV infected animals 14 dpi were labeled with 0.5μM of CFSE at 37°C for 10 minutes. 5×105 of labeled cells were cultured in presence of IL-2 (100U/ml), anti-CD3 (1μg/ml) and anti-CD28 (1μg/ml) mAb for three days. After three days of incubation, dilution of CFSE was analyzed in stained Foxp3− and Foxp3+ CD4+ T cells.
Ex vivo apoptosis assay
Draining lymph node cells and splenocytes isolated from uninfected and HSV infected C57B/6 mice at 8 dpi were incubated with various concentrations of galectin-9 and galectin-3 for 8 hrs in 96 well flat bottomed plates in humidified incubators in presence of 5% CO2. After incubation period was over cells, were stained for annexin V using a kit from BD Bioscience. Additionally, cells were also co-stained for TIM-3 and Annexin V. Stained cells were analyzed immediately by flow cytometry.
In vitro assays for VEGF production
A mouse stromal fibroblast cell line (MK/T-1) kindly gifted by Dr Winston Kao, Department of Ophthalmology, University of Cincinnati, Cincinnati, OH, was used for studying the effect of galectin-9 on VEGF production. The cells were cultured in 10% DMEM and plated onto 24 well tissue culture plates. The cells at more than 90% confluency were infected with 5MOI of HSV KOS for one hour. Thereafter, galectin-9 was added into the cells at various concentrations. Untreated but infected cells served as positive control for these experiments. In initial experiments cells were harvested at different times and stored in RNA stabilizing solution obtained from Quiagen. For most of the subsequent experiments cells were harvested after 12 hrs.
Real time RT-PCR
RNA was extracted from the cells stored in RNA later using RNeasy mini kit (Quiagen). Total cDNA was made with 1μg of RNA using oligod(T) primer. Real time PCR was performed using SYBR Green PCR Master Mix with iQ5 Real-Time PCR Detection System (BioRad, Hercules, CA). VEGF expression levels of each samples were normalized to HPRT using ΔCt calculations. Relative VEGF expression between control and experimental groups were calculated using 2−ΔΔCt formula. The sequences of the primers were: VEGF 5′-ACACAGGACCGCTTGAAGAT-3′ and 5′-CTGCACCCACGACAGAAG-3′. HPRT 5′-GACCGGTCCCGTCATGC-3′ and 5′-TCATAACCTGGTTCATCATCGC-3′.
ELISA
The corneal samples were pooled group wise and homogenized using a tissue homogenizer (Pellet pestle mortar, Kontes). The concentrations of various cytokines and VEGF was measured by sandwich ELISA kits from eBioscience (IL-6), and BD Bioscience (IL-12, TGF-β) and Quantikine (VEGF-A). For TGF-β quantitation, samples were first acidified as per manufacturer’s instructions.
Flow cytometric analysis
Cell preparation
Single-cell suspensions were prepared from the cornea, draining cervical lymph nodes (DLN), and spleen of mice at different time points p.i. Corneas and trigeminal ganglia were excised, pooled group wise and digested with 60 U/ml Liberase (Roche Diagnostics) for 60 min at 37°C in a humidified atmosphere of 5% CO2 as described earlier (17, 18). After incubation, the corneas and trigeminal ganglia were disrupted by grinding with a syringe plunger on a cell strainer and a single-cell suspension was made in complete RPMI 1640 medium.
Staining for flow cytometry
The single-cell suspension obtained from LNs, spleen, and corneal samples were stained for different cell surface molecules for FACS. All steps were performed at 4°C. Briefly, a total of 1×106 cells were first blocked with an unconjugated anti-CD32/CD16 mAb for 30 min. in FACS buffer. After washing with FACS buffer, fluorochrome-labeled respective Abs was added for 30 min. Finally, the cells were washed three times and resuspended in 1% paraformaldehyde.
To enumerate the number of IFN-γ and TNF-α producing T cells, intracellular cytokine staining was performed as previously described (18). In brief, 106 freshly isolated splenocytes, lymph node cells were cultured in U bottom 96-well plates. Cells were left untreated or stimulated with 2 MOI of UV inactivated HSV I and incubated overnight at 37°C in 5% CO2. Brefeldin A (10 μg/ml) was added for the last five hours of the culture period. After this period, cell surface staining was performed, followed by intracellular cytokine staining using a Cytofix/Cytoperm kit (BD PharMingen) in accordance with the manufacturer’s recommendations. The Ab used were anti-IFN-γ-PE and anti-TNF-α-FITC. The fixed cells were resuspended in 1% paraformaldehyde. For in vitro induced cultures, cells were stimulated with PMA (50ng) and ionomycin (500ng) for 5 hours in presence of brefeldin A (10 μg/ml). Subsequently, cells were first stained as described above for surface CD4 and intracellular cytokines (IL-17 or IFN-γ). The stained samples were acquired with BD FACSCalibur and the data were analyzed using the Flowjo software.
Statistical analysis
Most of the analyses for determining the level of significance were performed using Student’s t test. P ≤ 0.001 = *** P ≤ 0.01 = ** and P ≤ 0.05 = * were considered significant. Results are expressed as mean ± SD. For some experiments, as mentioned in the fig. legends, one way ANOVA test was applied.
Results
1. TIM-3 expression is up regulated on T cells after HSV infection
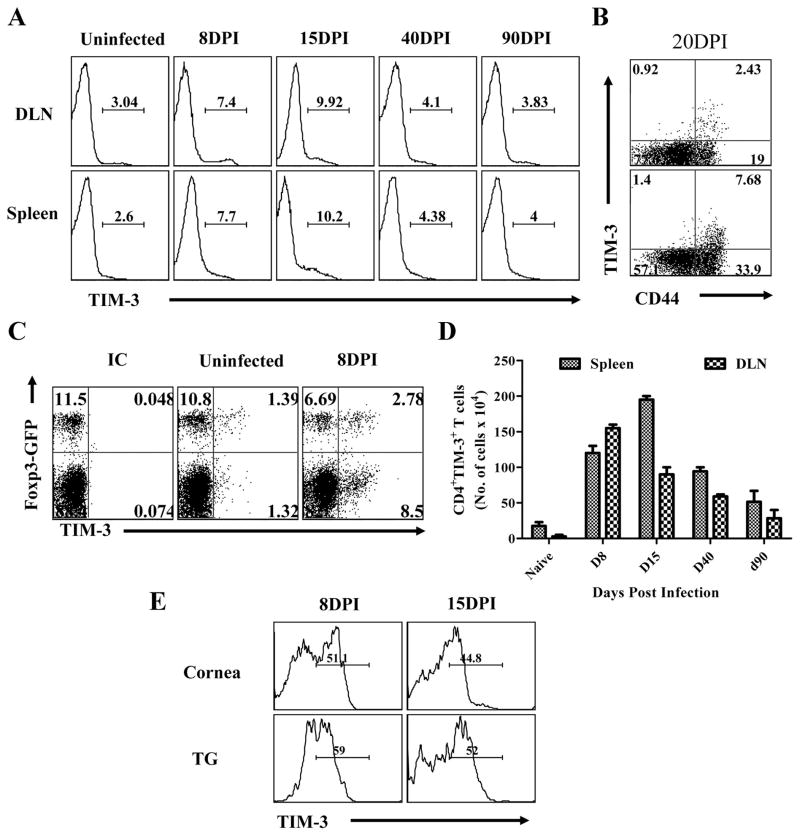
As a prelude to exploring the value of manipulating TIM-3/galectin-9 interaction to influence the outcome of stromal keratitis, mice were ocularly infected with HSV and the expression pattern of TIM-3 on CD4+T cells was measured at various times in lymphoid tissues, as well as the eye and trigeminal ganglion. Naïve animals lack inflammatory cells in the corneal stroma but invasion by many cell types, including CD4+ T cells, is fully evident by day 8 p.i. At this time, replicating virus is usually cleared, although the extent of the ocular inflammatory response usually continues to progress and peaks in severity between 15 to 21 days (5). Spontaneous regression, especially to a fully resolved state, usually does not occur without treatment. The results in fig 1A and D record the frequency and numbers of CD4+TIM-3+ T cells in the draining lymph nodes (DLN) and spleens at various times after HSV infection. In naïve animals, few CD4+ T cells expressed TIM-3 (between 0.5 to 3.0% in different experiments) and many that do were Foxp3+ (around 30 % of CD4+TIM-3+) (Fig 1C). By day 8, significant increases in the frequencies of TIM-3+CD4+ T cells were evident in both lymph nodes and spleen. The pattern was similar on day 15 but by day 40 had declined markedly. Analysis of lymph node and spleen cells at the time of peak responses revealed that most of the TIM-3+CD4+ T cells expressed the activation marker CD44hi (Fig 1B). Although we lack the reagents to prove it, we suspect that the increased populations of TIM-3+CD4+ T cells were HSV specific cells.
Fig. 1. Kinetic analysis of TIM-3 expression on CD4+ T cells after ocular HSV infection.
C57B/6 mice were infected with 5×103 of HSV. Three animals were sacrificed at each indicated time point and their spleens and draining cervical LN cells were analyzed for surface expression of CD4 and TIM-3 by flow cytometry. A. Histogram showing the percent of CD4+TIM-3+ T cells in spleen and DLN at indicated time points is shown. Data are shown from one representative experiment. B. Dot plot shows the expression of TIM-3 on CD4+CD44hi T cells. C. The expression of TIM-3 on CD4+Foxp3-GFP+ and CD4+Foxp3-GFP− from uninfected and HSV infected animals at 8dpi. D. Absolute numbers of TIM-3+CD4+ T cells in spleen and DLNs at indicated time point are shown. The expression of TIM-3 on CD4+ T cells isolated from inflamed pooled corneas and trigeminal ganglia at day 8 and day 15 post infection is shown. All kinetic experiments were repeated at least two times.
Ocular as well as trigeminal ganglion samples were also analysed using tissue pools from several animals after collagenase digestion (Fig. 1E). At both time points examined, around 50% of CD4+ T cells were TIM-3+. Taken together these results demonstrate that TIM-3 is up regulated on CD4+ T cells following HSV infection. This was especially evident at the inflammatory ocular and trigeminal ganglia sites.
2. TIM-3 signaling may control stromal keratitis lesion severity
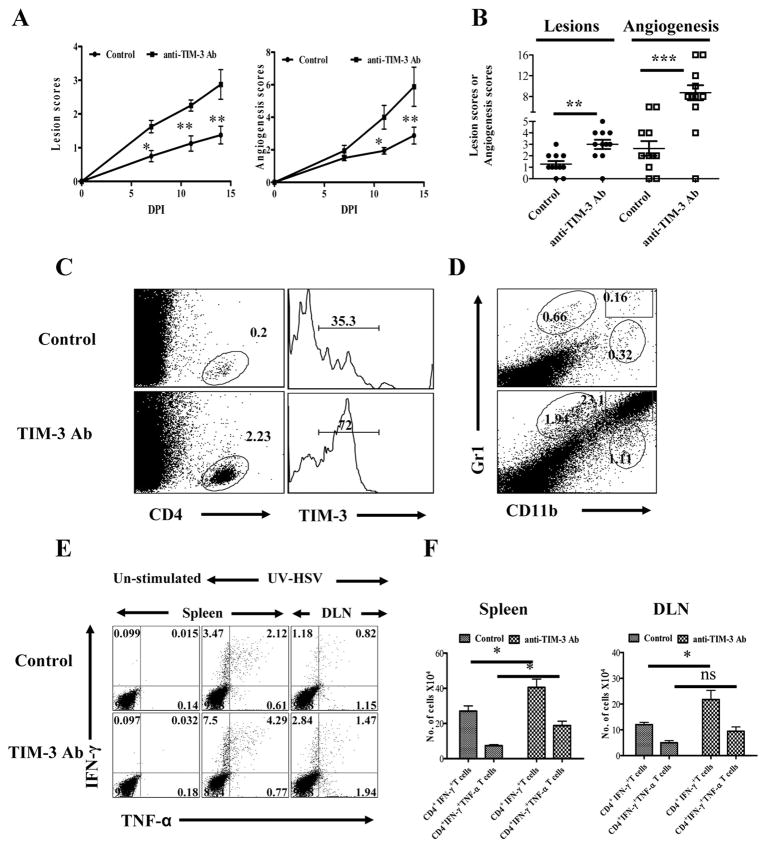
Studies on autoinflammatory lesions have indicated that TIM-3 signaling may represent a mechanism by which lesions are regulated under physiological conditions (8, 11, 19). The results expressed in figures 2A and B indicate that a similar situation likely occurs with HSV induced ocular lesions. This was shown by comparing the outcome of ocular infection in mice given a TIM-3 blocking monoclonal antibody (mAb) with those that received an isotype control mAb. Treatment was begun 3 days pi and the severity of HSK, as well as the extent of neovascularization, compared over a 15 day observation period. The results were clear cut and supported the notion that blocking TIM-3 led to enhanced HSV lesions and increased levels of neovascularization (Fig.2A and B). In two separate experiments involving 14 eyes, positive SK lesions (a scores of ≥ 2) were evident earlier in mice that received anti-TIM-3 (8 out of 14 eyes positive at day 7) than those given the isotype control Ab (0 out of 14 eyes at day 7). Lesions also became significantly more severe in anti-TIM-3 recipients with a higher percentage of eyes showing positive lesions overall (12 out of 14 with scores of ≥ 2 and 10 out of 14 with a score of ≥ 3 ) compared to isotype control Ab recipients (2 out of 14 with scores of ≥ 3) at day 14. A similar pattern of events was noted with the neovascularization scores.
Fig. 2. Effects of TIM-3 blockade on corneal inflammation and HSV-specific CD4+ T cell immune response.
C57B/6 animals infected with 5×105 PFU of HSV were given either anti-TIM-3 monoclonal antibody or isotype control antibody every alternate day starting from day 3 until day 13. The disease progression and immune parameters at day 14 were analyzed. A. The SK lesion severity and magnitude of angiogenesis is shown. B. Cumulative scores of lesion severity and angiogenesis at 14 days post infection. C. Percentages and phenotype (surface TIM-3) of CD4+ T cells in the corneas of control (upper panel) and antibody treated (lower panel) animals is shown D. CD11b+Gr1+ PMN infiltrated into cornea of control (upper panel) and antibody treated (lower panel) animals is shown. E. HSV-specific CD4+ T immune response in control and anti-TIM-3 antibody treated animals is shown at day 14. Dot plots depicting the percentages of IFN-γ+ TNF-α+ CD4+ T cells is shown. F. Total number of HSV-specific CD4+IFN-γ + and CD4+IFN-γ+TNF-α+ T cells in spleen and DLN at day 14 is shown.
At day 15, experiments were terminated and corneas were pooled and collagen digested to enumerate and phenotype the recovered cells. In addition, lymph nodes and spleens were collected from animals with positive lesions to measure HSV specific immune responses. As seen in figure 2C and D, the number of both CD4+ T cells and granulocytes was far greater in the ocular samples from anti-TIM-3 treated animals than controls (a more than 10 fold increase in CD4+ T cells and granulocytes). With regard to phenotype, the frequency of CD4+ T cells that were TIM-3+ was far higher in the anti-TIM-3 MAb group (72% in one experiment) than in controls (35%). The reason for this observation was not clear, but conceivably it could have reflected the finding that the magnitude of anti-HSV CD4+ T cell responses was higher in the anti-TIM-3 MAb treated animals (Fig 2E and F). Thus cells isolated from individual spleens and draining LNs at 15 days p.i. of control Ab and TIM-3 antibody recipients, when stimulated with HSV antigens and the numbers of IFN-γ and TNF-α producing cells measured, revealed a 2.5 fold increase in the frequencies and numbers of each of the cytokine producing cell populations in anti-TIM-3 treated animals (Fig. 2E and F)
These results indicated that TIM-3 signaling of CD4+ T cells in lymphoid organs or perhaps corneal lesions via its ligand under natural conditions may serve to regulate the extent of stromal keratitis and that this may proceed at least in part by influencing the magnitude of the immune response.
3. Galectin-9 Administration suppresses HSK lesions
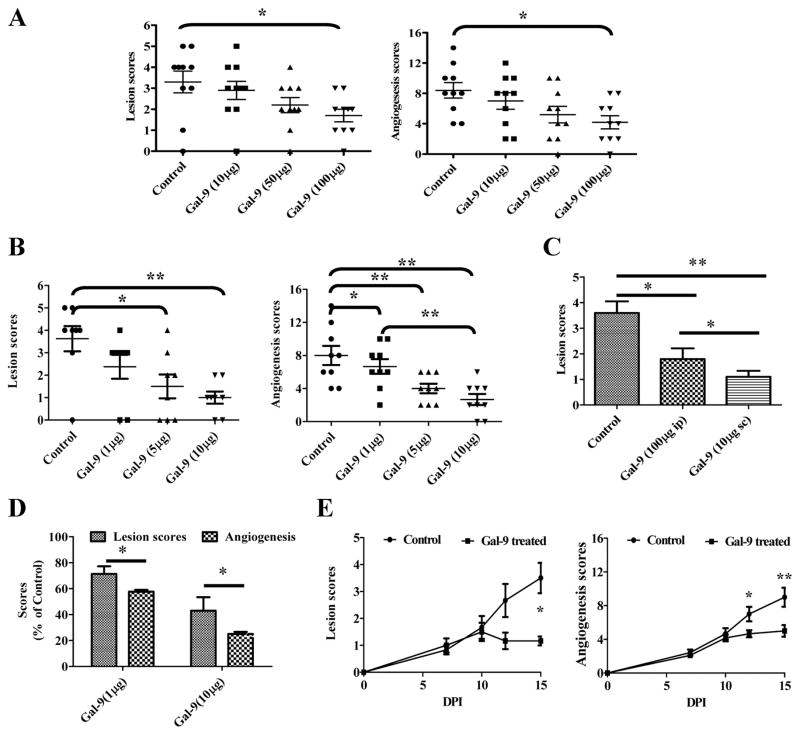
Recently galectin-9 was shown to be the natural ligand of TIM-3 and that signaling via galectin-9, at least to some T cell subsets, may cause them to undergo apoptosis (8). To evaluate if the administration of galectin-9 could influence the expression of HSK, two types of experiments were preformed in HSV ocularly infected mice. Animals were either treated i.p. with different doses of galectin-9 starting at day 4 or were given different doses of the drug injected subconjunctively starting at day 4 or day 8 until mice were sacrificed to evaluate different parameter at day 15. Both treatment modalities inhibited SK lesions as well as the extent of neovascularization, but the efficacy of the local subconjunctival administration was the greater (Fig 3A, B and C). The results shown in figure 3A demonstrated that following systemic administration, significant levels of lesion inhibition were only observed at the highest doses of galectin-9 evaluated (100μg) but a trend for suppressed lesions was evident at lower doses.
Fig 3. Effect of galectin-9 treatment on the severity of SK.
C57B/6 animals (n=12) infected with 5×103 PFU of HSV were treated with galectin-9 (10μg, 50μg and 100μg) daily starting from day 4 until day 14. A. Lesion and angiogenesis scores of control and galectin-9 treated animals at day 14 are shown. B. C57B/6 animals (n=9) infected with 5×103 PFU of HSV were injected subconjuctivally with 1μg, 5μg or 10μg of galectin-9 daily starting from day 4 until day 13. Lesion and angiogenesis scores of control and treated animals at day 14 are shown. C. The comparative lesion scores of HSV infected animals at 14 dpi when treated with galectin-9 either 10μg sub-conjuctivally or 100μg i.p. is shown D. The comparative reduction in lesion severity and angiogenesis scores of HSV infected animals treated locally with 10μg of galectin-9 daily is shown. E. Therapeutic effect of galectin-9 (10μg) administration on SK in C57B/6 animals infected (n=8) with 5×103 PFU of HSV. Kinetics of lesion and angiogenesis expression in control and galectin-9 treated animals (8–14 days pi) at 15 dpi is shown. All experiments were repeated at least three times. Data was analysed using one way ANOVA test with Dunnett’s post settings except in E where Student ‘t’ test was used.
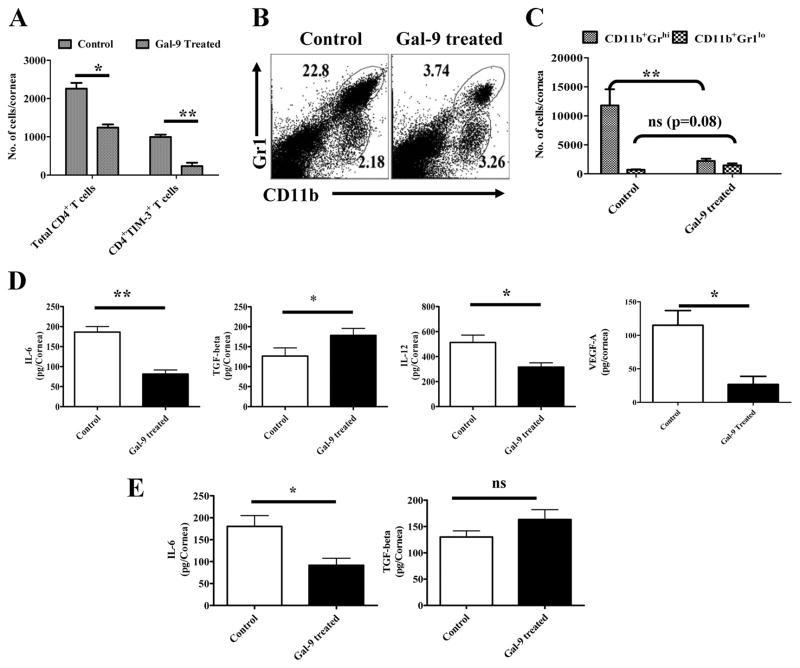
With local administration of galectin-9, a dose dependent inhibition of SK lesions and neovascularization also occurred with the differences compared to controls even more apparent then observed with systemic administration (Fig 3B and C). Accordingly, in animals given galectin-9 locally starting on day 4, lesions were reduced almost 3 fold on average compared to untreated controls with these differences being highly significant (p ≥ 0.01 ). The numbers of CD4+TIM-3+ cells recovered from eyes were significantly reduced compared to untreated controls (Fig 4A). In addition the frequencies and numbers of PMNs were reduced 6–7 fold as a consequence of galectin-9 treatment (Fig 4B and C). Along with clinical changes occurring in response to local galectin-9 treatment, the levels of cytokines as well as the angiogenesis factor VEGF were measured in pooled corneal extracts. As shown in Fig 4D, whereas levels of the proinflammatory cytokines IL-6 and IL-12 were less, TGF-β levels were higher than in controls. VEGF levels were also lower in galectin-9 treated pooled samples.
Fig 4. Effects of galectin-9 treatment on cellular infiltration and cytokine levels in corneas of HSV infected animals.
C57B/6 animals infected with 5×103 PFU of HSV were given 10μg of galectin-9 subconjuctivally daily starting from day 4 until day 13 as described above in Fig 3B. A. Total numbers of CD4+ and CD4+TIM-3+ T cells in the corneas of control and galectin-9 treated animals is shown at day 14. B–C. The frequencies and numbers of infiltrated PMNs (CD11b+Gr1hi) and a cell type akin to MSCs (CD11b+Gr1lo) is shown in control and galectin-9 treated animals. D. Levels of cytokines (IL-6, TGF-β and IL-12) and angiokine (VEGF-A) in pooled corneal samples each consisting of four cornea/group isolated from control and galectin-9 treated animals as analyzed by sandwich ELISA are shown. E. Levels of IL-6 and TGF-β in pooled corneal samples isolated from control and therapeutically treated HSV infected animals are shown. Experiments were repeated twice. Statistical levels of significance were estimated by Student’s ‘t’ test
Of notable interest, if local treatment was begun on day 8, the time when lesions become clinically evident, they failed to progress and even slightly diminished in severity compared to untreated animals (Fig 3F). Furthermore, as shown in fig. 4E, pooled corneal samples from treated animals had lower levels of proinflammatory (IL-6) and higher levels of anti-inflammatory (TGF-β). These data strongly indicate that TIM-3/galectin-9 interaction serves to induce resolution of viral immunoinflammatory lesions.
4. Possible mechanisms by which galectin-9 therapy functions
I. Induction of apoptosis of TIM-3+CD4+ T cells
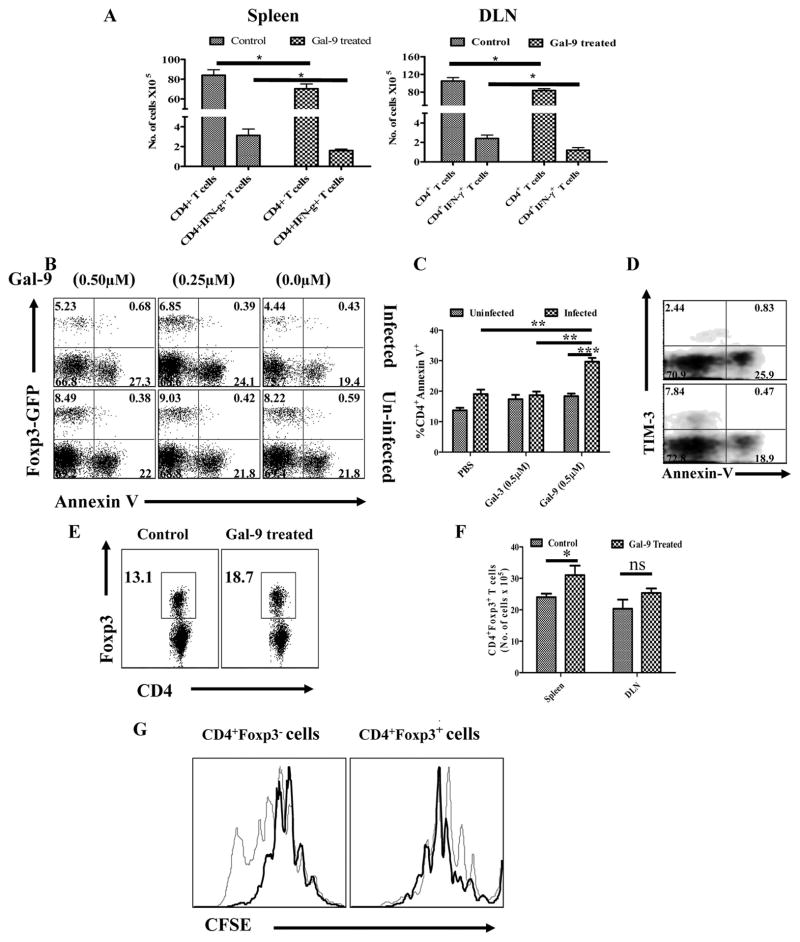
Prior in vitro studies have indicated that galectin-9 triggering of proinflammatory CD4+ T cells causes them to undergo apoptosis (8). Since in the HSK model, lesions appeared to be mainly orchestrated by IFN-γ producing CD4+ T cells (6), lesions could be reduced if orchestrating T cells were destroyed. We attempted to demonstrate such an effect in vivo by examining single cell suspensions from lymphoid organs by flow cytometry or tissue sections of treated eyes for signs of apoptotic cells. Such could not be demonstrated (data not shown). However, it was evident that the total numbers of CD4+ T cells as well as HSV-specific CD4+IFN-γ+ T cells were significantly reduced in animals treated systemically with galectin-9 which could have been the consequence of apoptosis (Fig 5A). In addition, we were able to demonstrate CD4+ T cells that express TIM-3 as a consequence of HSV infection in vivo could be induced to undergo apoptosis when exposed to galectin-9 in vitro (Fig 5B–D).
Fig. 5. Differential effects of galectin-9 therapy on regulatory T cells and effector CD4+ T cells in HSV infected animals.
A. Total numbers of CD4+ T cells and CD4+IFN-γ+ T cells in the DLNs and spleens of control and galectin-9 treated (systemic) animals in shown. B–D. Induction of apoptosis of CD4+Foxp3−TIM-3+ T cells by galectin-9. Cells isolated from cervical LNs of HSV infected Foxp3-GFP knock in animals (day 8) and uninfected animals were cultured in the presence of PBS, galectin-3 and galectin-9 for 8 hours and thereafter stained for annexin-V, B. Annexin-V+CD4+Foxp3− and Foxp3+ T cells isolated from infected (upper panel) and uninfected (lower panel) animals incubated with PBS and galectin-9 animals is shown. C. The bar diagram showing the percentages of annexin-V+ cells under indicated conditions is shown. D. Co-staining of CD4+ T cells for annexin-V and TIM-3 is shown. E–G. Proliferative responses of Foxp3- and Foxp3+CD4+ T cells in HSV infected animals after galectin-9 therapy. Frequencies (E) and absolute numbers (F) of CD4+Foxp3+ T cells in the spleens (E and F) and DLNs (F) of control and galectin-9 treated animals are shown. G. DLN cells isolated from control and galectin-9 treated animals were labeled with CFSE and their proliferative response in the presence of anti-CD3 and anti-CD28 were analyzed after 3 days. CD4+ gated population analyzed for the dilution of CFSE in Foxp3+ and Foxp3− CD4+ T cells from control (thin line) and galectin-9 treated (thick line) animals is shown.
As TIM-3 expression was also evident on about 10–15% of Foxp3+CD4+ T in lymphoid organs, we investigated if these cells are also susceptible to killing by galectin-9 ligation. Thus, DLN cells were isolated from either infected or uninfected GFP-Foxp3 knock-in animals and treated with various doses of galectin-9 in vitro for eight hours. As shown in fig 5B, while more than 90% of CD4+TIM-3+Foxp3− cells underwent apoptosis, less than 10% of CD4+TIM-3+Foxp3+ T cells were annexin V+. Furthermore, CD4+TIM-3+Foxp3+ T cells showed minimal level of expression of annexin V on their surface as measured by the mean fluorescence intensity. This could mean that as a consequence of galectin-9 treatment with Foxp3+ cells showing more resistance to apoptosis, the ratio of Tregs: Teffector would increase perhaps accounting in part for the anti-inflammatory effect of galectin-9.
II. Expansion or induction of Foxp3+ Tregs
Prior experiments have established that the severity of HSK lesions can be modulated by Foxp3+ regulatory T cells either induced in vivo or by adoptive transfers of such cells (15, 17). There is some indirect evidence that galectin-9 could be involved in the induction of Foxp3+ cells since such cells were reduced in numbers in galectin-9 knockout animals (12). To determine if galectin-9 administration to HSV infected animals had any influence on the Treg response, experiments were done in which animals were infected with HSV after which from day 4 to day 14 one group was injected systemically with a dose of galectin-9 shown to be effective at diminishing lesion severity and the other group received diluent. Experiments were terminated on day 15 and the cell types in individual DLN and spleen were collected to quantify CD4+ T cells that were Foxp3+. As shown in Fig. 5E and F, significant increases (P ≥ 0.05) in the frequencies and numbers of CD4+Foxp3+ T cells were evident in the spleens of galectin-9 treated animals. Additionally the numbers of Foxp3+ cells in the DLNs of galectin-9 treated animals were also increased, but the numbers compared to controls were not significant. This effect of galectin-9 treatment might be the consequence of expansion of preexisting Foxp3+ cells or perhaps conversion of some Foxp3− cells to become Foxp3+.
Evidence that galectin-9 could be causing some expansion of Foxp3+ cells was shown by comparing the proliferative capacity in vitro of Foxp3+ cells from control and galectin-9 treated animals. As shown in fig. 5G, CFSE dilution in Foxp3+ cells TCR stimulated for 3 days, in the presence of IL-2 was greater in the cell population from galectin-9 treated animals than from controls. Curiously, the effect of galectin-9 treatment on the response of Foxp3− cells was to diminish their proliferation capacity compared to that occurring in the same cell population from controls. These observations support the concept that the enrichment of Foxp3+ cells in galectin-9 treated animals could in part be explained by expansion of previously Foxp3+ cells. However, the relative contribution of increased proliferative response of Foxp3+ and decreased proliferative response of Foxp3−CD4+ T cells could not be established in these studies.
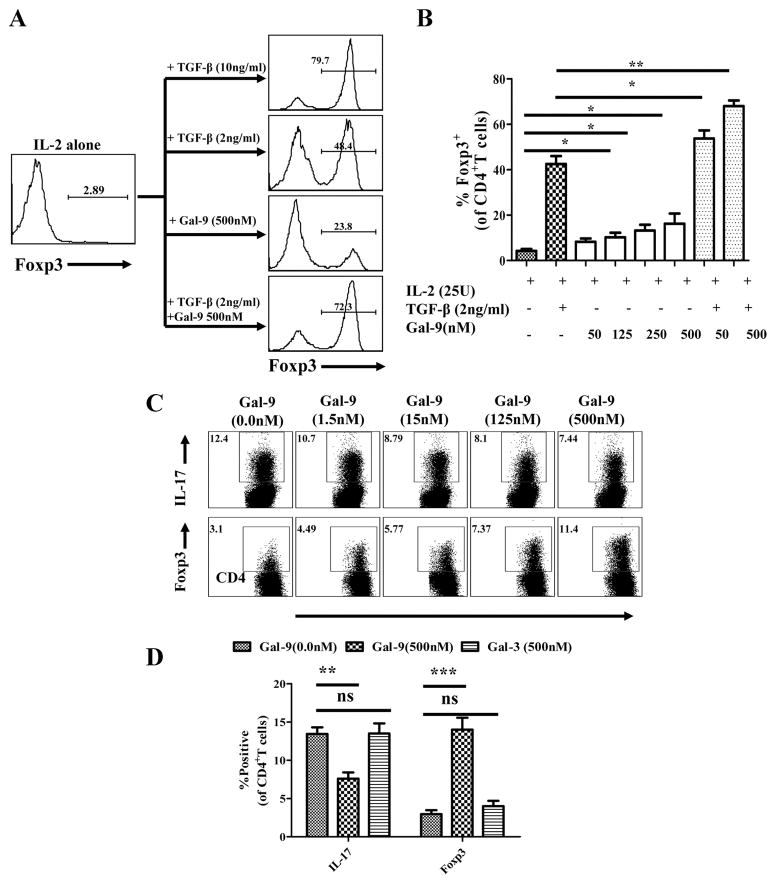
III. Galectin-9 may promote Foxp3+ Treg conversion and limit Th17 generation in vitro
To directly assess whether or not galectin-9 can cause some TCR stimulated conventional T cells to become Foxp3+, an in vitro culture system was used which in previous studies demonstrated Foxp3+ conversion (17). Briefly, splenocytes from DO11.10 RAG−/− animals (that lack Treg) were stimulated in vitro with plate bound anti-CD3 antibody, optimal amounts of IL-2 and various doses of galectin-9. TGF-β at optimal amounts was used instead of galectin-9 as a positive control system for Foxp3+ Treg induction. In such experiments galectin-9 did cause significant numbers of cells to convert to become Foxp3+ although the conversion was around four to five fold less effective than occurred with TGF-β (Fig.6A and B). When experiments were done using suboptimal amounts of TGF-β, the addition of galectin-9 increased the conversion beyond the various doses of galectin-9 in the absence of TGF-β. This observation may mean that galectin-9 might be inducing TGF-β in the cultures which in turn could enhance the conversion efficiency although this needs to be formally confirmed.
Fig 6. Effects of galectin-9 treatment on the generation of CD4+Foxp3+ regulatory T cells and Th17 cells in vitro.
Splenocytes isolated from DO11.10RAG2−/− animals were cultured with IL-2 alone, IL-2+TGF-β 2ng/ml, IL-2+Galectin-9, IL-2+TGF-β+galectin-9, IL-2+TGF-β +IL-6 with or without galectin-9. A. Representative FACS plots from more than 6 experiments showing the Foxp3 induction in TCR stimulated CD4+ T cells isolated from DO11.10RAG2−/− animals under indicated conditions. B. The bar diagram shows the percentage of CD4+ T cells expressing Foxp3 under indicated conditions. The levels of significance were calculated by ANOVA test C. Cells were cultured under Th17 differentiating conditions (IL-2 (50U/ml) + TGF-β (10ng/ml) + IL-6 (30ng/ml)) with or without galectin-9. The dot plots show the inhibitory effects on the generation of Th17 cells (upper panel) and the stimulatory effects on the generation of Foxp3+ T cells (lower panel) of various doses of galectin-9 in in vitro cultures. D. The bar diagram shows the percentages of Th17 or Foxp3+ Tregs induced under indicated conditions. The experiments were repeated at least four times and the level of significance was determined by Student ‘t’ test.
In another approach splenocytes from DO11.10 RAG−/− animals, were cultured in conditions that resulted in the induction of Th17 cells. Basically such cultures were OVA323–339 stimulated whole splenocytes in the presence of IL-2, TGF-β and IL-6. Curiously, the addition of galectin-9 in these cultures inhibited the induction of Th17 cells in a dose dependent manner (Fig 6C and D). It was of particular interest to note that the addition of galectin-9 in these cultures increased the production of Foxp3+ cells up to four fold. The results could mean, however, that the anti-inflammatory effects of galectin-9 will not be negated by the presence of proinflammatroy cytokines such as IL-6 in the inflamed tissue. We did not investigate the mechanism of Th17 suppression, but conceivably it could involve galectin-9 apoptosis signals delivered to TIM-3 expressed by developing Th17 cells. We are currently attempting to verify this.
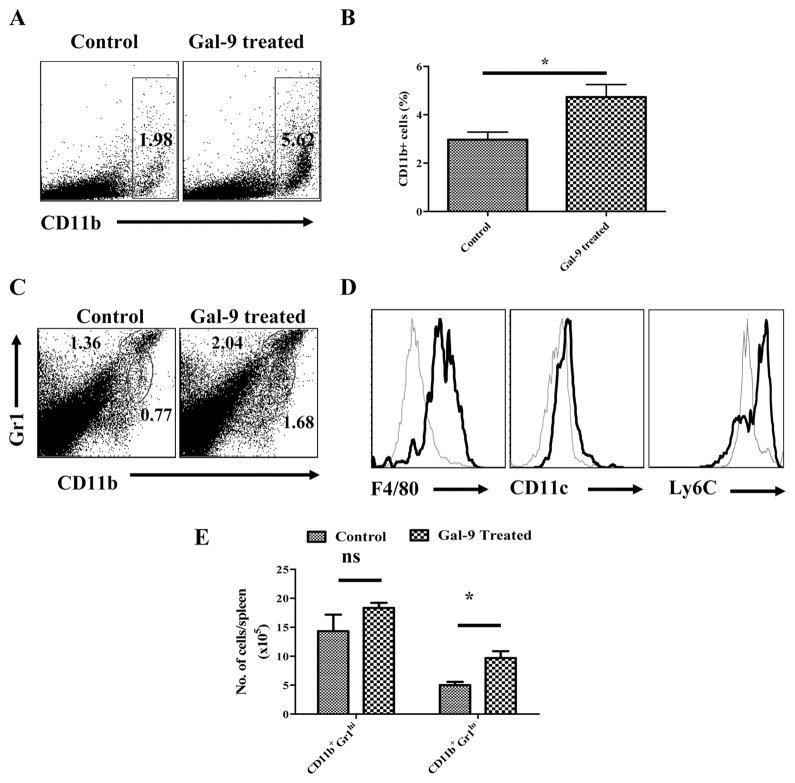
IV. Expansion of Myeloid suppressor cells
It was of interest to note that galectin-9 treated animals, as compared to control animals showed significantly expanded populations of CD11b+ cells especially in the spleens (Fig. 7A and B). As shown in the fig 4C–E, a significant proportion of these cells also expressed Gr1lo, F4/80+ and Ly6Chi a phenotype possessed by myeloid suppressor cells which have been shown to inhibit the function of conventional CD4+ and CD8+ T cells (20, 21). Interestingly, the frequencies of cells of this phenotype also increased in when galectin-9 treatment was performed locally in the corneas of infected animals (Fig. 4B and C). Future studies will attempt to measure if the myeloid suppressor cells play any regulatory effect in HSK.
Fig. 7. Effects of galectin-9 administration on CD11b+ Myeloid suppressor cells.
C57B/6 animals infected with 5×103 PFU of HSV were treated i.p. with 100μg of galectin-9 daily starting from day 4 until day 14 as described in Fig 3A and CD11b+ cells from control and galectin-9 treated animals were phenotypically characterized at day 15 post infection. A–B. FACS plots (A) and bar diagram (B) depicting the percentages of CD11b+ cells in the spleens of control and galectin-9 treated animals at day 14 are shown. C–D. Phenotypic characterization of CD11b+ cells from control and galectin-9 treated animals with respect to the expression of Gr1, F4/80, CD11c and Ly6C is shown. C. Bold lines in hitograms in D show the expression of the indicated marker on CD11b+Gr1lo gated population while thin lines show the expression of indicated surface molecules on CD11b+Gr1hi gated population from the spleens of galectin-9 treated animals. E. Total numbers of cells in gated CD11b+Gr1hi and CD11b+Gr1lo cells in the spleens of control and galectin-9 treated animals as shown in C. Student ‘t’ test was used to calculate significance level between groups.
Taken together our results may indicate that a consequence of galectin-9 therapy is that there is an overall increase in the frequency of regulatory cells that include both Foxp3+ Tregs and CD11b+Gr1loF4/80+Ly6Chi myeloid suppressor cells. Expanded population of Foxp3+ Tregs may represent conversion of conventional cells to become Treg and perhaps reflect a disappearance of CD4+ Th1 cells because many TIM-3+ cells are triggered to die by apoptosis.
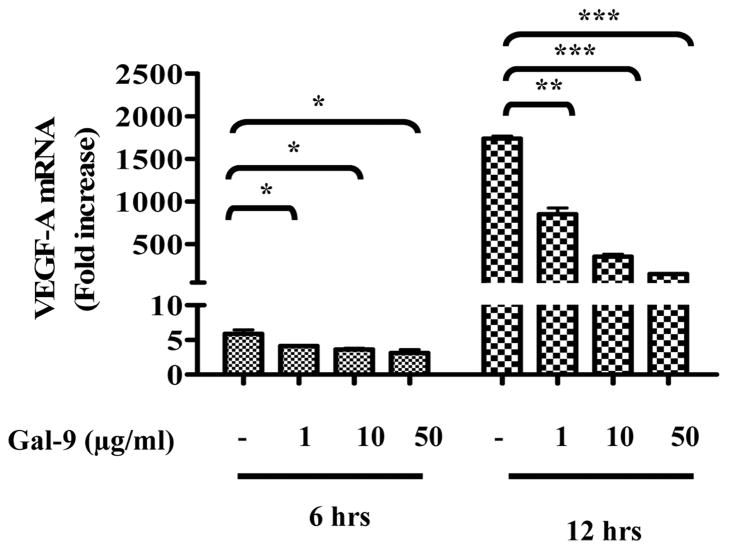
5. Galectin-9 may also inhibit the production of angiogenic factors
As indicated above, galectin-9 treatment of HSV infected mice also led to a reduction in the extent of ocular neovascularization. Indeed there was a trend for the effect on this necessary step in HSK pathogenesis to be greater than was observed on SK lesion severity (Fig 3D). This effect could be explained by the fact that some angiogenic factors derive from the inflammatory cells themselves and these responses were reduced in galectin-9 treated animals. Alternatively, galectin-9 might have some direct inhibitory effects on cells in the eye that produce angiogenesis factors in response to infection. To mimic the latter possibility, the effects of galectin-9 on the induced production of VEGF-A mRNA in stromal fibroblast cell line was measured. These cells can be induced to produce the angiogenic factor VEGF-A upon exposure to IL-6 or HSV infection (22). Experiments were performed in which MKT-1 cells were exposed to a high multiplicity of HSV-KOS (5MOI) which resulted in cells upregulating VEGF-A mRNA. This increase which reached 1800 fold in some instance was suppressed up to 100 fold by galectin-9. Similar doses of galectin-3 were almost without inhibitory effects. These results support the possibility that galectin-9 can function as an angiogenesis factor inhibitor although it must mediate its function by binding to a receptor distinct from TIM 3 since the latter molecule was not detectable on the MKT-1 cells (data not shown).
Discussion
Controlling chronic immunoinflammatory diseases represents a major therapeutic challenge. Such an example is lesions in the eye caused by HSV infection which commonly result in blindness. These lesions are strongly suspected to result from T cell mediated responses to the infection. Since the main T cell subset responsible for orchestrating lesions of stromal keratitis appear to be CD4+ T cells of Th1 type with perhaps some involvement by Th17CD4+ T cells, a logical approach to therapy would be to suppress or delete the function of activated CD4+ T cell subsets and increase the representation of cells that express regulatory function. We show in this report that this outcome can be achieved by therapy, systemic or local, with the lectin family member galectin-9. This molecule, which is natural product of cell types such as several cells of innate immune system, endothelial, epithelia cells etc., acts as a ligand to the inhibitory molecule TIM-3 that is expressed by activated but not naïve T cells. We show that 50% or more of T cells in ocular lesions caused by HSV in mice express TIM-3 and that blocking signals from its natural ligand with a monoclonal antibody results in more severe lesions. More importantly, however, the provision of additional galectin-9 either systemically or more effectively by local subconjuctival administration diminished SK lesion severity as well as the extent of corneal neovascularization. The mechanisms by which the galectin-9 therapy functioned were shown to be multiple. These involved apoptosis of the orchestrating effector T cells with consequent reduction of proinflammatory cytokines, an increase in the representation of two separate subtypes of regulatory cells as well as inhibitory effects on the production of molecules involved in ocular neovascularization, an essential component of HSK pathogenesis. Our results indicate that galectin-9 therapy may represent a useful approach to control HSV induced lesions, the commonest infectious cause of blindness in the Western World.
A number of previous reports have shown that activated T cells, both CD4+ and CD8+ T cells (8, 13), may up regulate TIM-3 and that the engagement of the receptor with its ligand galectin-9 causes cells to undergo apoptosis (23). Currently, TIM-3 was also shown to be one of many molecules up regulated on T cells that are chronically stimulated during HIV infection (24). Since galectin-9 is a product of several cell types with its production increased upon exposure to some cytokines released from activated T cells, the TIM-3/galectin-9 interaction may represent a physiological means by which effector T cell responses are terminated (8, 11). Thus the severity of some autoimmune inflammatory responses may be more severe in galectin-9 knock out animals (12). Also of therapeutic relevance, it has been observed that administration of galectin-9 may suppress the severity of some autoimmunities even when given quite late in the disease process (12). Our studies too, in which we investigate the relevance of TIM-3/galectin-9 in a chronic viral induced inflammatory disease, galectin-9 administration caused the suppression of HSK lesions even when the drug was given 8 days after virus infection. At this stage, virus is usually no longer present in the eye although without treatment lesions continue to advance in severity and usually do not resolve spontaneously (25). In some instances we observed that galectin-9 therapy did cause almost complete resolution.
We presume that the efficacy of galectin-9 therapy could have resulted from causing apoptosis of the CD4+ T cells responsible for orchestrating lesions. However, we failed to show any evidence of T cell apoptosis in vivo which may not be too surprising since apoptotic cells are rapidly phagocytosed in vivo (26, 27). We could readily show apoptosis of TIM-3+ cells in populations of lymphoid cells taken from HSV infected animals when exposed to galectin-9 in vitro. However in such experiments, many TIM-3 positive cells resisted apoptosis even at highest doses of galectin-9 investigated. Curiously, in our in vitro studies we observed that TIM-3+Foxp3+CD4+ T cells showed more resistance to galectin-9 induced apoptosis than did Foxp3−CD4+TIM-3+ T cells. The molecular explanation for this interesting observation is currently under further investigation. However, if it turns out that Foxp3+ regulatory T cells are more resistant to galectin-9 induced apoptosis in vivo, this might explain why the treatment resulted in suppressed SK lesions. Thus, as we have shown previously, the Treg response serves to modulate the severity of SK lesions (18). Differential susceptibility of T effectors to Treg would result in Treg enrichment. In line with this, we did observe that frequencies of Foxp3+ T cells in lymphoid tissues as well as ocular inflammatory populations were increased in galectin-9 treated infected animals. That galectin-9 administration may result in the apparent induction of Foxp3+ regulatory T cells was also reported recently by Seki et al although the mechanisms responsible for the effect were not investigated (12).
In our studies, we observed that galectin-9 could affect the Treg responses by mediating at least two effects in addition to the differential susceptibility to apoptosis. Accordingly, we could show that the proliferative capacity in vitro of TCR stimulated Foxp3 cells was greater in populations taken from galectin-9 treated compared to control animals. However, perhaps of more importance we could also demonstrate that galectin-9 was able to induce conventional naïve TCR stimulated CD4+ T cells to convert to become Foxp3+. This was shown using TCR transgenic T cells TCR stimulated in vitro in the presence of galectin-9. Significant levels of conversion were observed, although this was less than that could be achieved with optimal doses of TGF-β. In fact our results could mean that the galectin-9 conversion effect involved the production of TGF-β, since when cells were stimulated in the presence of suboptimal levels of TGF-β, galectin-9 addition caused higher levels of conversion than could be achieved with galectin-9 or TGF-β alone. However, at present other possibilities can not be ruled out.
Whether or not galectin-9 administration succeeds in converting conventional cells to become Foxp3+ and their expansion in vivo is currently under investigation. If it does occur, it might help explain why some parasitic infections appear to be potent inducers of Treg responses, which in turn play an important role during pathogenesis (28, 29). Thus, many parasites express high levels of lectins that include galectin-9 in their surface components (29). Furthermore, galectin-9 is expressed abundantly in the gut mucosa (30) which is a site where the peripheral generation of regulatory T cells readily occurs (31–33). The presence of galectin-9 may help counteract an inflammatory environment that favors the induction of Th17 cells as we were able to demonstrate in our in vitro studies.
Whereas previous reports have noted that the TIM-3/galectin-9 interaction can have a notable effect on inflammatory cell function, any effect on angiogenesis was not noted. In our study, we observed that galectin-9 administration had an even greater effect on angiogenesis than it did on the severity of the SK lesions. The reasons for this may be that galectin-9 may be inhibiting the production of angiogenesis factors such as VEGF responsible for causing the new blood vessel development that plays an essential part of SK pathogenesis. Exactly how galectin-9 causes its inhibitory effects on angiogenesis factor induction still require further investigation. Of particular interest in our model systems, we demonstrated an inhibitory effect of galectin-9 on the induced production of VEGF from cells that lacked demonstrable TIM-3.
Taken together our results are consistent with the observation that the TIM-3/galectin-9 interaction plays a critical role at influencing the expression of HSV induced ocular lesions. It seems likely that the interaction affects lesion severity under normal circumstances since lesions become more severe if signals from endogenous galectin-9 are blocked with anti-TIM-3 mAb. Moreover, the interaction can be exploited for therapeutic purposes since treatment with galectin-9 can diminish lesions and can even result in their resolution. The mechanisms by which therapy succeeds are multiple and involve a change in balance between proinflammatory effector cells and regulators as well as effects on the production of angiogenesis factors responsible for causing neovascularization of the eye. It remains a challenge to sort out the relative importance in vivo that the several beneficial effects that galectin-9 mediates against viral immunoinflammatory lesions.
Fig 8. Effects of galectin-9 on VEGF-A production.
MKT (stromal fibroblast cell line) cells were used to observe the effect of galecetin-9 on VEGF production. The cells were infected with 5MOI of HSV KOS and were then treated with various doses of galectin-9. A VEGF-A mRNA expression was measured at 6 hours and 12 hours from control and galectin-9 (1μg, 10μg and 50μg/ml) treated cells as normalized with HPRT expression is shown. One was ANOVA was used to calculate the level of significance.
Acknowledgments
We thank Nancy Neilsen for excellent technical support with flow cytometry. We also thank Naveen Rajasagi, and Stacy Grimshaw for invaluable assistance in many ways.
Footnotes
Work supported by AI 063365 and EY 05093
References
- 1.Kim B, Kaistha SD, Rouse BT. Viruses and autoimmunity. Autoimmunity. 2006;39:71–77. doi: 10.1080/08916930500484708. [DOI] [PubMed] [Google Scholar]
- 2.Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein BI, Schwartz J, Gordon GG, Dominguez MO, Varma S, Dunn MW, Southren AL. Characterization of a glucocorticoid receptor and the direct effect of dexamethasone on herpes simplex virus infection of rabbit corneal cells in culture. Invest Ophthalmol Vis Sci. 1982;23:651–659. [PubMed] [Google Scholar]
- 4.Deshpande SP, Zheng M, Lee S, Rouse BT. Mechanisms of pathogenesis in herpetic immunoinflammatory ocular lesions. Vet Microbiol. 2002;86:17–26. doi: 10.1016/s0378-1135(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 5.Niemialtowski MG, Rouse BT. Phenotypic and functional studies on ocular T cells during herpetic infections of the eye. J Immunol. 1992;148:1864–1870. [PubMed] [Google Scholar]
- 6.Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- 7.Kuchroo VK, Meyers JH, Umetsu DT, DeKruyff RH. TIM family of genes in immunity and tolerance. Adv Immunol. 2006;91:227–249. doi: 10.1016/S0065-2776(06)91006-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 9.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8:577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DE. TIM-3 as a therapeutic target in human inflammatory diseases. Expert Opin Ther Targets. 2007;11:1005–1009. doi: 10.1517/14728222.11.8.1005. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, Gutierrez-Ramos JC, Coyle AJ, Strom TB. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 12.Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, Ito K, Takeshita K, Niki T, Saita N, Nishi N, Yamauchi A, Katoh S, Matsukawa A, Kuchroo V, Hirashima M. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang F, He W, Zhou H, Yuan J, Wu K, Xu L, Chen ZK. The Tim-3 ligand galectin-9 negatively regulates CD8+ alloreactive T cell and prolongs survival of skin graft. Cell Immunol. 2007;250:68–74. doi: 10.1016/j.cellimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, He W, Yuan J, Wu K, Zhou H, Zhang W, Chen ZK. Activation of Tim-3-Galectin-9 pathway improves survival of fully allogeneic skin grafts. Transpl Immunol. 2008;19:12–19. doi: 10.1016/j.trim.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Sehrawat S, Rouse BT. Anti-inflammatory effects of FTY720 against viral-induced immunopathology: role of drug-induced conversion of T cells to become Foxp3+ regulators. J Immunol. 2008;180:7636–7647. doi: 10.4049/jimmunol.180.11.7636. [DOI] [PubMed] [Google Scholar]
- 16.Kim B, Tang Q, Biswas PS, Xu J, Schiffelers RM, Xie FY, Ansari AM, Scaria PV, Woodle MC, Lu P, Rouse BT. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–2185. doi: 10.1016/S0002-9440(10)63267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J Virol. 2008;82:6838–6851. doi: 10.1128/JVI.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 19.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, Freeman GJ, Kuchroo VK. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 20.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 21.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng M, Klinman DM, Gierynska M, Rouse BT. DNA containing CpG motifs induces angiogenesis. Proc Natl Acad Sci U S A. 2002;99:8944–8949. doi: 10.1073/pnas.132605599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, Nakamura T, Hirashima M. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol. 2003;170:3631–3636. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 24.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouse BT, Sarangi PS. Herpetic Keratitis. Elsevier Limited; London, England: In press. [Google Scholar]
- 26.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 27.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 28.Pelletier I, Hashidate T, Urashima T, Nishi N, Nakamura T, Futai M, Arata Y, Kasai K, Hirashima M, Hirabayashi J, Sato S. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9: possible implication of galectin-9 in interaction between L. major and host cells. J Biol Chem. 2003;278:22223–22230. doi: 10.1074/jbc.M302693200. [DOI] [PubMed] [Google Scholar]
- 29.Turco SJ, Descoteaux A. The lipophosphoglycan of Leishmania parasites. Annu Rev Microbiol. 1992;46:65–94. doi: 10.1146/annurev.mi.46.100192.000433. [DOI] [PubMed] [Google Scholar]
- 30.Wada J, Kanwar YS. Identification and characterization of galectin-9, a novel beta-galactoside-binding mammalian lectin. J Biol Chem. 1997;272:6078–6086. doi: 10.1074/jbc.272.9.6078. [DOI] [PubMed] [Google Scholar]
- 31.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204:1737–1739. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]