Abstract
Previous studies have identified differences in the expression of proteins that regulate myosin light chain phosphorylation and contraction in tonic and phasic smooth muscle. cGMP plays a critical role in smooth muscle relaxation and is important for optimal function of phasic and tonic smooth muscle. The intracellular cGMP levels are regulated by its hydrolysis via phosphodiesterase 5 (PDE5) and efflux via novel multidrug resistance protein 5 (MRP5). In the present study we tested the hypothesis that the differences in the phasic and tonic behavior of smooth muscles may be related to differences in mechanisms that terminate cGMP signaling. Expression of PDE5 and MRP5 was significantly (more than 2-fold) higher in fundus compared with antrum. The NO donor S-nitrosoglutathione (GSNO) caused an increase in PDE5 activity and intra- and extracellular cGMP levels in both fundus and antrum. Stimulation of PDE5 activity and increase in extracellular cGMP were significantly higher in fundus, whereas increase in intracellular cGMP was significantly higher in antrum. GSNO-induced increase in extracellular cGMP was blocked in dispersed cells by the cyclic nucleotide export blocker probenecid and in cultured muscle cells by depletion of ATP or suppression of MRP5 by siRNA, providing evidence that cGMP efflux was mediated by ATP-dependent export via MRP5. Consistent with the higher expression and activity levels of PDE5 and MRP5, GSNO-induced PKG activity and muscle relaxation were significantly lower in muscle cells from fundus compared with antrum. Thus higher expression of PDE5 and MRP5 in muscle cells from fundus correlates with tonic phenotype of muscle.
Keywords: cyclic nucleotides, MRP5, phasic muscle, tonic muscle
gastrointestinal tract smooth muscle possesses distinct regional and functional properties that distinguish it from other types of visceral and vascular smooth muscle. An essential step in smooth muscle contraction is phosphorylation of the 20-kDa regulatory myosin light chain (MLC20) by a Ca2+/calmodulin-dependent or -independent myosin light chain (MLC) kinase (MLCK) that transfers the phosphate group from ATP to Ser19 (16, 20, 30, 43, 45, 46). Smooth muscle relaxation is initiated by targeting MLC20 dephosphorylation (43). Most agents cause relaxation by stimulating the production of cAMP (e.g., vasoactive intestinal peptide and its homologue pituitary adenylate cyclase-activating peptide) or cGMP [e.g., nitric oxide (NO)] leading to activation of PKA, PKG, or both (25, 26, 30, 43). cAMP-activated PKA and cGMP-activated PKG are the main enzymes that induce relaxation in smooth muscle. They target different components of the contractile signaling pathways that attenuate MLCK activity and/or augment MLC phosphatase (MLCP) activity, which eventually induce dephosphorylation of MLC20 and thus smooth muscle relaxation (7, 30, 32, 43). Although generation of both nucleotides and activation of both kinases are the physiological norm, dysfunctions of gastrointestinal motility in vivo and nitrergic relaxation in vitro in mice lacking neuronal NO synthase (nNOS), soluble guanylyl cyclase (sGC), or PKG-I suggest an important role for cGMP signaling in smooth muscle relaxation (5, 11, 27, 35).
The levels of cAMP and cGMP in gastrointestinal smooth muscle depend on the rates of their synthesis by cyclases and degradation by phosphodiesterases (PDEs) (3, 8, 10, 29, 40). cAMP is rapidly degraded by the cAMP-preferring PDE3 and cAMP-specific PDE4 (10, 33). On the other hand, cGMP is rapidly degraded by cGMP-specific PDE5. In addition to degradation by phosphodiesterases, cyclic nucleotide elimination pathways comprise active export into the extracellular space via members of the multidrug resistance protein (MRP) family (also known as ATP-binding cassette transporter family) (41, 44). MRPs are localized in the plasma membrane, bind and hydrolyze ATP, and provide the energy to transport their substrates across membrane barriers. Among MRP family members, MRP4 and MRP5 have been shown to be competent in the transport of cAMP and cGMP, respectively (48). MRP5 has higher affinity for cGMP (Km = 2 μM) than cAMP (Km = 400 μM) (18). Expression of transcripts for MRP5 and cellular cGMP export via MRP5 have been demonstrated (28, 34, 39). Nevertheless, to date, the role of MRP5 in the regulation of gastrointestinal smooth muscle tone has not been demonstrated.
On the basis of membrane properties, activation speed, and contractile behavior, smooth muscles have been classified as either phasic or tonic. The phasic and tonic behavior of smooth muscles may also be related to differences in content and isoform composition of contractile proteins and intracellular signaling molecules that regulate the activities of MLCK and MLCP (4, 6, 7, 13, 15, 17, 21–23, 36). However, differences in the mechanisms involved in the termination of cGMP signaling in phasic and tonic muscle is not known. The strength and duration of cGMP signaling is regulated by its degradation into inactive 5′-GMP via cGMP-specific PDE5, and efflux via ATP-dependent transporter, MRP5. In the present study, we postulated that the termination of cGMP effect by both PDE5 and MRP5 in tonic muscle could contribute to rapid return to tonic contractile phenotype following a relaxation. Insights into the mechanism of relaxation in tonic smooth muscle are important to understand the underlying mechanism involved in altered muscle contractions related to dysfunctions of sphincters such as achalasia. Our studies demonstrated higher expression of PDE5 and MRP5 in fundus compared with antrum. As a result, attenuation of intracellular cGMP levels in response to NO donors was greater in muscle cells from fundus compared with antrum.
MATERIALS AND METHODS
Materials.
[125I]cGMP and [γ-32P]ATP were obtained from PerkinElmer Life Sciences, Boston, MA; collagenase CLS type II and soybean trypsin inhibitor were obtained from Worthington, Freehold, NJ; Western blotting, Dowex AG 1-X8 resin (100–200 mesh in formate form), chromatography material, protein assay kit, and Tris·HCl Ready Gels were obtained from Bio-Rad Laboratories, Hercules, CA; antibodies to PDE5 and MRP5 were obtained from Santa Cruz Biotechnology, Santa Cruz, CA; cGMP and PKI(6–22) amide were obtained from Calbiochem, La Jolla, CA; RKRSRAE (Arg-Lys-Arg-Ser-Arg-Ala-Glu) was obtained from Peninsula Laboratories, Belmont, CA; RNAqueous kit was obtained from Ambion, Austin, TX; Lipofectamine 2000 Transfection Reagent was from Invitrogen, Grand Island, NY; QIAEXII Gel extraction kit and QIAprepSpin Miniprep kit were obtained from Qiagen Sciences, Germantown, MD; PCR reagents were obtained from Applied Biosystems, Foster City, CA; SuperScript II Reverse Transcriptase and TOPO TA Cloning Kit Dual Promoter were obtained from Invitrogen, CA; Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Fisher Scientific, Pittsburgh, PA. All other chemicals were obtained from Sigma, St. Louis, MO.
New Zealand white rabbits (weight: 4–5 lbs) were purchased from RSI Biotechnology, Clemmons, NC and euthanized by injection of Euthasol (100 mg/kg), as approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University. The animals were housed in the animal facility administered by the Division of Animal Resources, Virginia Commonwealth University. All procedures were conducted in accordance with the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
The present study characterized the termination mechanisms of cGMP signaling in smooth muscle cells of rabbit proximal stomach since the yield of dispersed muscle cells (30–40 × 106 cells/stomach) required for biochemical, molecular, and functional analysis is optimal and data are available for comparison from previous studies using rabbit. Studies were performed either in dispersed muscle cells or cells in culture to avoid confounding effect of other cell types such as enteric neurons and interstitial cells of Cajal that are present in the intact tissue. All experiments were done on cells in the first passage because repeated passages alter the expression of signaling molecules involved in contractile phenotype (26). Previous studies using freshly dispersed muscle cells and muscle cells in culture have characterized the signaling mechanism activated by excitatory (e.g., acetylcholine) or inhibitory (e.g., NO or vasoactive intestinal peptide) neurotransmitters to mediate muscle contraction and relaxation, respectively (29–32).
Preparation of dispersed gastric smooth muscle cells.
Smooth muscle cells from the circular muscle layer of the antrum and fundus were isolated by sequential enzymatic digestion of muscle strips, filtration, and centrifugation as described previously (29, 32). The tissue was cut into thin slices by use of a Stadie-Riggs tissue slicer and then the slices were incubated for 30 min in a smooth muscle buffer at 31°C containing 0.1% collagenase (300 U/ml) and 0.01% soybean trypsin inhibitor (wt/vol). The tissue was continuously gassed with 100% oxygen during the entire isolation procedure. The partly digested tissues were washed twice with 50 ml of collagenase-free smooth muscle buffer and the muscle cells were allowed to disperse spontaneously for 30 min in collagenase-free medium. Cells were harvested by filtration through 500 μm Nitex and centrifuged twice at 350 g for 10 min to eliminate broken cells and organelles. The cells were counted in a hemocytometer, and it is estimated that 95% of the cells excluded Trypan blue. The experiments were done within 2–3 h of cell dispersion.
Dispersed muscle cells isolated from the antrum and fundus were resuspended in DMEM containing penicillin (200 U/ml), streptomycin (200 μg/ml), gentamycin (100 μg/ml), amphotericin B (2.5 μg/ml), and 10% fetal bovine serum (DMEM-10). The muscle cells were plated at a concentration of 5 × 105 cells/ml and incubated at 37°C in a CO2 incubator. DMEM-10 medium was replaced every 3 days for 2–3 wk until confluence was attained. The muscle cells in confluent primary cultures were trypsinized (0.5 mg trypsin/ml), replated at a concentration of 2.5 × 105 cells/ml, and cultured under the same conditions. All experiments were done on cells in the first passage. Previous studies have determined the purity of cultured muscle cells with smooth muscle-specific γ-actin (47). Cultured muscle cells were starved in serum-free medium for 24 h before each use.
Expression of MRP5 by RT-PCR.
Total RNA was isolated from freshly dispersed smooth muscle cells with TRIzol reagent (Invitrogen) and cultured gastric muscle cells from both antrum and fundus regions of the stomach by use of ULTRASPECT reagent and then treated with TURBO DNase (Ambion). RNA from each preparation was reversely transcribed by using the SuperScript II system containing 50 mM Tris·HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 0.5 mM deoxynucleoside triphosphates (dNTP), 2.5 μM random hexamers, and 200 units of reverse transcriptase in a 20-μl reaction volume. The reactions were carried out at room temperature for 10 min and at 42°C for 50 min, and terminated by heating at 70°C for 15 min. Three microliters of the reversely transcribed cDNA were amplified in a final volume of 50 μl by PCR in standard conditions (2 mM MgCl2, 200 μM dNTP, 2.5 units Taq polymerase) with specific primers designed based on conserved sequences in human, rat, and mouse cDNAs (F′: 5′ GCAAGAGCCCTGCTGCGTCA 3′, MRP5 R′: 5′ CTGTGTGCAGGCGATGGGCA 3′). PCR was performed for 30 cycles. For each experiment, a parallel control without reverse transcriptase was processed. The amplified PCR products were analyzed on 1.5% agarose gel containing 0.1 μg/ml ethidium bromide (32, 33, 47).
Expression of PDE5 by real-time PCR.
Real-time PCR was performed on cDNA samples synthesized from total RNA isolated from antrum and fundus cultured muscle cells with the StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA) and the intercalating dye, SYBRgreen. PCR conditions were optimized on the gradient thermal cycler on the StepOne Real-Time PCR System. For each cDNA sample, real-time PCR was conducted in a 20-μl reaction volume containing Quantitect SYBRgreen PCR Mastermix (Qiagen, Mississauga, ON, Canada). The following time and temperature profile was used for the real-time PCR reactions: 95°C for 5 min; 50 cycles of a series consisting of 15 s at 94°C, 30 s at 52°C, 30 s at 72°C; and a final extension of 5 min at 72°C. The optimal annealing temperatures were determined empirically for each primer set. The sequences of specific primers for PDE5 are F′: 5′ CTATTCCCTGTTCCTTGTCTGTGA 3′, R′: 5′ CAAAGAGGCGGCTGATAAGAA 3′. Real-time PCR reactions were performed in triplicate. Each primer set generated only one PCR product, and the identity and integrity of these products were confirmed by electrophoresis on 1.5% agarose gel containing 0.1 μg/ml ethidium bromide and sequencing of the individual bands. The fluorescent threshold value was calculated by use of the StepOne Real-Time PCR System software. The absence of peaks in water controls suggested a lack of primer-dimer formation. GAPDH was selected as reference gene. Cycle threshold (Ct) values were obtained and the relative fold change in gene expression was calculated as 2−ΔΔCt.
Western blot analysis.
Muscle cells were solubilized in Triton X-100-based lysis buffer plus protease and phosphatase inhibitors (100 μg/ml PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 30 mM sodium fluoride, and 3 mM sodium vanadate). After centrifugation of the lysates at 20,000 g for 10 min at 4°C, the protein concentrations of the supernatant were determined with a DC protein assay kit from Bio-Rad. Equal amount of proteins were fractionated by SDS/PAGE and transferred to nitrocellulose membrane. Blots were blocked in 5% (wt/vol) nonfat dried milk/TBS-T [Tris-buffered saline (pH 7.6) plus 0.1% Tween-20] for 1 h and then incubated overnight at 4°C with various primary antibodies in TBS-T plus 1% (wt/vol) nonfat dried milk. After incubation for 1 h with horseradish peroxidase-conjugated corresponding secondary antibody (1:2,000; 10 μg/ml, Pierce) in TBS-T plus 1% (wt/vol) nonfat dried milk, immunoreactive proteins were visualized by use of a SuperSignal Femto maximum sensitivity substrate kit (Pierce). All washing steps were performed with TBS-T. The protein bands were identified by enhanced chemiluminescence reagent. Quantification of protein bands obtained on Western blot was done by densitometric analysis. The average intensity obtained for each band was normalized to that of β-actin for the same lane. The band intensity of each treatment was then calculated as a percent value of normalized value of the control lane. The percent value of the control was used for statistical analysis (29, 31).
Transfection of cultured smooth muscle cells with MRP5 siRNA.
Confluent smooth muscle cells in the first passage on six-well plates were transiently transfected with the control siRNA or MRP5 siRNA by using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). Briefly, 100 pmol of the siRNA in 125 μl Opti-MEM medium were mixed with 5 μl Lipofectamine 2000 in 125 μl Opti-MEM. The mixture was incubated at room temperature for 20 min and added to wells containing 1.5 ml DMEM with 10% FBS for 1 day. The medium was then replaced with DMEM with 10% FBS plus antibiotics for 2 days. Cells were maintained for a final 24 h in DMEM without FBS before experiments were started (32).
[3H]cGMP transport in plasma membrane vesicle.
Plasma membrane vesicle were prepared from muscle cells from fundus and antrum as described previously (9). Cells were homogenized in 10 mM Tris·HCl medium (pH 7.4) containing 250 mM sucrose and protease inhibitors and were centrifuged at 9,000 g for 15 min. The supernatant was centrifuged at 100,000 g for 30 min, and the resultant pellet was suspended in Tris·HCl medium with a Dounce B homogenizer. The suspension was layered on 38% sucrose in 5 mM HEPES medium (pH 7.4) and centrifuged at 290,000 g, and the turbid layer at the interface was collected to pass through the 27-gauge needle nearly 20 times to prepare the membrane vesicle.
Plasma membrane vesicles (100 μg) were incubated in a 10 mM Tris·HCl (pH 7.4) medium containing 4 mM ATP (or 4 mM 5′AMP), 10 mM MgCl2, 10 mM creatine phosphate, 100 μg/ml creatine kinase, 4 μM of [3H]cGMP, and 250 mM sucrose. At different times 15 μl of aliquots were diluted in 1 ml of ice-cold medium and rapidly filtered through nitrocellulose filters (0.2 μm) and the radioactivity on the filters was measured. ATP-dependent transport was calculated by subtracting the counts obtained in the absence of ATP and in the presence of 5′-AMP; results are expressed as pmol/mg protein.
Radioimmunoassay for cGMP.
cGMP levels were measured by radioimmunoassay, as previously described (29). To study efflux and intracellular accumulation of cyclic nucleotides, cells were washed twice and incubated with NO donor in the absence or presence of 100 μM IBMX, a PDE inhibitor. At the end of the incubation period, medium was collected, cyclic nucleotide concentrations were determined in both supernatants and pellets by radioimmunoassay, and the results are expressed as picomoles per milligram of protein.
Assay for PDE5 activity.
PDE5 activity was measured in immunoprecipitates of PDE5 by the method of Wyatt et al. (29, 49). PDE5 was immunoprecipitated from lysates of dispersed gastric smooth-muscle cells (3×106 cells/ml) by using an anti-PDE5 antibody, and the immunoprecipitates were washed in a solution of 50 mM Tris·HCl (pH 7.5), 200 mM NaCl, and 5 mM EDTA. The immunoprecipitates were then incubated for 15 min at 30°C in a reaction mixture containing 100 mM Mes (pH 7.5), 10 mM EDTA, 0.1 M magnesium acetate, 0.9 mg/ml BSA, 20 μM cGMP, and [3H]cGMP. The samples were boiled for 3 min, chilled for 3 min, and then incubated at 30°C for 10 min in 20 mM Tris·HCl (pH 7.5) containing 10 μl of Crotalus atrox snake venom (10 μg/μl). The samples were added to DEAE-Sephacel A-25 columns and the radioactivity in the effluent was counted. The results were expressed as cpm/mg of protein.
cGMP-dependent PKG assay.
PKG activity was measured by the method of Jiang et al. (19, 31). One milliliter of cell suspension was incubated in HEPES medium with 8-Br-cGMP for 90 s at 31°C. The reaction was stopped by rapid centrifugation, and the pellet was rinsed with a medium containing 50 mM Tris·HCl (pH 7.4), 10 mM EDTA, 0.5 mM IBMX, 10 mM β-mercaptoethanol, and 100 mM NaCl and homogenized in 0.5 ml ice-cold medium. The mixture was centrifuged at 48,000 g for 15 min and the supernatant was used as a source of protein kinase. PKG activity was measured in a volume of 60 μl containing 50 mM Tris, 10 mM MgCl2, 100 μM [32P]ATP, 50 μM synthetic heptapeptide histone H2B (RKRSRAE), and 0.25 mg/ml of bovine serum albumin. The assay was done in the presence or absence of 10 μM of cGMP and 1 μM PKI (6–22 amide) and was initiated by the addition of 20 μl of cell supernatants (50 μg protein) to the reaction mixture. PKG activity was calculated as picomoles per milligram protein and expressed as the ratio of activity in the presence or absence of 10 μM cGMP (−cGMP/+cGMP).
Measurement of contraction and relaxation in dispersed smooth muscle cells.
Contraction in freshly dispersed gastric circular smooth muscle cells was determined by scanning micrometry (29, 32). An aliquot (0.4 ml) of cells containing ∼104 cells/ml was treated with 100 μl of medium containing acetylcholine (ACh, 0.1 μM) for 30 s and the reaction was terminated with 1% acrolein at a final concentration of 0.1%. The resting cell length was determined in control experiments in which muscle cells were incubated with 100 μl of 0.1% bovine serum albumin without the ACh. The mean lengths of 50 muscle cells treated with various agonists was measured by scanning micrometry and compared with the mean lengths of untreated cells. The contractile response to ACh was expressed as the percent decrease in mean cell length from control cell length. Relaxation was measured as decrease in response to ACh in the presence of S-nitrosoglutathione (GSNO). Relaxation was expressed as percent decrease in contractile response to ACh.
Statistical analysis.
The results were expressed as means ± SE of n experiments and analyzed for statistical significance by Student’s t-test for paired and unpaired values. Each experiment was performed on cells obtained from different animals. Differences among multiple groups were tested by ANOVA and checked for significance by Fisher′s protected least significant difference test. A statistical software program was used (GraphPad Software, San Diego, CA). A probability of P < 0.05 was considered significant.
RESULTS
The strength and duration of cGMP signaling is regulated by its degradation into inactive 5′-GMP via cGMP-specific PDE5 and efflux via ATP-dependent transporter MRP5. We postulated that the termination of the cGMP effect by both PDE5 and MRP5 in tonic muscle could contribute to rapid return to a tonic contractile phenotype following a relaxation.
PDE5 expression and activity.
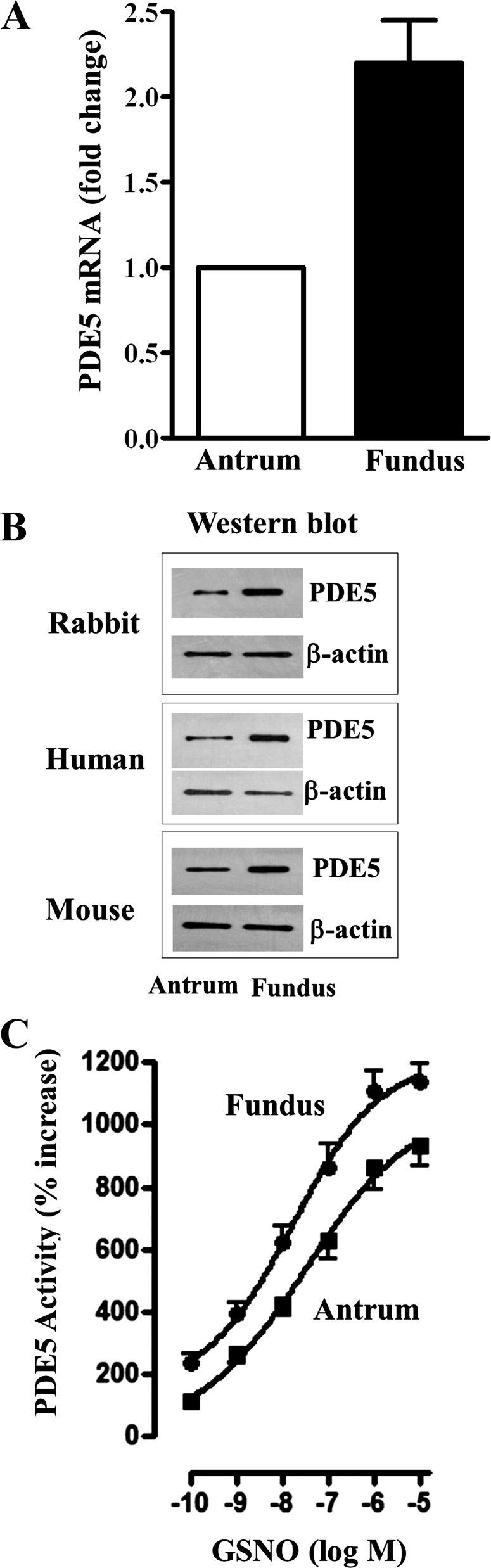
Expression of PDE5, by both quantitative RT-PCR and Western blot, and stimulation of PDE5 activity in response to the NO donor GSNO, were determined in muscle cells from antrum and fundus. PDE5 mRNA was expressed in both antrum and fundus and the expression was twofold higher in fundus compared with antrum (Fig. 1A). PDE5 protein expression was examined in the two regions of the stomach by Western blot using selective antibody to PDE5A, the smooth muscle predominant isoform. Results confirmed the expression of PDE5 of expected size (100 kDa) in the homogenates of smooth muscle cells from both antrum and fundus (Fig. 1B). Comparing the densities of protein bands in the two regions revealed a 3.2 ± 0.6-fold higher expression of PDE5 in fundus compared with antrum and this is consistent with the higher expression of PDE5 mRNA in fundus compared with antrum. Similar higher expression of PDE5 in muscle cells from fundus compared with antrum was obtained in the stomach of human (4.1 ± 0.6-fold higher) and mouse (2.8 ± 0.6-fold higher) (Fig. 1B).
Fig. 1.
Differential expression of phosphodiesterase 5 (PDE5) and S-nitrosoglutathione (GSNO)-stimulated PDE5 activity in fundus and antrum. A: total RNA isolated from cultured (first passage) muscle cells from antrum and fundus was reverse transcribed using 2 μg of total RNA. The cDNA was amplified with specific primers for PDE5A by real-time RT-PCR. Relative quantification of a target gene in relation to reference gene was calculated on the basis of ΔΔCt values. Results demonstrated that mRNA levels of PDE5A are significantly higher in fundus compared with antrum. Values represent means ± SE of 3 separate experiments. B: representative Western blot results of PDE5A expression. Cell lysates derived from dispersed muscle cells of antrum and fundus of rabbit, human, and mouse stomach containing equal amounts of total proteins were separated with SDS-PAGE and expression of PDE5A was analyzed by using selective antibody for PDE5A. Membranes were reblotted to measure β-actin. Protein bands were visualized with enhanced chemiluminescence, images were quantified, and densitometric values were calculated after normalization to β-actin density. Results are expressed as fold increase over the expression of PDE5 in antrum. C: 1 ml of cell suspension (2 × 106 cells/ml) of freshly dispersed muscle cells from antrum and fundus was treated with different concentrations of GSNO, an NO donor, for 60 s. The cells were homogenized in the lysis buffer and the protein content in the supernatants was measured. PDE5 was immunoprecipitated from lysates containing equal amount of protein, and the activity was measured in immunoprecipitates by liquid chromatography using [3H]cGMP as substrate. The amount of radioactivity in the elutes was measured by liquid scintillation and the results are expressed as percent increase above basal values (antrum: 238 ± 56 cpm/mg protein; fundus: 285 ± 45 cpm/mg protein). Values represent means ± SE of 5–6 separate experiments.
Basal and GSNO-stimulated PDE5 activity was measured by ion-exchange chromatography using [3H]cGMP as substrate. Although the expression levels are different, basal PDE5 activity was not significantly different in antrum (238 ± 56 cpm/mg protein) and fundus (285 ± 45 cpm/mg protein). Treatment of dispersed muscle cells with GSNO increased PDE5 activity in a concentration-dependent fashion in antrum and fundus. Stimulation of PDE5 activity was significantly higher at concentrations above 10 nM of GSNO in fundus compared with antrum (Fig. 1C). The maximal stimulation was also significantly higher (P < 0.05, n = 6) in fundus compared with antrum (Fig. 1C).
MRP5 expression.
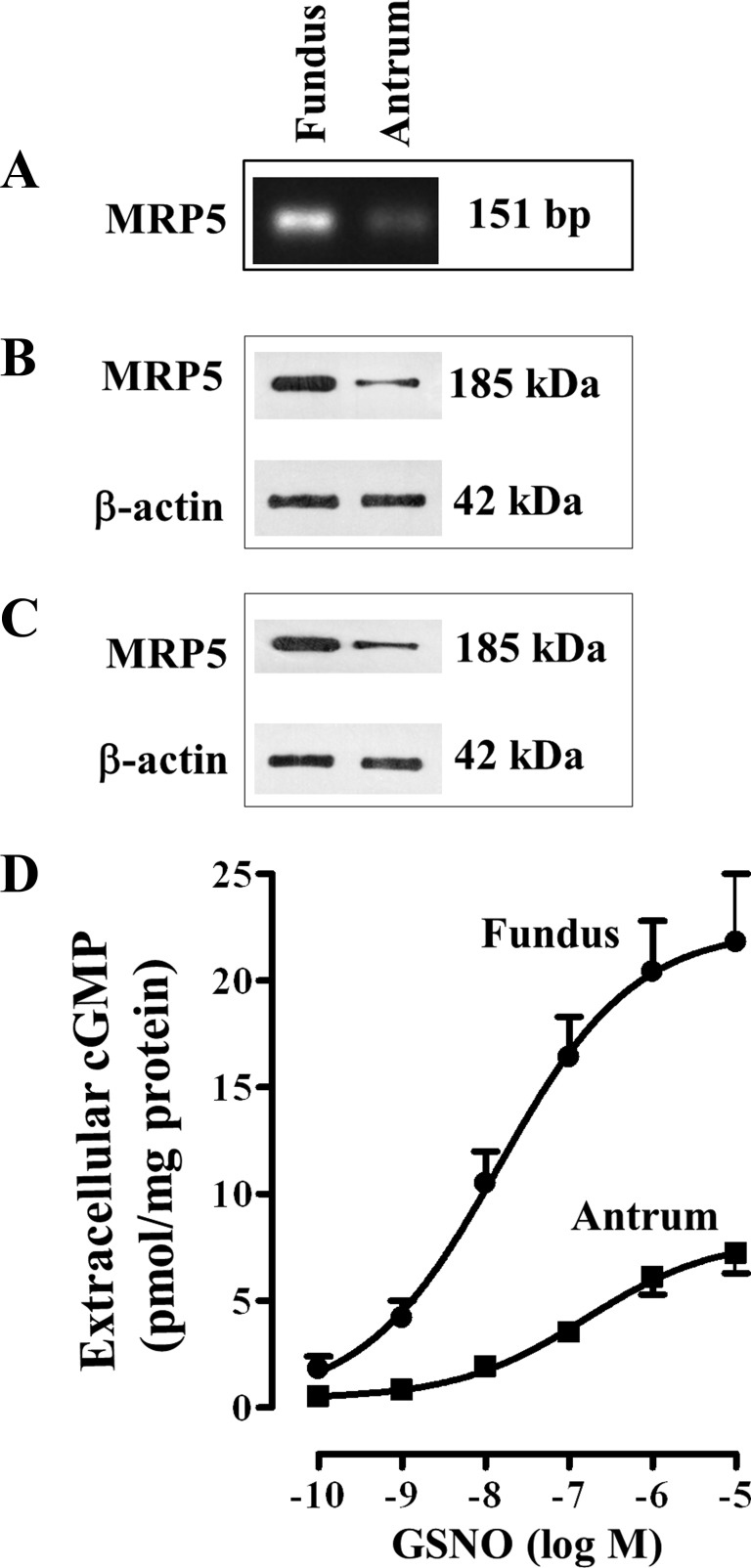
MRP5 mRNA levels were assessed by RT-PCR in RNA isolated from cultured muscle cells from fundus and antrum. MRP5 mRNA was detectable in both fundus and antrum. RT-PCR detected the expected 151-bp MRP5 mRNA amplification product (Fig. 2A). Cloning and sequence analysis of the RT-PCR product of MRP5 in the stomach was 98% similar to the corresponding amino acid sequences of human. Relative expression of MRP5 mRNA appears to be higher in fundus compared with antrum (Fig. 2A). This was corroborated by immunoblot analysis using MRP5-specific antibody. The 185-kDa MRP5 protein was detected in homogenates from both antrum and fundus (Fig. 2B). The blots were also probed with a monoclonal antibody against β-actin as a marker protein, which was used for normalization of the densitometric quantification. Expression of MRP5 proteins in muscle cells from fundus was significantly higher (3.8 ± 0.4-fold higher) compared with muscle cells from antrum of rabbit (Fig. 2B). Similar higher expression of MRP5 was obtained in muscle cells of fundus (4.2 ± 0.6-fold higher) compared with antrum of human stomach (Fig. 2C).
Fig. 2.
Differential expression of multidrug resistance protein 5 (MRP5) and GSNO-stimulated efflux of cGMP in fundus and antrum. A: total RNA isolated from cultured (first passage) muscle cells from antrum and fundus was reverse transcribed using 2 μg of total RNA. The cDNA was amplified with specific primers for MRP5 by RT-PCR. The primer set generated only one PCR product (151 bp), and the identity and integrity of these products were confirmed by electrophoresis in agarose gel in the presence of ethidium bromide and sequencing of the individual band. B and C: representative Western blot results of MRP5 expression. Cell lysates derived from dispersed muscle cells of antrum and fundus of rabbit (B) and human (C) stomach containing equal amounts of total proteins were separated with SDS-PAGE, and expression of MRP5 was analyzed with selective antibody for MRP5. Membranes were reblotted to measure β-actin. Protein bands were visualized with enhanced chemiluminescence, images were quantified, and densitometric values were calculated after normalization to β-actin density. D: 1 ml of cell suspension (2 × 106 cells/ml) of freshly dispersed muscle cells from antrum and fundus was treated with different concentrations of GSNO for 20 min in the presence of nonspecific PDE inhibitor, 100 μM IBMX. cGMP was measured by radioimmunoassay using [125I]cGMP in the supernatant to reflect and extracellular cGMP levels. The results are expressed as pmol/mg protein. Values represent means ± SE of 5 separate experiments.
cGMP efflux.
The function of MRP5 was analyzed by measuring intra- and extracellular cGMP levels in response to GSNO. To exclude the involvement of PDE5 and examine the singular contribution of MRP5 in the regulation of intracellular cGMP levels, experiments were done in the presence of a nonselective PDE inhibitor, IBMX (100 μM). Under these experimental conditions, changes in intracellular cGMP reflect efflux of cGMP and suggest potential contribution of MRP5 in the regulation of intracellular cGMP. Cells in cultures were washed twice and incubated for variable times with GSNO, and at the end of the incubation period medium and cells were collected separately and cyclic nucleotide concentrations were determined in both compartments by radioimmunoassay.
GSNO-induced increase in extracellular cGMP at 20 min was concentration dependent in both fundus and antrum, and the maximum increase was significantly higher in fundus compared with the maximum increase in antrum (Fig. 2D).
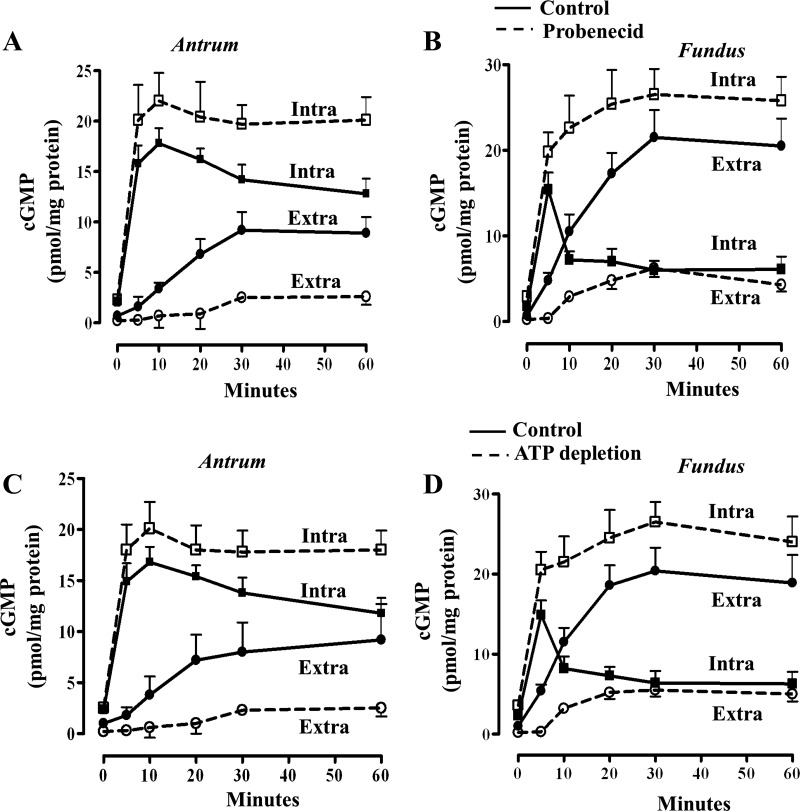
GSNO also caused an increase in intra- and extracellular cGMP levels in fundus and antrum. The increase in extracellular cGMP was greater in fundus, whereas the increase in intracellular cGMP was greater in antrum (Fig. 3, A–D). Incubation of cells with GSNO in the presence of probenecid, a common inhibitor of cyclic nucleotide efflux (1, 42), inhibited efflux of cGMP in both fundus and antrum, and the inhibition in cGMP efflux was accompanied by a proportional increase in intracellular levels of cGMP. The decrease in extracellular cGMP and the increase in intracellular cGMP in the presence of probenecid were significantly higher in fundus compared with antrum (Fig. 3, A and B).
Fig. 3.
ATP-dependent cGMP efflux. A and B: 1 ml of cell suspension (2 × 106 cells/ml) of freshly dispersed muscle cells from antrum and fundus was treated with GSNO for different times in the presence of nonspecific PDE inhibitor, 100 μM IBMX (solid line). Under these experimental conditions, the effect of PDE5 on cGMP degradation was precluded. In some experiments cells were incubated with probenecid (200 μM), a common cyclic nucleotide inhibitor (dashed line). C and D: 1 ml of cell suspension (2 × 106 cells/ml) of freshly dispersed muscle cells from antrum and fundus was treated with GSNO for different times in the presence of nonspecific PDE inhibitor, 100 μM IBMX under normal (solid lines) and under ATP-depleting conditions (dashed lines). cGMP was measured by radioimmunoassay using [125I]cGMP in the cell pellet and supernatant to reflect intracellular (Intra; squares) and extracellular (Extra; circles) cGMP levels, respectively. The results are expressed as pmol/mg protein. Values represent means ± SE of 5 separate experiments.
To determine whether cGMP was ATP dependent, we measured GSNO-stimulated cGMP efflux under normal and under ATP-depleting conditions (48). Time-dependent increase in extracellular cGMP in the presence of GSNO was significantly inhibited under ATP-depleting conditions, suggesting active transport of cGMP. The decrease in extracellular cGMP and the increase in intracellular cGMP under ATP-depleting conditions were significantly higher in fundus compared with antrum (Fig. 3, C and D).
cGMP added extracellularly to the cells in culture medium was stable for at least 3 h (less than 2% decrease), suggesting that the increased extracellular levels in the presence of GSNO reflect the actual amount effluxed from the cells.
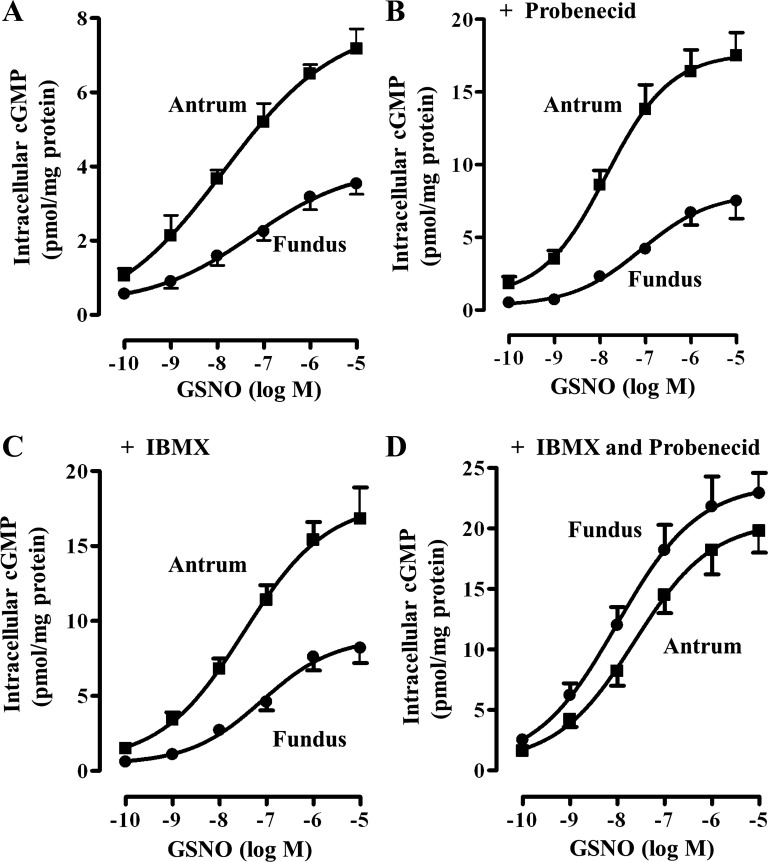
GSNO-induced increase in intracellular cGMP at 20 min was concentration dependent in both fundus and antrum and the maximum increase was significantly higher in antrum compared with fundus (Fig. 4). In the absence of IBMX and probenecid, GSNO caused an increase in intracellular cGMP in a concentration-dependent manner in muscle from fundus and antrum, and the maximal increase was significantly higher in antrum compared with the fundus, suggesting that lower levels of cGMP in fundus are due to higher PDE5 and MRP5 activity (Fig. 4A). Evidence for higher PDE5 activity in fundus was obtained by measurements of intracellular cGMP levels in response to different concentrations of GSNO in the absence of IBMX but in the presence of cGMP efflux blocker probenecid. Under these conditions, GSNO caused an increase in cGMP levels in fundus and antrum in a concentration-dependent fashion, and the maximal increase was significantly higher in antrum compared with the maximal increase in fundus (Fig. 4B). The results are consistent with the higher PDE5 expression and activity in fundus compared with antrum. Evidence for higher MRP5 activity in fundus was obtained by measurements of cGMP levels in response to different concentrations of GSNO in the absence of probenecid, but in the presence of IBMX. Under these conditions, GSNO caused an increase in cGMP levels in fundus and antrum in a concentration-dependent fashion, and the maximal increase was significantly higher in antrum compared with the maximal increase in fundus (Fig. 4C). The results are consistent with the higher MRP5 expression and activity in fundus compared with antrum. When cGMP levels are measured in the presence of both IBMX and probenecid, GSNO caused an increase in cGMP levels in a concentration-dependent manner, and the maximal increase was not significantly different in fundus compared with antrum (Fig. 4D).
Fig. 4.
GSNO-induced increase in intracellular levels of cGMP. One milliliter of cell suspension (2 × 106 cells/ml) of freshly dispersed muscle cells from antrum and fundus was treated with different concentrations of GSNO for 20 min and the reaction was terminated with 10% trichloroacetic acid. Intracellular cGMP levels in response to GSNO were measured in the absence of IBMX or probenecid (A) and in the presence of probenecid (200 μM) alone (B), IBMX (100 μM) alone (C), or both probenecid and IBMX (D). cGMP was measured by radioimmunoassay using [125I]cGMP and the results were expressed as pmol/mg protein. Values represent means ± SE of 5 separate experiments.
MRP5-dependent cGMP efflux.
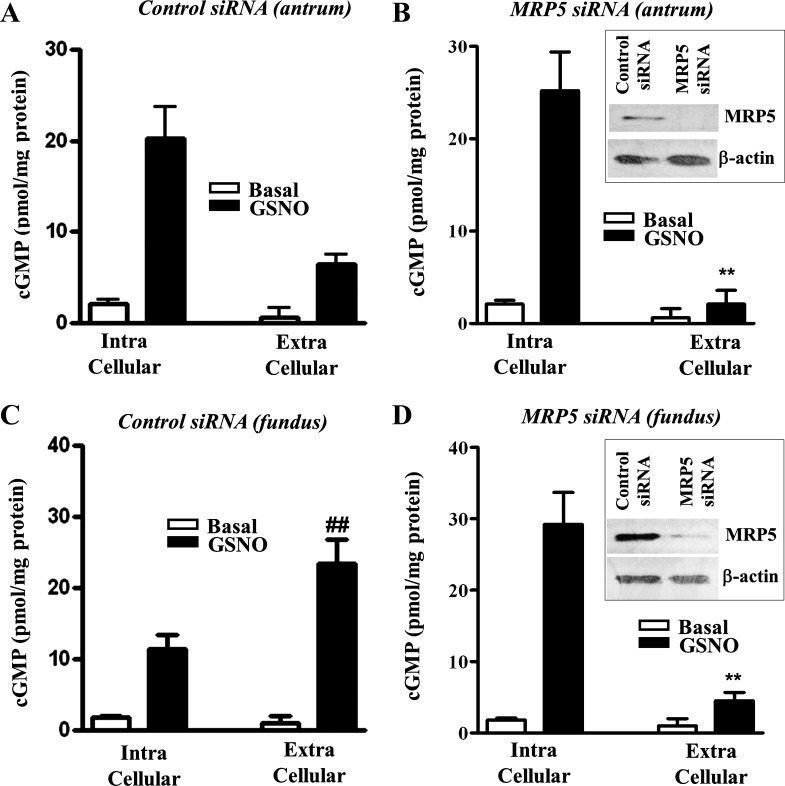
The specific involvement of MRP5 in cGMP export was analyzed by transfection of cells with MRP5 siRNA. Western blot analysis revealed downregulation of MRP5 expression in cells transfected with MRP5 siRNA.
GSNO induced an increase in intracellular cGMP levels in muscle cells from antrum and fundus (Fig. 5). Transfection of MRP5 siRNA had no effect on basal intracellular levels of cGMP. GSNO-induced increase in intracellular cGMP, however, was significantly augmented by MRP5 siRNA in muscle cells from fundus (15.8 ± 1.9-fold increase vs. 6.3 ± 0.7-fold increase in control cells) (Fig. 5, C and D) compared with muscle cells from antrum (11.8 ± 1.6-fold increase vs. 9.6 ± 0.8-fold increase in control cells) (Fig. 5, A and B). GSNO also induced an increase in extracellular cGMP levels in muscle cells from antrum and fundus (Fig. 5). Transfection of MRP5 siRNA had no effect on basal extracellular cGMP levels. GSNO-induced increase in extracellular cGMP, however, was significantly attenuated by MRP5 siRNA in muscle cells from fundus (4.2 ± 0.8-fold increase vs. 22.8 ± 3.2-fold in control cells) (Fig. 5, C and D) and antrum (3.8 ± 0.6-fold vs. 10.2 ± 1.2-fold increase in control cells) (Fig. 5, A and B). Thus, in muscle cells from fundus, augmentation (151 ± 6% increase) of intracellular cGMP by MRP5 siRNA is accompanied by significant attenuation (82 ± 7% decrease) of extracellular cGMP levels. Similarly, in muscle cells from antrum, augmentation (20 ± 6% increase) of intracellular cGMP by MRP5, siRNA was accompanied by significant attenuation (61 ± 7% decrease) of extracellular cGMP levels. The effect of MRP5 siRNA on changes in intracellular and extracellular cGMP levels in response to GSNO was greater in muscle cells from fundus compared with antrum. The results are consistent with the higher expression of cGMP-specific MRP5 in fundus compared with antrum.
Fig. 5.
MRP5-mediated cGMP efflux. Cultured muscle cells from antrum (A and B) or fundus (C and D) were transfected with control siRNA (A and C) or MRP5 siRNA (B and D) for 48 h. Cells were treated with GSNO for 20 min in the presence of nonspecific PDE inhibitor, 100 μM IBMX. cGMP was measured by radioimmunoassay using [125I]cGMP in the cell pellet and supernatant to reflect intracellular and extracellular cGMP levels, respectively. The results are expressed as pmol/mg protein. Values represent means ± SE of 5 separate experiments. **P < 0.001 significant decrease extracellular cGMP levels in MRP5 siRNA cells compared with control cells. ##P < 0.01 significant increase in extracellular cGMP in fundus compared with antrum. Inset: Western blot analysis of MRP5 expression in cells transfected with control siRNA or MRP5 siRNA.
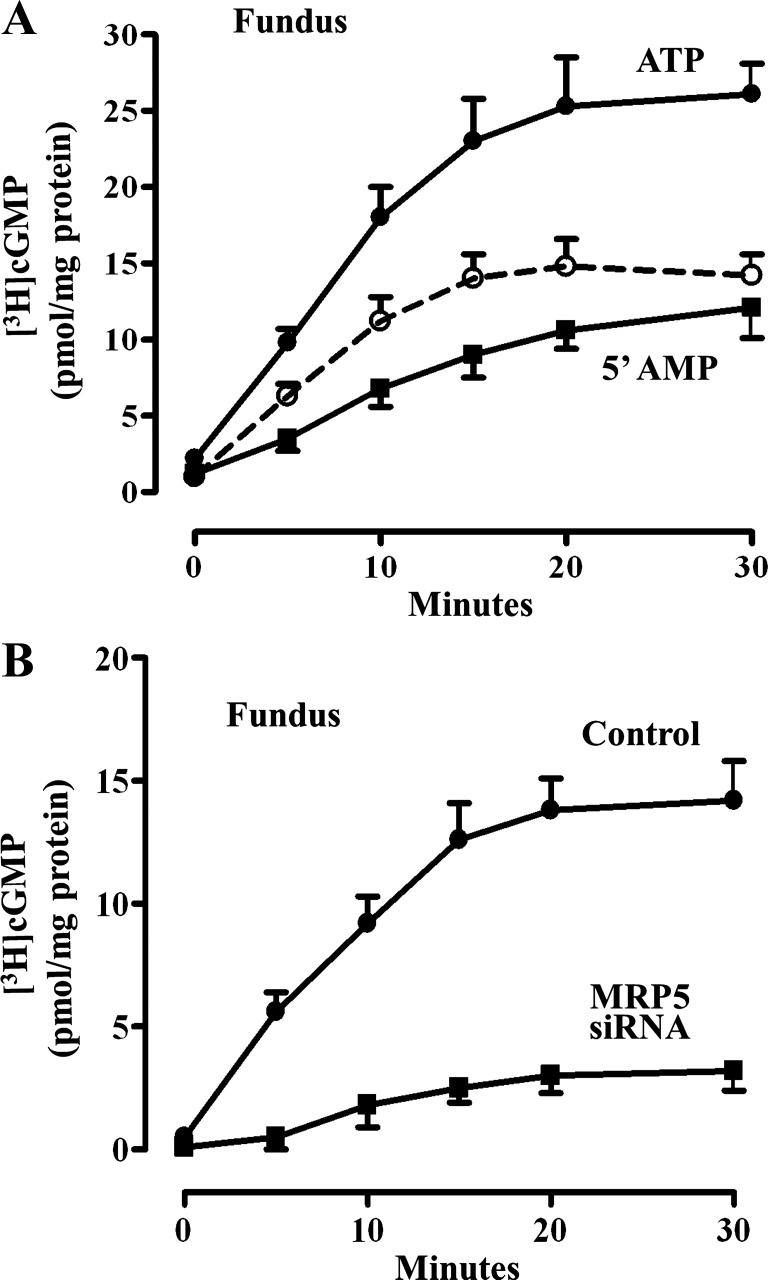
Further evidence for ATP-dependent cGMP was obtained in plasma membrane vesicles isolated from muscle cells of fundus with [3H]cGMP for 30 min and the increase in the association of [3H]cGMP with the membrane in the presence of ATP reflected active transport of cGMP. There was time-dependent increase in the association of [3H]cGMP with the vesicles in the presence of ATP or 5′-cGMP; however, the increase in association was significantly higher in the presence of ATP (Fig. 6A). The vesicle-associated radioactivity in the presence of ATP was subtracted from the nonspecific binding of [3H]cGMP in the presence of 5′-cGMP to obtain ATP-dependent transport. ATP-dependent cGMP transport was significantly reduced in plasma membrane vesicles isolated from muscle cells transfected with MRP5 siRNA (Fig. 6B).
Fig. 6.
cGMP transport in plasma membrane vesicle. A: membrane vesicle (100 μg protein) prepared from muscle cells isolated from fundus were incubated with [3H]cGMP (5 μM) in the presence of ATP (5 mM) or 5′-AMP (5 mM) for different times. Vesicle-associated [3H]cGMP was measured by rapid filtration through nucleopore filters as described in methods. ATP-dependent transport (dashed line) was calculated as the difference between transport in the presence of ATP and in the presence of 5′-AMP. The results are expressed as pmol/mg protein. Values represent means ± SE of 5 separate experiments. B: cultured muscle cells from fundus were transfected with control vector or MRP5 siRNA for 48 h. Membrane vesicles (100 μg protein) were incubated with [3H]cGMP (5 μM) in the presence of ATP (5 mM) or 5′-AMP (5 mM) for different times. Vesicle-associated [3H]cGMP was measured by rapid filtration through nucleopore filters as described in methods. ATP-dependent transport expressed as pmol/mg protein is shown in the figure. Values represent means ± SE of 5 separate experiments.
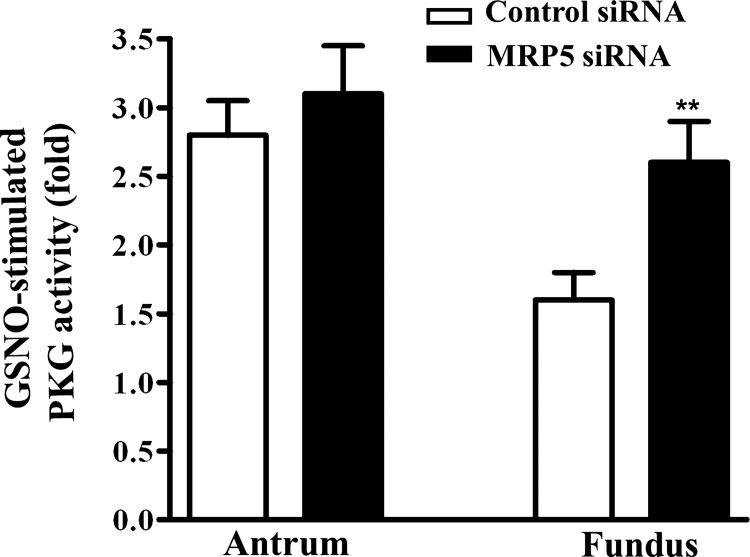
GSNO-induced PKG activity.
To examine the role of MRP5 in the regulation of cGMP signaling pathways, GSNO-induced PKG activity was measured in muscle cells from antrum and fundus. Stimulation of PKG activity in response to GSNO was significantly lower in fundus compared with antrum, consistent with the lower cGMP levels in fundus compared with antrum (Fig. 7). Transfection of MRP5 siRNA significantly augmented PKG activity in fundus compared with cells transfected with control siRNA (Fig. 7). These results suggest that higher expression of MRP5 in fundus plays an important role in regulating cGMP levels and PKG activity.
Fig. 7.
GSNO-induced PKG activity. Cultured muscle cells from fundus and antrum were transfected with control siRNA or MRP5 siRNA for 48 h. Cells were treated with GSNO (10 μM) for 10 min and then homogenized in the lysis buffer and the protein content in the supernatants was measured. PKG activity was measured in the presence or absence of cGMP by using a specific substrate [Arg-Lys-Arg-Ser-Arg-Ala-Glu (RKRSRAE)] and [32P]ATP, and the results are expressed as the ratio of activity in the absence or presence of cGMP. Values represent means ± SE of 4 separate experiments. **P < 0.01 significant increase in PKG activity in MRP5 siRNA-transfected cells compared with control cells.
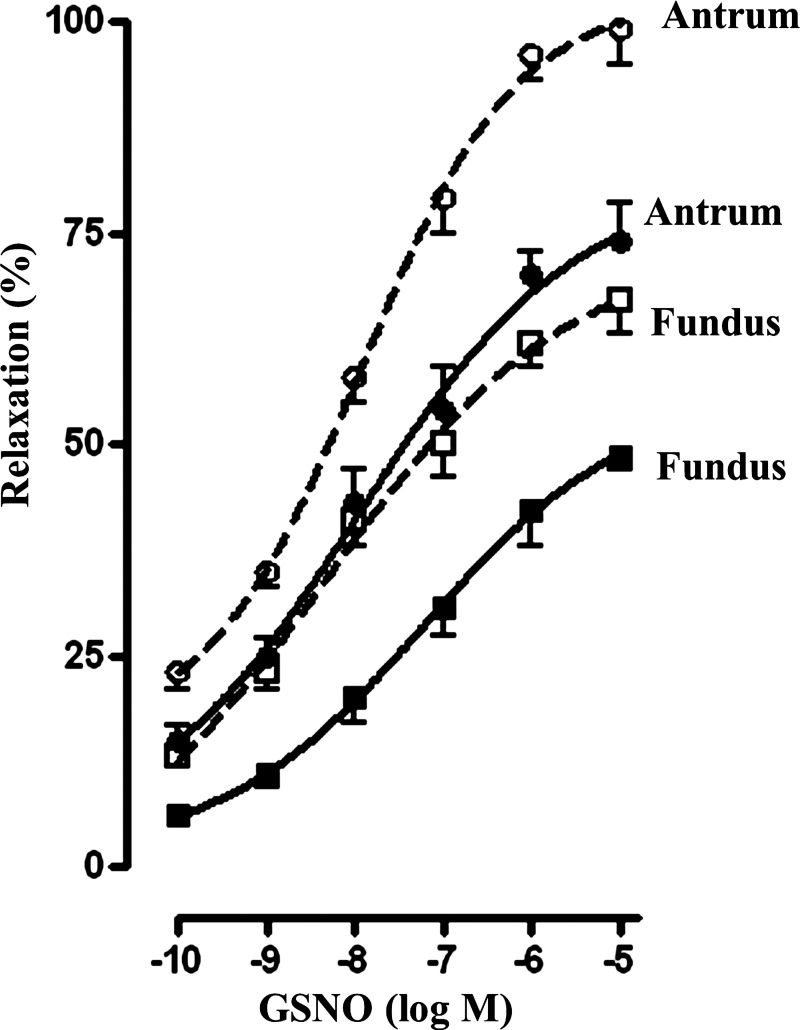
NO-induced smooth muscle relaxation.
To examine the role of PDE5 and MRP5 pathways in the regulation of muscle function, relaxation of muscle cells from antrum and fundus was measured by scanning micrometry. Muscle relaxation was measured as inhibition of ACh-induced contraction in response to GSNO as described previously (29). GSNO-induced muscle relaxation was concentration dependent in both antrum and fundus (Fig. 8). Relaxation was significantly lower in fundus compared with antrum, and the maximal response was 73 ± 3% in antrum and 48 ± 4% in fundus (Fig. 8). The results are consistent with the higher PDE5 and MRP5 functions resulting in lower intracellular cGMP levels in muscle cells from fundus compared with cells from antrum. GSNO-induced relaxation was significantly augmented in the presence of probenecid (200 μM) and IBMX (100 μM) (Fig. 8). Although GSNO-induced cGMP levels in the presence of probenecid and IBMX are higher in fundus compared with antrum, relaxation, in contrast, was significantly higher in antrum compared with fundus. This increase in relaxation in antrum reflects higher expression of telokin and lower expression of CPI-17, which activates and inhibits, respectively, MLCP activity in phasic muscle compared with tonic muscle (4, 22).
Fig. 8.
GSNO-induced muscle relaxation. Relaxation of dispersed muscle cells from antrum and fundus in response to different concentrations of GSNO was measured by scanning micrometry as decrease in ACh-induced contraction. In some experiments effect of GSNO was examined in the presence of probenecid (200 μM) and IBMX (100 μM) (dashed lines). Contraction was measured in response to maximal concentrations of ACh (0.1 μm) at 30 s as decrease in control cell length (antrum: control cell length 98 ± 3 μm; fundus: control cell length 91 ± 4 μm). Contraction was similar in muscle cells from antrum (28 ± 3% decrease) and fundus (29 ± 2% decrease). GSNO-caused relaxation was concentration dependent in both antrum and fundus and the relaxation was greater in antrum compared with fundus. Values represent means ± SE of 4 separate experiments.
DISCUSSION
Smooth muscle cells are the fundamental structural and functional units of the gastrointestinal system and exhibit distinct contractile phenotypes. The underlying features of phasic muscles (e.g., distal stomach and intestine) are the ability to generate rhythmic contractions and relaxations, whereas tonic muscles (e.g., sphincters and proximal stomach) have sustained tone and relax transiently in response to relaxant transmitters (4, 23, 30, 38). In both phasic and tonic muscle, phosphorylation of MLC20 is a prerequisite for contraction; the levels of MLC20 phosphorylation are determined by the opposing activities of MLCK and MLC phosphatase, and both of these enzymes’ activities are well regulated in smooth muscle. Although MLCK-mediated contraction is strictly dependent on intracellular Ca2+ concentration ([Ca2+]i), agonist-induced MLC20 phosphorylation and contraction can be maintained even after [Ca2+]i returns to basal levels via two ways: MLC20 phosphorylation by Ca2+-independent MLCKs and regulated inhibition of MLC phosphatase (12, 30, 38, 43).
The phasic and tonic behavior of smooth muscles may be related to differences in content and isoform composition of contractile proteins and intracellular signaling molecules that regulate the activities of MLCK and MLC phosphatase (4, 6, 7, 13, 15, 17, 21–24, 36). For example, previous studies have shown that phasic muscle differs from the tonic muscle in the relative abundance of the ratio of acidic to basic isoforms of the 17-kDa essential light chain, in the ratio of seven-amino acid inserted to noninserted myosin heavy chain isoforms (SMA/SMB isoforms) (2, 17, 21), in thin-filament-associated proteins such as caldesmon and calponin, and in the signaling pathways that regulate MLC20 phosphorylation (45, 46). These differences in the expression of contractile proteins and biochemical pathways highly correlate with the functional phasic phenotype of antrum and tonic phenotype of fundus. cGMP/PKG pathway plays an important role in smooth muscle relaxation and gastrointestinal motility. Dysfunctions in muscle in diseases such as achalasia and gastroparesis could reflect changes in the cGMP levels. It is not known, however, whether the expression and regulation of the signaling molecules that regulate cGMP are different in phasic and tonic smooth muscle.
Although generation of both cAMP and cGMP and activation of PKA and PKG are the physiological norm, studies in transgenic mice lacking nNOS (27), sGC (11, 14), and PKG-Iα (35) suggest that cGMP/PKG plays a critical role in relaxation of smooth muscle. cGMP levels in gastrointestinal smooth muscle are well controlled by the balance between the synthetic activities of sGC, and the degradative activities of specific PDE5, the main cGMP-specific PDE in smooth muscle (10, 29). Besides regulation via synthesis and degradation, cGMP elimination via MRP5-mediated active ATP-dependent export into the extracellular space has been found to be an important pathway in returning cyclic nucleotide levels back to basal state (4). MRP5 has been identified as a cGMP export pump in the heart and vasculature (50), but its expression and function in gastrointestinal smooth muscle are not known.
In the present study, we analyzed MRP5 expression and function in isolated muscle cells from fundus and antrum. Our studies demonstrate the expression of MRP5 in gastric smooth muscle cells by means of real-time RT-PCR and immunoblotting. Higher expression of MRP5 proteins in muscle cells from fundus correlated with the mRNA levels determined by real-time PCR. Efflux of cGMP was significantly higher in fundus consistent with the higher expression of MRP5 in this tissue. The siRNA knockdown of MRP5 reduced basal extracellular cGMP and greatly inhibited GSNO-stimulated increases in extracellular cGMP in intact cells and cGMP transport in plasma membrane vesicles. In GSNO-treated cells, MRP5 siRNA decreased the ratio of extracellular cGMP to intracellular cGMP. The importance of the efflux system in regulating intracellular cGMP levels should be interpreted with caution when considering the time course of accumulation inside and outside cells is compared. This is because accumulation of extracellular cGMP is degradation independent, whereas most of the intracellular cGMP is rapidly hydrolyzed by PDEs. The singular contribution of MRP5 in the regulation of intracellular cGMP level was measured in the presence of IBMX, which inhibits PDEs but not MRP5, and the singular contribution of PDE5 in the regulation of intracellular cGMP levels was measured in the presence of the cyclic nucleotide export inhibitor, which inhibits MRP5 but not PDE5 (1, 42). The intracellular levels of cGMP under both conditions were significantly lower in fundus compared with antrum, consistent with higher expression of PDE5 and MRP5 in fundus compared with antrum.
One of our most important results is that MRP5 inhibition alone was sufficient to modulate intracellular cGMP levels and cGMP-dependent protein kinase activity. GSNO-induced PKG activity in cells from fundus was significantly lower in fundus compared with antrum, and PKG activity in fundus was augmented by MRP5 siRNA. These findings suggest that MRP5 is present in gastric smooth muscles, and thus that the MRP5-dependent efflux of cGMP might contribute to the regulation of intracellular cGMP levels and cGMP-dependent signaling pathways and muscle relaxation. Our results point to MRP5 being an alternative or complement to PDEs, ensuring intracellular cGMP homeostasis. Because cGMP plays an important role in regulating smooth muscle functions, and because MRPs are suggested to serve as overflow pumps under conditions in which cyclic nucleotide production is strongly facilitated and PDE activity is limited, this transport system may be a novel target for the prevention and/or treatment of motility disorders. It is demonstrated that MRP5 is also inhibited by PDE inhibitors that are structurally related to cGMP such as trequinsin and sildenafil (48). An increase in cGMP with these compounds could be due to inhibition of PDE5 and efflux of cGMP. MRP5 mRNA has been detected in other tonic smooth muscle tissues with high transcript levels and has been shown to be competent in the transport of cGMP. Studies using immunofluorescence microscopy have shown that MRP5 is coexpressed with PDE5 in smooth muscle cells, providing strong evidence that these two pathways might complement with each other in keeping cGMP levels within a low range (34, 50).
Degradation of cGMP by PDE5 plays an important role in rapid termination of relaxation in smooth muscle. This is a more rapid and efficient system to regulate intracellular cGMP levels (3, 8, 10). PDE5 expression is abundant in smooth muscle and its activity is regulated by increase in cGMP levels. PDE5 is a dimer containing two allosteric cGMP-binding sites in its regulatory NH2-terminal domain, and a specific cGMP-binding site in its catalytic COOH-terminal domain that hydrolyzes cGMP (3). An increase in cGMP levels not only stimulates PKG, but also augments PDE5 activity by allosteric activation via binding to its regulatory domain, and by PKG-mediated phosphorylation of PDE5 at a conserved serine residue in the NH2-terminal region. Increase in cAMP also regulates cGMP via PKA since, in the presence of cGMP, PDE5 can also be phosphorylated and activated by the PKA (10, 29, 49).
Our studies demonstrated higher expression of both PDE5 and MRP5 in fundus compared with antrum. As a result, intracellular cGMP levels and relaxation in response to NO donors were attenuated in fundus compared with antrum. Accommodation of food by fundus is carried out by transient relaxation followed by sustained tone, whereas phasic activity of antrum with contraction and relaxation cycles mixes and propels chyme into the duodenum. Increased degradation and efflux of cGMP might play an important role in rapid termination of relaxation in fundus. Relaxation of tonic smooth muscle in response to NO is transient and the muscle regains contraction to prevent continuous distending forces or reflux of materials. Efficient regulation of cGMP levels is not only important to regulate PKG activity, it is also important to regulate PKA activity since cGMP was shown to increase cAMP, via inhibition of cAMP hydrolyzing PDE3, and thus PKA activity in gastric smooth muscle cells (33). The differences in the cGMP termination may provide a basis for the functional differences in the fundus and antrum of the stomach and tonic and phasic phenotype in general. Significant increase in relaxation of antrum in the presence of inhibitors of PDE5 and MRP5 suggests that differences in cGMP signaling alone cannot fully explain the differences in relaxation. Additional differences in the expression of contractile proteins and regulation of MLCK and MLCP activities also contribute to the differences in the functional phenotypes of phasic and tonic smooth muscle. At the tissue level, nonmuscle elements add more complexity to the above-mentioned biochemical differences in cGMP handling between phasic and tonic muscles. For example, it was found that the density of endothelial NO synthase-positive neurons was scarce in the fundus part of the rat stomach whereas their density in distal part was intensive (37). Thus it is possible that, in addition to increase in the termination of cGMP signaling, the mechanisms responsible for generation of cGMP such as generation of NO and activation of sGC are also constrained in fundus to facilitate tone and optimal organ function.
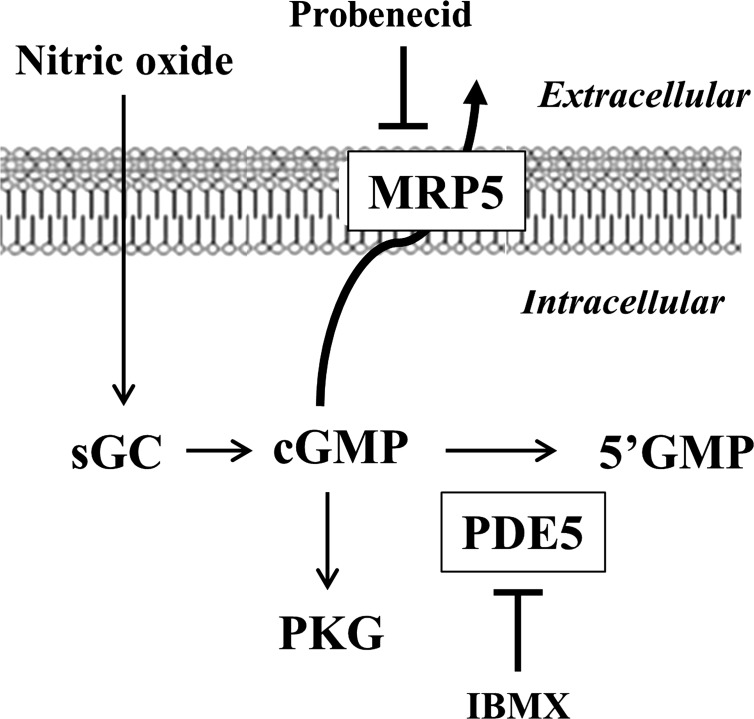
In summary, our studies demonstrate the importance of MRP5, together with PDE5, in the regulation of cGMP levels, cGMP-dependent signaling pathways, and smooth muscle tone (Fig. 9). Further studies are needed to assess the relative significance of the differences in cGMP termination in defining the differences in contractile phenotype of phasic and tonic smooth muscle.
Fig. 9.
Termination of cGMP signaling in smooth muscle. cGMP produced from activation of soluble guanylyl cyclase (sGC) is rapidly degraded via PDE5 and exported via MRP5. Expression of PDE5 and MRP5 is higher, and termination of cGMP signaling is greater in muscle cells from fundus compared with antrum.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant to K. S. Murthy (DK28300).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
O.A.-S., S.M., and W.S. performed experiments; O.A.-S., S.M., W.S., J.R.G., and K.S.M. analyzed data; O.A.-S. prepared figures; O.A.-S., S.M., and W.S. drafted manuscript; O.A.-S., S.M., W.S., J.R.G., and K.S.M. approved final version of manuscript; J.R.G. and K.S.M. conception and design of research; J.R.G. and K.S.M. interpreted results of experiments.
REFERENCES
- 1. Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology 147: 3435–3445, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Babu GJ, Loukianov E, Loukianova T, Pyne GJ, Huke S, Osol G, Low RB, Paul RJ, Periasamy M. Loss of SM-B myosin affects muscle shortening velocity and maximal force development. Nat Cell Biol 3: 1025–1029, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 75: 725–748, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Bhetwal BP, An CL, Fisher SA, Perrino BA. Regulation of basal LC20 phosphorylation by MYPT1 and CPI-17 in murine gastric antrum, gastric fundus, and proximal colon smooth muscles. Neurogastroenterol Motil 23: e425–e436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonnevier J, Fassler R, Somlyo AP, Somlyo AV, Arner A. Modulation of Ca2+ sensitivity by cyclic nucleotides in smooth muscle from protein kinase G-deficient mice. J Biol Chem 279: 5146–5151, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Chi M, Zhou Y, Vedamoorthyrao S, Babu GJ, Periasamy M. Ablation of smooth muscle myosin heavy chain SM2 increases smooth muscle contraction and results in postnatal death in mice. Proc Natl Acad Sci USA 105: 18614–18618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choudhury N, Khromov AS, Somlyo AP, Somlyo AV. Telokin mediates Ca2+-desensitization through activation of myosin phosphatase in phasic and tonic smooth muscle. J Muscle Res Cell Motil 25: 657–665, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76: 481–511, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Dazert P, Meissner K, Vogelgesang S, Heydrich B, Eckel L, Bohm M, Warzok R, Kerb R, Brinkmann U, Schaeffeler E, Schwab M, Cascorbi I, Jedlitschky G, Kroemer HK. Expression and localization of the multidrug resistance protein 5 (MRP5/ABCC5), a cellular export pump for cyclic nucleotides, in human heart. Am J Pathol 163: 1567–1577, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Francis SH, Blount MA, Corbin JD. Mammalian cyclic nucleotide phosphodiesterases: molecular mechanisms and physiological functions. Physiol Rev 91: 651–690, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Friebe A, Mergia E, Dangel O, Lange A, Koesling D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci USA 104: 7699–7704, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuglsang A, Khromov A, Torok K, Somlyo AV, Somlyo AP. Flash photolysis studies of relaxation and cross-bridge detachment: higher sensitivity of tonic than phasic smooth muscle to MgADP. J Muscle Res Cell Motil 14: 666–677, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem 267: 14662–14668, 1992 [PubMed] [Google Scholar]
- 14. Groneberg D, Konig P, Wirth A, Offermanns S, Koesling D, Friebe A. Smooth muscle-specific deletion of nitric oxide-sensitive guanylyl cyclase is sufficient to induce hypertension in mice. Circulation 121: 401–409, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol 288: G407–G416, 2005 [DOI] [PubMed] [Google Scholar]
- 16. He ZH, Ferenczi MA, Brune M, Trentham DR, Webb MR, Somlyo AP, Somlyo AV. Time-resolved measurements of phosphate release by cycling cross-bridges in portal vein smooth muscle. Biophys J 75: 3031–3040, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Q, Babu GJ, Periasamy M, Eddinger TJ. SMB myosin heavy chain knockout enhances tonic contraction and reduces the rate of force generation in ileum and stomach antrum. Am J Physiol Cell Physiol 304: (2) C194–C206, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275: 30069–30074, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Jiang H, Colbran JL, Francis SH, Corbin JD. Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem 267: 1015–1019, 1992 [PubMed] [Google Scholar]
- 20. Kamm KE, Stull JT. Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem 286: 9941–9947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem 268: 12848–12854, 1993 [PubMed] [Google Scholar]
- 22. Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci USA 103: 2440–2445, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim N, Cao W, Song IS, Kim CY, Harnett KM, Cheng L, Walsh MP, Biancani P. Distinct kinases are involved in contraction of cat esophageal and lower esophageal sphincter smooth muscles. Am J Physiol Cell Physiol 287: C384–C394, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Leguillette R, Zitouni NB, Govindaraju K, Fong LM, Lauzon AM. Affinity for MgADP and force of unbinding from actin of myosin purified from tonic and phasic smooth muscle. Am J Physiol Cell Physiol 295: C653–C660, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J 7: 328–338, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Lincoln TM, Komalavilas P, Boerth NJ, MacMillan-Crow LA, Cornwell TL. cGMP signaling through cAMP- and cGMP-dependent protein kinases. Adv Pharmacol 34: 305–322, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology 119: 766–773, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Meissner K, Kessler W, Meyer zu Schwabedissen HE, Schuster K, Saalfeld K, Grube M, Buck A, Jedlitschky G, Maier S, Traeger T, Mostertz J, Homuth G, Heidecke CD, Lehmann C, Kroemer HK. Sepsis affects cardiac expression of multidrug resistance protein 5 (MRP5, ABCC5), an ABC-type CGMP export pump. Shock 28: 564–569, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Murthy KS. Activation of phosphodiesterase 5 and inhibition of guanylate cyclase by cGMP-dependent protein kinase in smooth muscle. Biochem J 360: 199–208, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Murthy KS, Makhlouf GM. Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cells. Am J Physiol Cell Physiol 268: C171–C180, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol 284: G1006–G1016, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Murthy KS, Zhou H, Makhlouf GM. PKA-dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am J Physiol Cell Physiol 282: C508–C517, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Nies AT, Spring H, Thon WF, Keppler D, Jedlitschky G. Immunolocalization of multidrug resistance protein 5 in the human genitourinary system. J Urol 167: 2271–2275, 2002 [PubMed] [Google Scholar]
- 35. Ny L, Pfeifer A, Aszodi A, Ahmad M, Alm P, Hedlund P, Fassler R, Andersson KE. Impaired relaxation of stomach smooth muscle in mice lacking cyclic GMP-dependent protein kinase I. Br J Pharmacol 129: 395–401, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parisi JA, Eddinger TJ. Smooth muscle myosin heavy chain isoform distribution in the swine stomach. J Histochem Cytochem 50: 385–393, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Peng X, Feng JB, Yan H, Zhao Y, Wang SL. Distribution of nitric oxide synthase in stomach myenteric plexus of rats. World J Gastroenterol 7: 852–854, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rattan S, Singh J. Basal internal anal sphincter tone, inhibitory neurotransmission, and other factors contributing to the maintenance of high pressures in the anal canal. Neurogastroenterol Motil 23: 3–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H, Grube M, Kock K, Kroemer HK. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab Rev 37: 253–278, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res 93: 280–291, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Sager G. Cyclic GMP transporters. Neurochem Int 45: 865–873, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Schultz C, Vaskinn S, Kildalsen H, Sager G. Cyclic AMP stimulates the cyclic GMP egression pump in human erythrocytes: effects of probenecid, verapamil, progesterone, theophylline, IBMX, forskolin, and cyclic AMP on cyclic GMP uptake and association to inside-out vesicles. Biochemistry 37: 1161–1166, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Sundkvist E, Jaeger R, Sager G. Pharmacological characterization of the ATP-dependent low K(m) guanosine 3′,5′-cyclic monophosphate (cGMP) transporter in human erythrocytes. Biochem Pharmacol 63: 945–949, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Szymanski PT, Chacko TK, Rovner AS, Goyal RK. Differences in contractile protein content and isoforms in phasic and tonic smooth muscles. Am J Physiol Cell Physiol 275: C684–C692, 1998 [DOI] [PubMed] [Google Scholar]
- 46. Szymanski PT, Szymanska G, Goyal RK. Differences in calmodulin and calmodulin-binding proteins in phasic and tonic smooth muscles. Am J Physiol Cell Physiol 282: C94–C104, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 275: G342–G351, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem 278: 17664–17671, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Wyatt TA, Naftilan AJ, Francis SH, Corbin JD. ANF elicits phosphorylation of the cGMP phosphodiesterase in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 274: H448–H455, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Xu HL, Gavrilyuk V, Wolde HM, Baughman VL, Pelligrino DA. Regulation of rat pial arteriolar smooth muscle relaxation in vivo through multidrug resistance protein 5-mediated cGMP efflux. Am J Physiol Heart Circ Physiol 286: H2020–H2027, 2004 [DOI] [PubMed] [Google Scholar]