Abstract
We have reported that transmembrane mucin MUC17 binds PDZ protein PDZK1, which retains MUC17 apically in enterocytes. MUC17 and transmembrane mucins MUC3 and MUC12 are suggested to build the enterocyte apical glycocalyx. Carbachol (CCh) stimulation of the small intestine results in gel-forming mucin secretion from goblet cells, something that requires adjacent enterocytes to secrete chloride and bicarbonate for proper mucin formation. Surface labeling and confocal imaging demonstrated that apically expressed MUC17 in Caco-2 cells and Muc3(17) in murine enterocytes were endocytosed upon stimulation with CCh. Relocation of MUC17 in response to CCh was specific as MUC3 and MUC12 did not relocate following CCh stimulation. MUC17 colocalized with PDZK1 under basal conditions, while MUC17 relocated to the terminal web and into early endosomes after CCh stimulation. CCh stimulation concomitantly internalized the Na+/H+ exchanger 3 (NHE3) and recruited cystic fibrosis transmembrane conductance regulator (CFTR) to the apical membranes, a process that was important for CFTR-mediated bicarbonate secretion necessary for proper gel-forming mucin unfolding. The reason for the specific internalization of MUC17 is not understood, but it could limit the diffusion barrier for ion secretion caused by the apical enterocyte glycocalyx or alternatively act to sample luminal bacteria. Our results reveal well-orchestrated mucus secretion and trafficking of ion channels and the MUC17 mucin.
Keywords: MUC17; Muc3(17), PDZK1; NHE3; CFTR
mucins are large o-glycosylated proteins and the major components of the mucus that covers surfaces of luminal organs such as the lungs as well as the gastrointestinal and urogenital tracts. There are four human gel-forming mucins (MUC2, MUC5AC, MUC5B, and MUC6) that form large polymeric complexes with gel-like properties when secreted from goblet cells or glands (20). A second group of mucins is the transmembrane mucins that either belong to the NIDO (nidogen)-AMOP (adhesion associated domain in MUC4 and other proteins)-vWD (von Willebrand factor type D) family (MUC4) or the SEA (sea urchin sperm protein, enterokinase, and agrin) family (MUC1, MUC3, MUC12, MUC13, MUC16, and MUC17), as determined by the juxtamembrane extracellular functional domain(s) (24).
MUC17, and its murine orthologue Muc3(17), are the main transmembrane mucins expressed in the human and mouse small intestines (12, 32). MUC17 has a domain structure typical for most transmembrane mucins (Fig. 1A). An NH2-terminal signal sequence is followed by a repeated PTS (proline-, serine-, and threonine-rich) sequence, rich in proline, threonine, and serine amino acid residues. The serine and threonine residues carry O-glycans, which are added during posttranslational processing of the mucin in the Golgi apparatus to generate an extended bottle-brush-shaped mucin domain (2, 10). A SEA domain is located between the mucin domain and transmembrane domain. This conserved domain is cleaved in an autocatalytically strain-dependent manner in the endoplasmic reticulum, a process that is necessary for correct folding of the SEA domain (28, 30, 38). The cytoplasmic tails (CTs) of the SEA transmembrane mucins vary both in length and sequence. In MUC1, the CT harbors several phosphorylation sites involved in signaling and control of MUC1CT translocation to the cell nucleus (41). Moreover, MUC1CT has a binding site for β-catenin, Grb2, adaptor protein-2 (AP-2), and heat-shock proteins (37, 41, 45, 47, 48). There is limited information on functional motifs in CTs of other transmembrane mucins, with the exception of our previous discovery of a PDZ (PSD-95, Dlg, and ZO-1) class I binding motif in the COOH termini of MUC3, MUC12, and MUC17 (33).
Fig. 1.
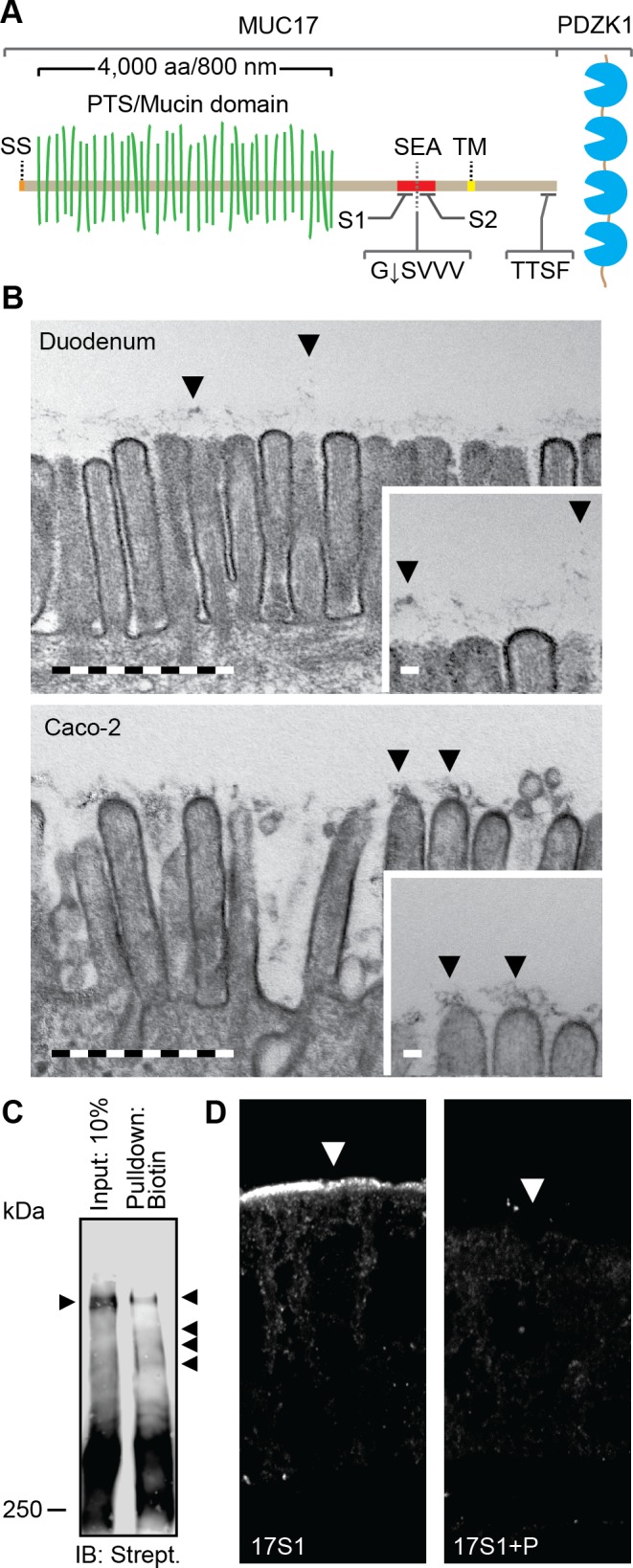
The transmembrane mucin MUC17 is a component of the glycocalyx covering apical membranes of Caco-2 cells. A: schematic view of the domain organization of MUC17. SS, signal sequence; PTS, proline-, threonine-, and serine-rich region called the mucin domain when O-glycosylated; SEA, SEA domain; TM, transmembrane domain. G↓SVVV represents the consensus sequence of the native SEA cleavage site. S1 and S2 indicate the position of epitopes recognized by anti-MUC17S1 and anti-MUC17S2 pAbs. TTSF represents the class I PDZ binding motif that allows binding to the scaffolding protein PDZK1. B: transmission electron microscopy of microvilli distributed on the apical surface of human duodenal enterocytes (top) and polarized Caco-2 cells (bottom). Insets: magnifications of the larger micrographs. Arrows point at the glycocalyx. Bars in large micrographs = 1 μm. Bars in insets = 100 nm. C: expression of surface glycoproteins on apical surface of Caco-2 cells assessed by surface biotinylation using biotin hydrazide. Biotinylated high-molecular glycoproteins were detected using streptavidin HRP. Arrows indicate separated biotinylated glycoproteins. IB, immunoblot. D: staining of MUC17 on apical surface of polarized Caco-2 cells (left). The specificity of anti-MUC17S1 pAb against MUC17 was assessed by neutralizing the antibody with its corresponding immunizing peptide (P) (right). Arrows point towards the apical plasma membrane.
The cystic fibrosis transmembrane conductance regulator (CFTR) and Na+/H+ exchanger 3 (NHE3) are expressed in the apical brush border membrane (BBM) of enterocytes. Mutations in the CFTR gene cause cystic fibrosis (CF), an autosomal recessive genetic disorder, defined by decreased chloride and bicarbonate ion conductance across the apical plasma membrane of epithelial cells in the lungs, pancreas, small intestine, and sweat glands. CFTR has been proposed to be important for mucus hydration and expansion after mucus discharge from goblet cells. In fact bicarbonate has been shown to be able to dissolve packed recombinant MUC2 NH2-terminal domains in vitro (1). This finding is supported by ex vivo experiments where the attached mucus phenotype in CF is rescued by addition of bicarbonate (13). NHE3 is a member of a family of exchangers that perform electroneutral exchange of extracellular Na+ for intracellular H+ and is thus able to neutralize secreted bicarbonate (49).
To maintain cell homeostasis, the expression as well as spatial distribution and activity of ion channels, exchangers, and other membrane proteins is under strict regulation. PDZ adaptor proteins are the most abundant protein-protein interaction modules involved in regulation of membrane proteins. PDZ domains are classified according to their ability to bind peptide sequences in the extreme COOH terminus of their corresponding ligands. One common class of PDZ binding motifs is the class I motif, characterized by X-[S/T]-X-Φ, where X is any amino acid and Φ is a hydrophobic amino acid residue at position 0 (16, 19, 46). CFTR (-DTRL, where L is at position 0) is a class I PDZ ligand for at least four PDZ adaptor proteins (35). Golgi-associated PDZ and coiled-coil motif-containing protein (GOPC) entraps CFTR in the trans-Golgi network and targets the channel for lysosomal degradation (7, 8). Three members of the Na+/H+ exchange regulatory cofactor (NHERF) family, NHERF1, NHERF2, and PDZK1 (NHERF3), bind CFTR in the BBM and modulate channel retention, conductivity, and dimerization as well as interaction with additional transporter proteins (44). Also, NHE3, which has a class I PDZ binding motif (-STHM), is a ligand for the NHERF family of adapter proteins that mediate acute regulation of the exchanger by modulating complex formation and rates of endo- and exocytosis (22, 25, 27, 43). For example, PDZK1 binds directly to NHE3 and the dissociation of PDZK1 from NHE3 results in internalization of the exchanger, thus reducing basal Na+/H+ exchange (50).
We have previously reported two novel class I PDZ interactions involving the transmembrane mucins MUC3 and MUC17. MUC3 (-SSSV) is captured by GOPC in the trans-Golgi network and targeted for degradation, a process that is bypassed when CFTR competes with MUC3 for binding to GOPC (39). MUC17 (-TTSF) and murine Muc3(17) (-MTSL) are ligands for BBM-resident PDZK1/Pdzk1, which, via a direct interaction also conserved in humans, retain transmembrane mucins in the apical membrane of cells (33).
The cholinergic agonist carbachol (CCh) has been reported to induce internalization of Nhe3 and simultaneous membrane recruitment of Cftr in rat ileum (18). Since both CFTR and NHE3 share PDZK1 as an adaptor protein with MUC17 and since CCh stimulation abolishes NHE3-PDZK1 interaction, we set out to study the effect of CCh stimulation on MUC17 distribution in BBM of enterocytes. Caco-2 cells, widely used as a model for polarized intestinal enterocytes (9, 26, 34), were stimulated with CCh, and the localization of MUC17, NHE3, and CFTR was studied by surface biotinylation and confocal microscopy. Here, we show that MUC17 colocalized with PDZK1 under basal conditions and that CCh inhibited this colocalization as MUC17 was internalized into the terminal web region and further into early endosomes. Furthermore, CCh-induced MUC17 endocytosis was specific and occurred concomitantly with NHE3 internalization and recruitment of CFTR to the apical membrane of enterocytes.
MATERIALS AND METHODS
Cell lines.
Caco-2 cells were cultured at 37°C in 5% CO2 in Iscove's modified Dulbecco's medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% (vol/vol) FCS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Caco-2 cells were grown on Transwell-COL semipermeable inserts (0.4-μm pores, 12 mm in diameter) (Corning, Corning, NY) for a minimum of 16 days post confluency to allow cell differentiation.
Mouse tissue and mucus thickness measurements.
Ethical approval for the animal experiments was granted by the Animal Ethics Committee, University of Gothenburg. Duodenum from male wild-type C57/Bl6 mice, 12–14 wk old, were harvested and mounted on a vertical Ussing chamber. The apical side of the tissue was bathed in KREB-mannitol solution, and the basolateral side was bathed in KREB-glucose solution. The solutions were gassed with 95% O2-5% CO2 at 37°C, pH 7.4. Tissues were equilibrated for 20 min before stimulation with 100 μM CCh for 30 min on the serosal side of the tissue. After stimulation, tissues were fixed in methanol-Carnoy's fixative, embedded in paraffin, and sectioned. Mucus thickness measurements were performed as previously described by Gustafsson et al. (14). Briefly, charcoal particles were applied to visualize the translucent mucus surface and a glass capillary, connected to a micromanipulator, was used for measurements of mucus thickness. Initial mucus thickness was measured between the mucus surface and tip of the intestinal villi. Upon removal of mucus, the villus height, i.e., the distance between the villus tip and the surface epithelium in between the villi, was determined. Total mucus thickness was defined as the sum of the villus height and the initial mucus thickness. Mucus secretion was induced by serosal stimulation using 100 μM CCh.
Primary and secondary antibodies.
Anti-MUC17S2 polyclonal antibody (pAb) was used to detect the COOH-terminal subunit of MUC17 (39). Anti-Muc3(17)S2 pAb was used to detect murine Muc3(17) (32). Anti-MUC17S1 pAb against the sequence LKNHSSQEFQEFKQTFTEQMNIC and anti-MUC12S2 pAb against the sequence DYTLEYEELFENLAEIVKAKIMNEC were raised by conjugating 2 mg of each peptide to keyhole limpet hemocyanin using its terminal cysteine and maleimide crosslinker and injecting the purified peptide into two rabbits. Primary antibodies anti-early endosome antigen 1 (anti-EEA1) mAb (1G11; Abcam, Cambridge UK), anti-NHE3 pAb (V16; Santa Cruz Biotechnology, CA), anti-PDZK1 mAb (1E9; Sigma-Aldrich, Oakville, St. Louis, MO), and anti-F-actin mAb (NH3, Abcam) were used to detect EEA1, NHE3/Nhe3, PDZK1, and F-actin, respectively. Anti-CFTR M3A7 mAb was a generous gift from Dr. John R. Riordan (University of North Carolina, Chapel Hill, NC; Ref. 21). The secondary antibodies goat anti-rabbit Alexa Fluor 488, goat anti-mouse Alexa Fluor 546, donkey anti-rabbit Alexa Fluor 488, and donkey anti-goat Alexa Fluor 555 were purchased from Invitrogen Life Technologies. Streptavidin horseradish peroxidase (HRP) antibody and goat anti-rabbit HRP were purchased from DAKO (Hamburg, Germany) and Pierce (Rockford, IL), respectively.
Measurement of surface MUC17.
Differentiated Caco-2 cells were fed with fresh culture medium 30 min before stimulation with 20 μM CCh for 20 min or 20 μM CCh for 20 min together with 80 μM Dynasore (Sigma-Aldrich) for 45 min at 37°C in 5% CO2. Cells were washed three times with PBS and incubated with 1 mM sodium meta-periodate for 15 min in the dark on ice. After oxidation of cell surface glycoproteins, cells were washed once with PBS and three times with 10 mM MES buffer [10 mM 2(N-morpholino)ethanesulfonic acid, pH 6.5, and 140 mM NaCl]. Biotinylation of surface glycoproteins was carried out by incubating cells with 1 mM biotin hydrazide (Sigma-Aldrich) in MES buffer for 1 h on ice. After biotin labeling, cells were washed three times with PBS (pH 8.0) and incubated with 0.5 mM sodium borohydride in PBS (pH 8.0). Cells were then washed three times with PBS (pH 8.0) and lysed with IP Lysis Buffer (25 mM Tris·HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, and 5% glycerol) for 10 min on ice. Lysates were homogenized, centrifuged at 16,000 g for 15 min at 4°C, and cleared from free biotin hydrazide by centrifugation through NanoSep 10K MWCO OMEGA columns (Pall, MI) for 15 min at 14,000 g. Biotinylated glycoproteins from 130 μg of the retentate were bound to 75 μl of Dynabeads Streptavidin M280 magnetic beads (Invitrogen Life Technologies). Unbound material was diluted with 2× reducing sample buffer containing DTT and saved for analysis. Bound material was eluted from magnetic beads with reducing sample buffer. Seventy-five micrograms of protein were mixed with reducing sample buffer and used as input loading control. Samples were boiled at 95°C for 5 min before they were loaded on a 3–5% or 10% SDS-PAGE. Separated proteins were transferred to PVDF P or pSQ membranes (Millipore, Billerica, MA) and blocked with 2% BSA in PBS. Total biotinylated proteins in samples were detected with streptavidin HRP whereas the COOH-terminal subunit of MUC17 was detected with anti-MUC17S2 pAb and goat anti-rabbit secondary antibody conjugated to HRP. Protein bands were detected in a LAS-4000 scanner (Fujifilm, Tokyo, Japan). Band densities were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Immunofluorescence.
Caco-2 cells grown on semipermeable supports were incubated with fresh culture medium for 30 min before treatment with 20 μM CCh for 20 min at 37°C in 5% CO2. Following treatment, cells were washed three times with PBS, fixed with 4% formaldehyde in PBS for 10 min at room temperature (RT), washed another round with PBS, and permeabilized with 1% Triton X-100 in PBS for 5 min at RT. After permeabilization, cells were washed and blocked with culture medium containing 10% (vol/vol) FCS for 30 min at RT. Cells were then incubated with 1 U/ml Alexa Fluor 647 Phalloidin (Invitrogen Life Technologies) for 30 min in the dark at RT. After three washes with PBS, cells were stained with primary antibodies overnight at 4°C, washed three times with PBS, and stained with secondary antibodies for 2 h in the dark at RT. Finally, cell nuclei were stained with DAPI for 5 min at RT. Semipermeable supports were washed, excised, and mounted on glass using Prolong Gold antifade (Invitrogen Life Technologies) and polymerized overnight. Sections of mouse duodenum were deparaffinized and rehydrated in xylene substitute (2 × 10 min), 100% ethanol (2 × 5 min) and 95% (vol/vol) ethanol (5 min), 70% (vol/vol) ethanol (5 min), 50% (vol/vol) ethanol (5 min), 30% (vol/vol) ethanol (5 min), and washed with water. Antigen retrieval was performed in boiling 0.01 M citric acid (pH 6.0) for 30 min followed by a wash in PBS for 5 min. Tissues were enclosed with a PAP pen and blocked with 5% FCS in PBS. Primary and secondary antibodies were diluted in 5% FCS in PBS. Coverslips were mounted using Prolong Gold antifade. Samples were acquired at room temperature using a Zeiss LSM700 upright confocal microscope (Carl Zeiss, Oberkochen, Germany) with an oil immersion Plan-Apochomat ×40/1.3 DIC objective lense (Carl Zeiss) and the ZEN 2009 software (Carl Zeiss). For neutralization of anti-MUC17S1 pAb before immunocytochemistry on differentiated Caco-2 cells, anti-MUC17S1 pAb was diluted in 5% FCS in PBS and incubate for 90 min with 1 μg/ml of immunizing peptide. Anti-MUC17S1 pAb diluted in 5% FCS in PBS was used to detect endogenous MUC17 in Caco-2 cells.
Quantification of membrane protein distribution in BBM.
The intensity profiles of MUC17, PDZK1, NHE3, CFTR, or F-actin in Caco-2 cells were analyzed by acquiring a minimum of three Z-stack images (0.43 μm sections, 20 sections per image) from each experiment. For each Z-stack, the intensity profile for the protein of interest along the terminal web-microvilli-axis was obtained from three 5 × 9 μm wide rectangles in a minimum of three XZ and three YZ images using ImageJ. After background subtraction, the intensities were normalized, averaged, and plotted against the distance along the terminal web-microvilli-axis. Amounts of each protein in terminal web region and microvillar region were determined by calculating the area under the curve in each region. The maximum intensity peak of F-actin was defined as the interface between the terminal web and the microvilli. A 2-μm broad region below the maximum intensity peak of F-actin was defined as the terminal web region and more intracellular compartments, whereas a 2-μm broad region above the F actin intensity maximum was defined as a region spanning the microvillar region and extracellular luminal compartment. The F-actin peak was set to zero on the X-axis, which represented the distance along the terminal web-microvilli-axis. The intensity profiles of Muc3(17) and F-actin in sections of mouse duodenum were analyzed by collecting a minimum of three images from each experiment. Five 2.5 × 8.0 μm rectangles were collected from each image, and the intensity profiles for Muc3(17) and F-actin were measured along the terminal web-microvilli-axis using ImageJ. After background subtraction, the intensities were normalized to 100, averaged, and plotted against the distance along the terminal web-microvilli-axis. Amounts of each protein in terminal web region and microvillar region were determined by calculating the area under the curve in each region. The peak of the F-actin signal was defined as the terminal web region (6).
Colocalization studies.
Colocalization of proteins was assessed using Volocity 3D Imaging Analysis Software (PerkinElmer, MA). For each experiment, a minimum of 25 cells from 3 Z-stack images were analyzed, and the degree of colocalization between two proteins was quantified using thresholded Pearson's correlation coefficient (PCC) that measures the correlation between signals in channels 1 and 2. PCC ranges between 1 and −1, where 1 defines a perfect positive correlation between the two channels and −1 defines a perfect inverse correlation. In our analysis, a positive correlation between two channels was defined as PCC ≥ 0.50 (3). Mander's colocalization coefficients (MCCs), M1 and M2, were used to determine the ratio between the total colocalized intensity and the total intensity for each of the two channels (3, 4).
Transmission electron microscopy.
Duodenal tissue from wild-type mice or Caco-2 cells cultured on semipermeable supports were fixed in Karnovsky fixative (2% paraformaldehyde and 2.5% glutaraldehyde in 0.05 M, pH 7.2, sodium cacodylate buffer) for 24 h, followed by staining using 1% OsO4 for 4 h, 1% tannic acid for 3 h, and 1% uranyl acetate overnight. Samples were dehydrated and embedded in epoxy resin (Agar 100). Ultrathin sections (50 nm; Reichert Ultracut E) were collected on mesh copper support grids, followed by contrasting using lead citrate and tannic acid. Samples were examined under a 902 electron microscope (Carl Zeiss).
Statistics.
Data are represented as means ± SE. Two-tailed Mann-Whitney U-test, Students t-test, and ANOVA with a Dunnett's post hoc test were applied where appropriate, using the GraphPad Prism 5 (La Jolla, CA). Values of P < 0.05 were considered significant.
RESULTS
Transmembrane mucin MUC17 is a component of the apical enterocyte glycocalyx (fuzz).
The three transmembrane mucins, MUC3, MUC12, and MUC17, share the same genomic locus, general structure, and length. MUC17 is large due to the PTS sequence that makes up 4,000 of its 4,500 amino acids. After O-glycosylation the extended mucin domain adopts a bottle-brush-rod-like shape where every amino acid has an estimated length of ∼0.2 nm, giving the MUC17 a total length of ∼800 nm (Fig. 1A) (1, 5, 11). MUC12 is slightly larger with a PTS sequence of 5,000 amino acids, whereas MUC3 is still not fully sequenced.
Enterocytes in the small intestine polarize into distinct apical and basolateral membrane domains. The surface of the microvilli has an attached filamentous layer, called the “fuzz” or glycocalyx, shown to contain large amounts of carbohydrates anchored to membrane proteins (17, 40). When duodenal enterocytes was analyzed by transmission electron microscopy, the apical plasma membrane was found to be covered by a dense layer of filamentous material, stretching ∼500 nm from the microvilli into the lumen (Fig. 1B, top). The apical plasma membrane of polarized Caco-2 cells was covered by a similar, but less dense, filamentous material attached to the microvilli that could reach a similar length (Fig. 1B, bottom). As the thickness of this filamentous layer is that high, it is most likely made up by the transmembrane mucins that can reach such length.
To confirm the existence of large glycoproteins, e.g., transmembrane mucins, in the glycocalyx covering the apical membrane of enterocytes, surface glycoproteins on Caco-2 cells were biotinylated using biotin hydrazide. Biotinylated surface glycoproteins were affinity-precipitated and separated using SDS-PAGE where visualization with streptavidin HRP revealed several heavy bands representing surface glycoproteins expressed apically in Caco-2 cells (Fig. 1C). Next, a polyclonal antibody anti-MUC17S1, against an NH2-terminal peptide in MUC17 SEA domain, stained the apical membrane of polarized Caco-2 cells, thus confirming the expression of MUC17 in the apical membrane (Fig. 1D, left). The specificity of the anti-MUC17S1 polyclonal antibody (pAb) was evaluated by neutralizing the pAb with the corresponding immunizing peptide before immunocytochemistry where the immunizing peptide blocked antibody specificity (Fig. 1D, right). The transmembrane mucin MUC17 is thus a component of the glycocalyx covering the apical plasma membrane of polarized Caco-2 cells and together with MUC3 and MUC12 likely makes up the thick enterocyte microvilli filamentous glycocalyx (fuzz).
CCh stimulation relocates MUC17 from the cell surface to the terminal web.
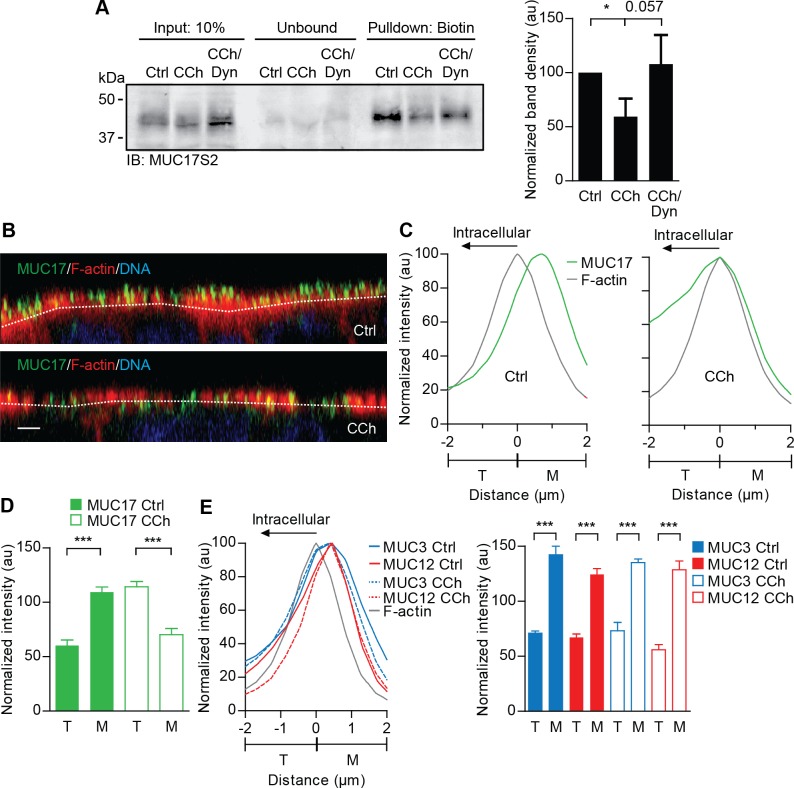
To study the effect of CCh on MUC17 surface expression, polarized Caco-2 cells were treated with CCh or a combination of CCh and the dynamin-inhibitor Dynasore, which prevents clathrin-mediated endocytosis (31). Biotin-labeled surface glycoproteins were separated on a SDS-PAGE before levels of MUC17 were assessed by Western blot. CCh stimulation significantly reduced surface expression of MUC17 compared with nonstimulated cells, whereas Dynasore prevented CCh-induced endocytosis of MUC17, although this effect was not statistically significant (P = 0.057; Fig. 2A). Confocal microscopy revealed that MUC17 in nonstimulated cells was localized to the outermost part of the apical membrane, i.e., the microvillar region as defined in materials and methods (Fig. 2B, top). Upon stimulation with CCh, MUC17 was redistributed to the F-actin-positive compartment defined as the terminal web region underlying the base of the microvilli (Fig. 2B, bottom). Intensity profiles showed that MUC17 relocated towards the terminal web region and overlapped with the F-actin peak after stimulation with CCh (Fig. 2C). Quantitative analysis indicated that the amount of MUC17 in microvilli decreased with ∼40% and increased with 62% in the terminal web (Fig. 2D). Finally, the specificity of CCh-induced endocytosis of transmembrane mucins was determined. We have previously shown that the transmembrane mucin MUC3 is endogenously expressed in Caco-2 cells (39). An assessment of the spatial distributions of MUC3 as well as MUC12 revealed that they were unaffected by CCh stimulation, as shown by intensity profiles and quantitative analysis (Fig. 2E). In summary, these results suggest that MUC17, but not MUC3 and MUC12, is redistributed from the microvilli to the terminal web in a dynamin-dependent endocytosis process in response to CCh stimulation.
Fig. 2.
MUC17 is endocytosed and redistributes to F-actin-rich terminal web upon stimulation with carbachol (CCh). A: nonstimulated polarized Caco-2 cells (Ctrl) and cells stimulated with CCh (CCh) or a combination of CCh and Dynasore (CCh/Dyn) were analyzed for apical MUC17 expression. Cell surface glycoproteins were biotinylated and MUC17 was detected by Western blot using anti-MUC17S2 pAb. Left: representative Western blot showing input controls, unbound material after affinity precipitation and the precipitated material. Right: bar graph summarizing means ± SE of 4 independent experiments (n = 4; *P < 0.05 is compared with control; au, arbitrary units). B: nonstimulated Caco-2 cells (Ctrl) and CCh-stimulated cells stained for MUC17 (green), F-actin (gray), and DNA (blue). Dashed white lines indicate F-actin intensity maximum. Scale bar = 2 μm. C: normalized intensity profiles obtained from 3 independent experiments showing distribution of MUC17 (green line) in relation to F-actin (gray line) in nonstimulated (left) and cells stimulated with CCh (right). D: bar graph summarizing means ± SE of 3 independent experiments. Solid bars represent amount of MUC17 in nonstimulated cells (Ctrl), whereas open bars represent amount of MUC17 in cells treated with CCh (n = 3 in each group, ***P < 0.001). E, left: normalized intensity profile of MUC3 (blue lines) and MUC12 (red lines) in relation to F-actin (gray line) in nonstimulated cells and CCh-stimulated cells. Right: bar graph summering means ± SE from 3 independent experiments. Solid bars represent amount of MUC3 (blue) and MUC12 (red) in nonstimulated cells, and open bars represent amount of MUC3 (blue) and MUC12 (red) in CCh-treated cells. T, terminal web; M, microvilli.
CCh-induced endocytosis of MUC17 reduces colocalization with PDZK1 and transfers the mucin to early endosomes.
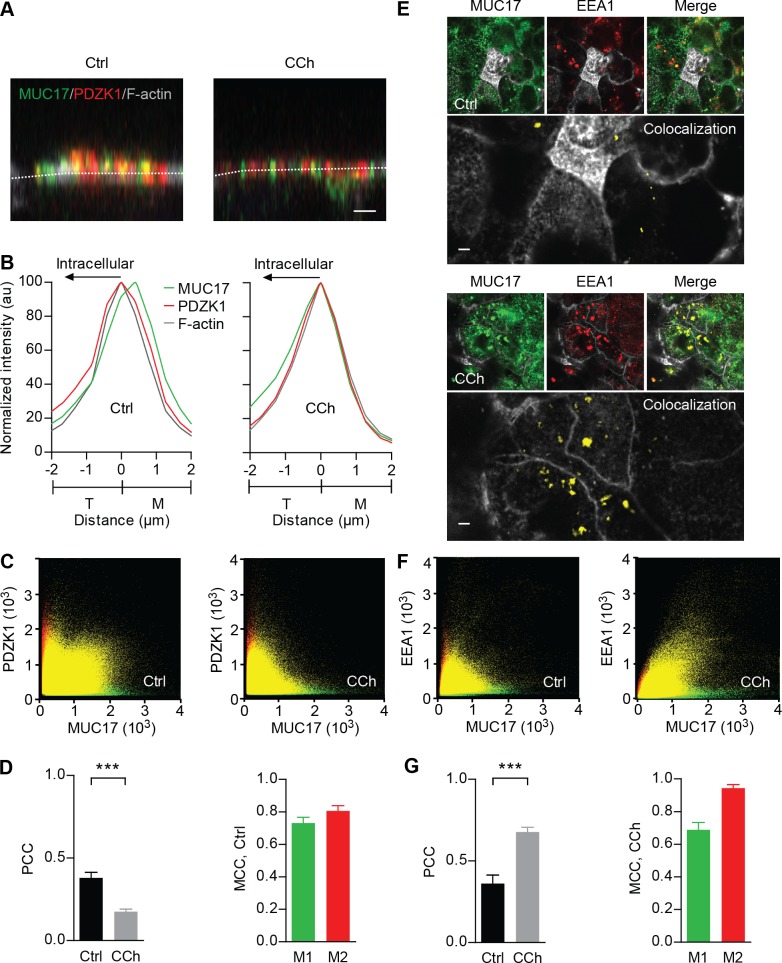
Lack of Pdzk1 in the mouse small intestinal enterocytes redistributes Muc3(17) from its normal apical surface localization to intracellular vesicles (33). We have now studied if CCh could redistribute MUC17 in relation to PDZK1 in Caco-2 cells. In nonstimulated cells, MUC17 was expressed in microvilli and a major fraction of MUC17 colocalized with PDZK1 (Fig. 3A). In contrast, following CCh stimulation, MUC17 relocated to the terminal web region, displaying reduced colocalization with PDZK1. The intensity profiles revealed that MUC17 shifted towards the terminal web in the presence of CCh, while the maximum intensity of PDZK1 overlapped with the terminal web region both under basal conditions and after CCh treatment (Fig. 3B). Also, CCh stimulation did not affect the amount of PDZK1 in the terminal web (results not shown). The loss of colocalization between MUC17 and PDZK1 in CCh-treated cells was confirmed by two-dimensional scatter plots (Fig. 3C). Thresholded PCC revealed a correlation, although weak, between MUC17 and PDZK1 in nonstimulated cells (PCC = 0.37 ± 0.03), while PCC significantly dropped to 0.18 ± 0.02 after CCh stimulation (Fig. 3D, left). MCC M1 in nonstimulated cells, i.e., the total colocalized intensity of MUC17 as a fraction of the total intensity of MUC17, was 0.73 ± 0.08, indicating that a majority of total MUC17 colocalized with PDZK1 (Fig. 3D, right).
Fig. 3.
Subcellular localization of MUC17 in relation to PDZK1 and EEA1-positive early endosomes. A: nonstimulated and CCh-stimulated Caco-2 cells stained for MUC17 (green), PDZK1 (red), and F-actin (gray). Dashed white lines indicate F-actin intensity maximum. Scale bar = 2 μm. B: normalized intensity profiles for MUC17 (green line) and PDZK1 (red line) plotted in relation to F-actin (gray line). C: 2-dimensional (2D) scatter plots showing correlation between MUC17 (green channel) and PDZK1 (red channel) in nonstimulated (left panel) and CCh-stimulated cells (right). D, left: bar graph summarizing thresholded PCC for colocalization of MUC17 with PDZK1 in nonstimulated and CCh-stimulated cells. Right: MCC M1 for MUC17 and M2 for PDZK1 in nonstimulated cells (n = 3 in each group; ***P < 0.001). E: Caco-2 cells stimulated with CCh and stained for MUC17 (green), EEA1 (red) and F-actin (gray). Top: MUC17 and EEA1 and the merge of the 2 channels in nonstimulated cells. Bottom: staining of MUC17 and EEA1 and the merge of the 2 channels in CCh-stimulated cells. Magnifications show colocalization of MUC17 with EEA1 in yellow. Scale bar = 2 μm. F: 2D scatter plots showing correlation between MUC17 (green channel) and EEA1 (red channel) in nonstimulated cells (left) and CCh-treated cells (right). G: bar graph summarizing thresholded Pearson's correlation coefficient (PCC) obtained from colocalization of MUC17 with EEA1 in nonstimulated and CCh-stimulated cells (left). Mander's correlation coefficients (MCC) M1 for MUC17 and M2 for EEA1 in CCh-stimulated cells are displayed at right (n = 3 in each group; ***P < 0.001).
Confocal images following CCh treatment showed increased colocalization of MUC17 with EEA1, a marker for early endosomes (Fig. 3E). In nonstimulated cells, MUC17 showed weak correlation with EEA1 as PCC was below 0.50 (0.36 ± 0.05). In contrast, the correlation between MUC17 and EEA1 increased significantly in CCh-treated cells, showing a PCC of 0.68 ± 0.03 (Fig. 3G, left). Two-dimensional scatter plots of the intensities for the two proteins confirmed the positive correlation between MUC17 and EEA1 upon CCh stimulation (Fig. 3F). The total colocalized intensity of MUC17 as a fraction of the total intensity of MUC17 (MCC M1) in CCh-treated cells was 0.69 ± 0.05 (Fig. 3G, right). In summary, colocalization of MUC17 with PDZK1 decreased following CCh stimulation, where nearly 70% of total MUC17 ended up in EEA1-positive vesicles.
CCh-induced endocytosis of MUC17 is concomitant with NHE3 internalization and CFTR membrane recruitment.
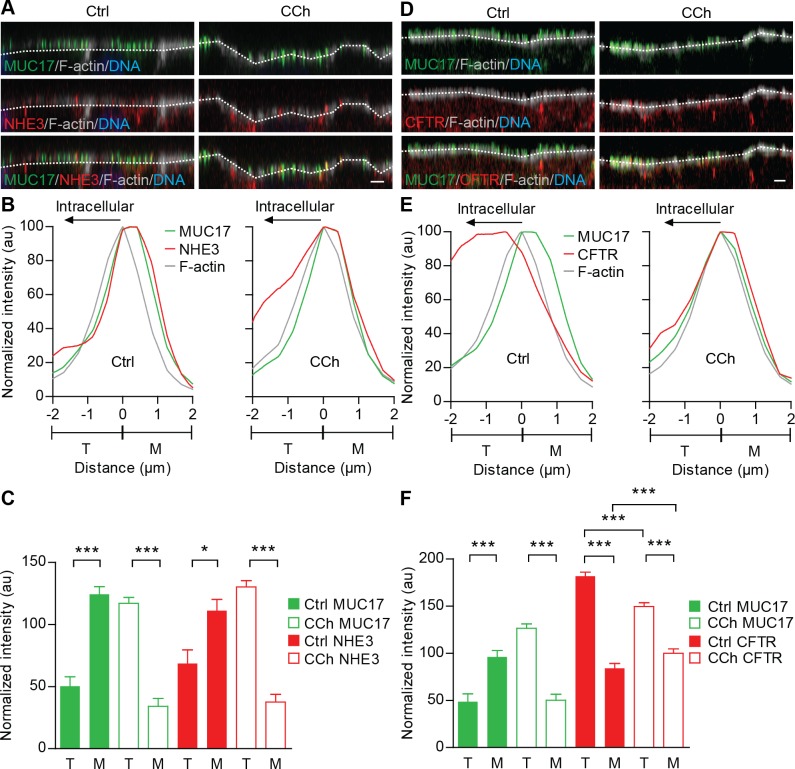
It was recently indicated that CCh regulates the spatial distribution of Nhe3 and Cftr in rat ileum, where Nhe3 undergoes endocytosis and Cftr is mobilized to the apical surface in response to CCh (18). Moreover, NHE3 interacts with PDZK1, which under basal conditions anchors the exchanger to Caco-2 apical membrane (50). These observations led us to study the effect of CCh stimulation on NHE3 and CFTR distribution in relation to MUC17 in the BBM of Caco-2 cells. In nonstimulated cells, MUC17 and NHE3 resided in the microvillar region whereas both proteins relocated to the terminal web region 20 min after CCh stimulation (Fig. 4, A and B). MUC17 and NHE3 levels in the microvillar region decreased with 73 and 66%, respectively, while levels of MUC17 increased 2.3-fold and NHE3 1.9-fold in the terminal web region compared with control cells (Fig. 4C). In contrast to NHE3 under basal conditions, the major fraction of CFTR resided intracellularly below the terminal web. Following CCh stimulation, CFTR was recruited to the apical membrane (Fig. 4D). Intensity profiles showed that membrane-recruited CFTR showed maximum intensity in the interface between the terminal web and microvilli (Fig. 4E). This indicated that membrane-recruited CFTR was located at the base of microvilli, just above the terminal web region. The amount of CFTR in the terminal web and lower compartments decreased significantly from 181.6 ± 4.4 to 149.4 ± 4.0 while CFTR levels in the microvilli increased from 84.1 ± 4.9 to 99.9 ± 4.5 (Fig. 4F). In summary, it is suggested that CCh-induced redistribution of MUC17 is concomitant with NHE3 internalization to the terminal web and recruitment of CFTR to the base of the microvilli.
Fig. 4.
MUC17 endocytosis occurs simultaneously with Na+/H+ exchanger 3 (NHE3) internalization and CFTR membrane recruitment. A and D: nonstimulated (left) and CCh-stimulated Caco-2 cells (right) stained for F-actin (gray) as well as MUC17 (green) and NHE3 (red) or MUC17 (green) and CFTR (red). Dashed white lines indicate F-actin intensity maximum. Scale bar = 2 μm. B and E: normalized intensity profiles for nonstimulated (left) and CCh-treated cells (right) from 3 independent experiments showing the distribution of MUC17 (green line) and NHE3 (red line) or MUC17 (green line) and CFTR (red line) in relation to F-actin (gray line). C and F: bar graphs summarizing means ± SE of 3 independent experiments. Solid bars represent distribution of MUC17 (green) and NHE3 (red) or MUC17 (green line) and CFTR (red line) in nonstimulated cells (Ctrl), while open bars show distribution of each protein after CCh stimulation (n = 3 in each group; *P < 0.05, ***P < 0.001).
Murine Muc3(17) is internalized upon CCh stimulation of duodenal enterocytes.
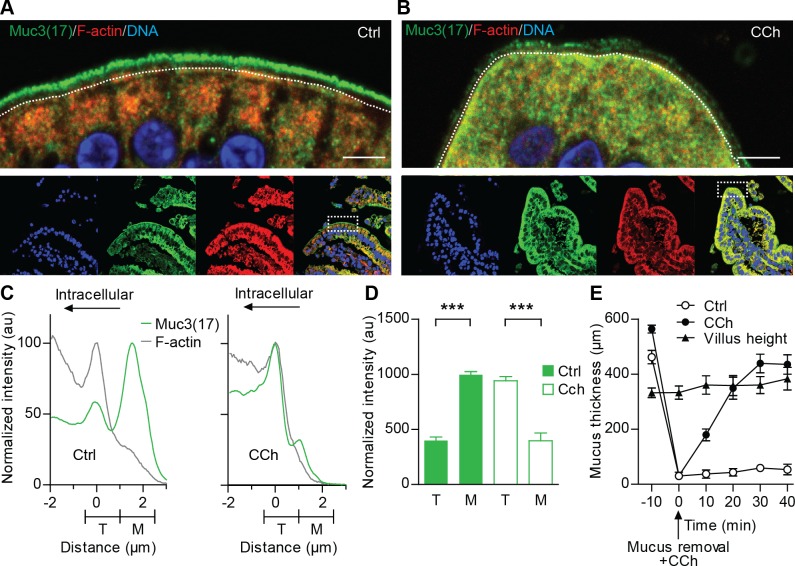
Next, we tested whether our observations in the Caco-2 intestinal model could be repeated in the mouse duodenum, which expresses high levels of Muc3(17). In nonstimulated duodenal explants, Muc3(17) was separated from F-actin and was predominantly localized to the microvilli of the enterocytes (Fig. 5A). In contrast, Muc3(17) relocated to the terminal web region and intracellular vesicles below this region in the CCh-stimulated tissue (Fig. 5B). Normalized intensity profiles showed that Muc3(17) was redistributed toward the terminal web region defined as the region where F-actin reaches its maximum intensity (Fig. 5C). The amount of Muc3(17) in microvilli was reduced with 60% whereas the amount of Muc3(17) in the terminal web region increased nearly 2.4-fold compared with nonstimulated tissue (Fig. 5D).
Fig. 5.
Murine Muc3(17) redistributes to the F-actin-rich terminal web of enterocytes upon stimulation with carbachol. Sections from nonstimulated (A) and CCh-stimulated mouse duodenum (B) were stained for Muc3(17) (green), F-actin (red), and DNA (blue). Dashed white lines indicate F-actin intensity maximum. Scale bar = 5 μm. C: normalized intensity profile for Muc3(17) (green line) in relation to F-actin (gray line) in nonstimulated (Ctrl) and tissue stimulated with CCh. D: bar graphs summarizing means ± SE for amount of Muc3(17) in nonstimulated (Ctrl) and CCh-stimulated tissue. (n = 3; ***P < 0.001). E: mucus thickness in mouse duodenal explants, mounted in a horizontal chamber at time point −10 min. ●, Mucus was removed and 100 μM CCh were added at 0 min. Mucus expansion and thickness were measured during 40 min. ○, Nonstimulated tissue. ▲, Villus height (n = 5).
Recently, it was indicated that CCh stimulation results in CFTR-mediated ion and fluid secretion, which enables proper unfolding of secreted mucins (13). To place CCh-induced endocytosis of Muc3(17) in the context of gel-forming mucin release from the goblet cells, unfolding, and subsequent growth, we measured gel-forming mucus release and expansion in mouse duodenum at different time points. Under basal conditions, the mucus covering intestinal cells measured 462–564 μm, ∼181 μm above the villi tips (Fig. 5E). At time 0 min, the overlying mucus was removed and the tissue was stimulated with 100 μM CCh. After mucus removal and stimulation, the mucus thickness increased from 31.2 μm (0 min) to 348.9 μm during a 20-min period, corresponding to the time point when Muc3(17) endocytosis was assessed. The mucus thickness reached equilibrium at 435.3 μm 40 min after CCh stimulation. In summary, we suggest that CCh-induced endocytosis of MUC17 observed in Caco-2 cells is valid in the mouse duodenum where Muc3(17) undergoes endocytosis in response to CCh. Finally, Muc3(17) endocytosis occurs at the same time as the gel-forming mucins expelled from goblet cells expand due to fluid and ion secretions into the intestinal lumen.
DISCUSSION
We have previously shown that MUC17 is a binding partner for the scaffolding protein PDZK1 (33). Pdzk1 retains Muc3(17) at the apical membrane of enterocytes in the mouse small intestine, as lack of Pdzk1 results in its redistribution into intracellular vesicles. Interestingly, PDZK1 plays a comparable role in NHE3 regulation. Under basal conditions in Caco-2 cells, PDZK1 anchors NHE3 to the apical cell membrane where the ion channel exchanges extracellular Na+ for intracellular H+ (50). Following knockdown of PDZK1, NHE3 leaves BBM most likely due to endocytosis. The same process can be observed when Caco-2 cells are stimulated with CCh that gives increased intracellular Ca2+, leading to dissociation of NHE3 from PDZK1 and the subsequent internalization of NHE3. The molecular mechanisms behind this finding is still unclear, but the Ca2+-dependent uncoupling of PDZK1 from NHE3 occurs in vivo but not in vitro, as purified recombinant NHE3 and PDZK1 proteins interact independent of Ca2+ (50). Thus Ca2+-dependent dissociation of NHE3 from PDZK1 requires additional proteins, most likely additional adapter and effector protein such as PDZ proteins and kinases, existing in vivo. In fact, PDZK1 undergoes serine phosphorylation in its COOH terminus, a feature that may be involved in uncoupling from NHE3 (36).
The goal of the present study was to study the spatial distribution of endogenous transmembrane mucin MUC17 in relation to PDZK1, CFTR, and NHE3 when Caco-2 cells are exposed to CCh stimulation. PDZK1 resides in the interface between microvilli and the underlying terminal web, i.e., the base of microvilli, where it colocalizes with F-actin but also overlaps with ezrin and NHERF1 that reside more distally in microvilli (23). Thus PDZK1 is not only limited to the base of microvilli but a small portion of PDZK1 overlaps with more distal portions of microvilli. Under basal conditions, endogenous MUC17 is expressed in the microvilli, where a major fraction of MUC17 colocalizes with PDZK1. Also, a minor fraction of MUC17 resides in early endosomes, indicating that MUC17 is subjected to intrinsic membrane protein recycling. MUC17 also undergoes dynamin-dependent endocytosis in response to CCh, a process that was associated with loss of colocalization with PDZK1 and redistribution of MUC17 to the terminal web and further down into early endosomes. Interestingly, the localization of PDZK1 in the terminal web was unaffected upon stimulation with CCh. The fact that redistribution of MUC17 to the terminal web is not reflected by colocalization with PDZK1 suggests that MUC17 and PDZK1 reside in deferent localizations in the microenvironment of the terminal web. These observations are in line with the Pdzk1−/− mice where Muc3(17) resided in intracellular vesicles in the small intestine. The CCh-induced endocytosis of MUC17 observed in Caco-2 cells was also valid in mouse duodenum, where Muc3(17) was internalized in response to CCh. Interestingly, MUC3 and MUC12 did not undergo CCh-induced endocytosis. This finding could be explained by our previous observation that PDZK1 only entraps MUC17CT and not MUC3CT and MUC12CT, despite the fact that all three intestinal transmembrane mucins harbor a class I PDZ motif (33). MUC17CT contains at least one additional amino acid sequence motifs that relates to the mentioned results. Besides the class I PDZ motif, MUC17CT also contains a putative binding site for AP-2, an adaptor protein responsible for linking cargo to clathrin-coated pits during clathrin-mediated endocytosis (45), explaining the inhibitory effect of the dynamin-inhibitor Dynasore on CCh-induced endocytosis of MUC17.
In this study, MUC17 endocytosis occurred as NHE3 was removed from the apical membrane and as CFTR was recruited to the apical surface of Caco-2 cells. As protein relocation took place 20–30 min following CCh stimulation, we conclude that the observed altered expression patterns of MUC17, NHE3, and CFTR in response to CCh were the outcome of regulated intracellular trafficking and neither de novo protein synthesis nor degradation. A previous study on the effect of CCh on Cftr and Nhe3 distribution in the rat small intestine revealed that the ion channel and the exchanger display opposite distribution patterns in response to CCh stimulation (18). While Nhe3 is removed from the cell surface, subapical pools of Cftr are mobilized to apical membranes. Recently, it was suggested that CFTR is important for providing sufficient amounts of bicarbonate for removing Ca2+ and proper unfolding of gel-forming mucins (13). The lack of a functional CFTR channel results in mucus that is anchored to the epithelium due to dysfunctional mucin expansion, a phenomenon that can explain the distal intestinal obstruction syndrome observed in cystic fibrosis patients. Importantly, MUC2 mucin secretion in the absence of a CFTR channel can be normalized by high bicarbonate concentration at the luminal side of cells. As NHE3 is exchanging luminal Na+ for H+, it will neutralize luminal HCO3− and by this action block the necessary contribution of CFTR-mediated ion secretion to proper mucin secretion. Consequently, when secretory responses facilitated by CFTR are required, NHE3 has to be removed from the surface membrane and its action downregulated. This supports this study as it indicates opposite traffic patterns of CFTR and NHE3 in response to CCh stimulation that concomitantly induce goblet cell mucin secretion.
Our observations revealed that a majority of CFTR was localized below the terminal web under basal conditions and that the amount of CFTR expressed in the microvillar region increased by ∼18% as CFTR was recruited to the apical membrane following CCh stimulation. Is should also be pointed out that the CFTR channel is also opened by phosphorylation by this stimulation (42). We could also show that CCh stimulation resulted in elevated levels of CFTR in the terminal web-microvilli-interface, suggesting that CFTR resides at the base of the microvilli when recruited to the apical membrane.
Fluid secretion and mucus discharge into the intestinal lumen are likely to be important mechanism for clearance of bacteria and viruses. Our results indicate that fluid and Muc2 mucin secretion accompany CCh-induced endocytosis of Muc3(17) in enterocytes. The reason for this coupling between transmembrane mucin Muc3(17) and the gel-forming mucin Muc2 is not understood, as the physiological function of transmembrane mucins remains unclear. One possible explanation is to facilitate diffusion of secreted bicarbonate ions by limiting the number of molecules that act as a diffusion barrier in the apical glycocalyx. However, such a model requires all three transmembrane mucins to respond to secretory stimulus in the same manner. As MUC3 and MUC12 were not internalized, one should speculate of a more specific function for MUC17. The PDZ interactions related to CTs of transmembrane mucins are diverse, which argue for a sensory or signaling function associated with transmembrane mucin MUC17 (15, 30). As the overlying mucus layer in the small intestine is penetrable to bacteria, the densely O-glycosylated mucin domain of MUC17 is a potential attachment site for bacteria that are in close contact with the epithelial surface. In a scenario where bacteria induce secretion, i.e., membrane recruitment of CFTR and NHE3 endocytosis, the internalization of MUC17 could function as a means for enterocytes to sample luminal bacterial to remove these or modulate immune responses. Meanwhile, induced secretion via CFTR is responsible for clearance of residual bacteria in the proximity of the epithelial surface. Interestingly, a signal transduction machinery that is activated upon bacterial binding has been proposed for the transmembrane mucin MUC1 in lung epithelia, where Pseudomonas aeruginosa bacterial cells or flagellin induce serine and tyrosine phosphorylation on MUC1CT (29). Further studies on the role and function of transmembrane mucins are required for a deeper understanding of how the outermost molecules of the enterocyte, the transmembrane mucins, function.
In summary, this study demonstrates a close relationship between goblet cell mucin secretion and enterocyte transmembrane mucins and ion channels or exchangers that maintain intestinal mucus-associated protection and homeostasis.
GRANTS
This work was supported by the Swedish Research Council (no. 7461, 21027), Swedish Cancer Foundation, Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital (LUA-ALF), Wilhelm and Martina Lundgren's Foundation, Torsten och Ragnar Söderbergs Stiftelser, Sahlgrenska Academy, National Institute of Allergy and Infectious Diseases (U01AI095473, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health), and Swedish Foundation for Strategic Research–Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program, Swedish CF Foundation, Erica Lederhausen's Foundation, and Lederhausen's Center for CF Research at the University of Gothenburg.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.P. and G.C.H. conception and design of research; T.P., J.K.G., I.J.G., and A.E. performed experiments; T.P., J.K.G., I.J.G., A.E., and G.C.H. analyzed data; T.P., J.K.G., and G.C.H. interpreted results of experiments; T.P. prepared figures; T.P. and G.C.H. drafted manuscript; T.P. and G.C.H. edited and revised manuscript; T.P., J.K.G., I.J.G., A.E., and G.C.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Centre for Cellular Imaging and the Electron Microscopy Unit at the University of Gothenburg for technical help. We thank Dr. John R. Riordan for contributing with antibodies against CFTR.
REFERENCES
- 1.Ambort D, Johansson MEV, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, Koeck PJB, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA 109: 5645–5650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axelsson MA, Karlsson NG, Steel DM, Ouwendijk J, Nilsson T, Hansson GC. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology 11: 633–644, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Barlow AL, MacLeod A, Noppen S, Sanderson J, Guérin CJ. Colocalization analysis in fluorescence micrographs: verification of a more accurate calculation of Pearson's correlation coefficient. Microsc Microanal 16: 710–724, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Bustamante C, Marko JF, Siggia ED, Smith S. Entropic elasticity of lambda-phage DNA. Science 265: 1599–1600, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Carlstedt I, Herrmann A, Karlsson H, Sheehan J, Fransson LA, Hansson GC. Characterization of two different glycosylated domains from the insoluble mucin complex of rat small intestine. J Biol Chem 268: 18771–18781, 1993 [PubMed] [Google Scholar]
- 6.Chen M, Sultan A, Cinar A, Yeruva S, Riederer B, Singh AK, Li J, Bonhagen J, Chen G, Yun C, Donowitz M, Hogema B, deJonge H, Seidler U. Loss of PDZ-adaptor protein NHERF2 affects membrane localization and cGMP- and [Ca2+]- but not cAMP-dependent regulation of Na+/H+ exchanger 3 in murine intestine. J Physiol 588: 5049–5063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng J, Moyer BD, Milewski M, Loffing J, Ikeda M, Mickle JE, Cutting GR, Li M, Stanton BA, Guggino WB. A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J Biol Chem 277: 3520–3529, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cheng J, Wang H, Guggino WB. Modulation of mature cystic fibrosis transmembrane regulator protein by the PDZ domain protein CAL. J Biol Chem 279: 1892–1898, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Delie F, Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Cri Rev Ther Drug Carrier Syst 14: 221–286, 1997 [PubMed] [Google Scholar]
- 10.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol 57: 607–634, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Gerken TA, Butenhof KJ, Shogren R. Effects of glycosylation on the conformation and dynamics of O-linked glycoproteins: carbon-13 NMR studies of ovine submaxillary mucin. Biochemistry 28: 5536–5543, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Gum JR, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun 291: 466–475, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, Hebert H, Sjövall H, Hansson GC. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 209: 1263–1272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustafsson JK, Ermund A, Johansson MEV, Schütte A, Hansson GC, Sjövall H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol 302: G430–G438, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4: 45–60, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hung AY, Sheng M. PDZ domains: structural modules for protein complex assembly. J Biol Chem 277: 5699–5702, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Ito S. Structure and function of the glycocalyx. Fed Proc 28: 12–25, 1969 [PubMed] [Google Scholar]
- 18.Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol Gastrointest Liver Physiol 300: G82–G98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jelen F, Oleksy A, Smietana K, Otlewski J. PDZ domains–common players in the cell signaling. Acta Biochim Pol 50: 985–1017, 2003 [PubMed] [Google Scholar]
- 20.Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A, Subramani DB, Holmen-Larsson JM, Thomsson KA, Bergstrom JH, van der Post S, Rodriguez-Pineiro AM, Sjovall H, Backstrom M, Hansson GC. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 68: 3635–3641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kartner N, Augustinas O, Jensen TJ, Naismith AL, Riordan JR. Mislocalization of delta F508 CFTR in cystic fibrosis sweat gland. Nat Genet 1: 321–327, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Lee-Kwon W, Park JB, Ryu SH, Yun CH, Donowitz M. Ca2+-dependent inhibition of Na+/H+ exchanger 3 (NHE3) requires an NHE3–E3KARP-α-actinin-4 complex for oligomerization and endocytosis. J Biol Chem 277: 23714–23724, 2002 [DOI] [PubMed] [Google Scholar]
- 23.LaLonde DP, Garbett D, Bretscher A. A regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization. Mol Biol Cell 21: 1519–1529, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci USA 104: 16209–16214, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee-Kwon W, Kim JH, Choi JW, Kawano K, Cha B, Dartt DA, Zoukhri D, Donowitz M. Ca2+-dependent inhibition of NHE3 requires PKCα which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol 285: C1527–C1536, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Levy E, Mehran M, Seidman E. Caco-2 cells as a model for intestinal lipoprotein synthesis and secretion. FASEB J 9: 626–635, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Li X, Zhang H, Cheong A, Leu S, Chen Y, Elowsky CG, Donowitz M. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol 556: 791–804, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ligtenberg MJ, Kruijshaar L, Buijs F, van Meijer M, Litvinov SV, Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J Biol Chem 267: 6171–6177, 1992 [PubMed] [Google Scholar]
- 29.Lillehoj EP, Kim H, Chun EY, Kim KC. Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. Am J Physiol Lung Cell Mol Physiol 287: L809–L815, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Macao B, Johansson DG, Hansson GC, Hard T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat Struct Mol Biol 13: 71–76, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a Cell-Permeable Inhibitor of Dynamin. Dev Cell 10: 839–850, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Malmberg EK, Noaksson KA, Phillipson M, Johansson ME, Hinojosa-Kurtzberg M, Holm L, Gendler SJ, Hansson GC. Increased levels of mucins in the cystic fibrosis mouse small intestine, and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol 291: G203–G210, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Malmberg EK, Pelaseyed T, Petersson AC, Seidler UE, De Jonge H, Riordan JR, Hansson GC. The C-terminus of the transmembrane mucin MUC17 binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine. Biochem J 410: 283–289, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Meunier V, Bourrie M, Berger Y, Fabre G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol Toxicol 11: 187–194, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Moyer BD, Duhaime M, Shaw C, Denton J, Reynolds D, Karlson KH, Pfeiffer J, Wang S, Mickle JE, Milewski M, Cutting GR, Guggino WB, Li M, Stanton BA. The PDZ-interacting domain of cystic fibrosis transmembrane conductance regulator is required for functional expression in the apical plasma membrane. J Biol Chem 275: 27069–27074, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Shibata N, Nishimoto-Shibata T, Feng D, Ikemoto M, Motojima K, Iso-o N, Tsukamoto K, Tsujimoto M, Arai H. Regulation of SR-BI protein levels by phosphorylation of its associated protein, PDZK1. Proc Natl Acad Sci USA 102: 13404–13409, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandey P, Kharbanda S, Kufe D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res 55: 4000–4003, 1995 [PubMed] [Google Scholar]
- 38.Parry S, Silverman HS, McDermott K, Willis A, Hollingsworth MA, Harris A. Identification of MUC1 proteolytic cleavage sites in vivo. Biochem Biophys Res Commun 283: 715–720, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Pelaseyed T, Hansson GC. CFTR anion channel modulates expression of human transmembrane mucin MUC3 through the PDZ protein GOPC. J Cell Sci 124: 3074–3083, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Rambourg A, Neutra M, Leblond CP. Presence of a “cell coat” rich in carbohydrate at the surface of cells in the rat. Anat Rec 154: 41–71, 1966 [DOI] [PubMed] [Google Scholar]
- 41.Ren J, Raina D, Chen W, Li G, Huang L, Kufe D. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res 4: 873–883, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem 77: 701–726, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Sarker R, Valkhoff VE, Zachos NC, Lin R, Cha B, Chen TE, Guggino S, Zizak M, de Jonge H, Hogema B, Donowitz M. NHERF1 and NHERF2 are necessary for multiple but usually separate aspects of basal and acute regulation of NHE3 activity. Am J Physiol Cell Physiol 300: C771–C782, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh AK, Riederer B, Krabbenhoft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest 119: 540–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol 163: 203–208, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Ham M, Hendriks W. PDZ domains-glue and guide. Mol Biol Rep 30: 69–82, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Wen Y, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J Biol Chem 278: 38029–38039, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J Biol Chem 272: 12492–12494, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1–G11, 1995 [DOI] [PubMed] [Google Scholar]
- 50.Zachos NC, Li X, Kovbasnjuk O, Hogema B, Sarker R, Lee LJ, Li M, de Jonge H, Donowitz M. NHERF3 (PDZK1) contributes to basal and calcium inhibition of NHE3 activity in Caco-2BBe cells. J Biol Chem 284: 23708–23718, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]