Abstract
Saturated fatty acids activate the c-Jun NH2-terminal kinase (JNK) pathway, resulting in chronic low-grade inflammation and the development of insulin resistance. Mixed-lineage kinase 3 (MLK3) is a mitogen-activated protein kinase kinase kinase (MAP3K) that mediates JNK activation in response to saturated fatty acids in vitro; however, the exact mechanism for diet-induced JNK activation in vivo is not known. Here, we have used MLK3-deficient mice to examine the role of MLK3 in a saturated-fat diet model of obesity. MLK3-KO mice fed a high-fat diet enriched in medium-chain saturated fatty acids for 16 wk had decreased body fat compared with wild-type (WT) mice due to increased energy expenditure independently of food consumption and physical activity. Moreover, MLK3 deficiency attenuated palmitate-induced JNK activation and M1 polarization in bone marrow-derived macrophages in vitro, and obesity induced JNK activation, macrophage infiltration into adipose tissue, and expression of proinflammatory cytokines in vivo. In addition, loss of MLK3 improved insulin resistance and decreased hepatic steatosis. Together, these data demonstrate that MLK3 promotes saturated fatty acid-induced JNK activation in vivo and diet-induced metabolic dysfunction.
Keywords: mixed-lineage kinase 3, saturated fatty acids, insulin resistance, inflammation
obesity is a major health problem that has been associated with the development of metabolic syndrome, nonalcoholic fatty liver disease, type 2 diabetes, and cardiovascular disease (13, 14). In addition to genetic predisposition and sedentary lifestyle, chronic consumption of a diet high in fat is the leading contributor to the development of obesity. Understanding the link between high-fat diet-induced obesity and disease development is key to the treatment of obesity-related health problems (11). Low-grade inflammation is emerging as an important pathogenic link between disease development and obesity (43). Chronic low-grade inflammation is characterized by an increased number of adipose tissue macrophages (42) and conversion from alternatively activated M2 macrophages to classically activated M1 macrophages (26), with a concomitant increase in plasma levels of proinflammatory cytokines such as TNFα, IL-1β, and IL-6 (16). Proinflammatory cytokines activate inflammatory pathways such as the c-Jun NH2-terminal kinase (JNK) pathway (8). JNK is known to regulate insulin resistance directly by phosphorylating insulin receptor substrate-1 (IRS-1) at an inhibitory site that can block signal transduction by the insulin receptor (1) and indirectly by regulating cytokine secretion in immune cells (17). Jnk1−/− mice are protected against obesity-induced insulin resistance (18), and tissue-specific deletion of JNK1/2 in macrophages (17) is sufficient to inhibit the development of diet-induced insulin resistance.
It has become clear that, in addition to total fat content, fatty acid composition plays an important role in metabolic dysfunction (25). Saturated fatty acids are less efficiently oxidized than unsaturated fatty acids, and therefore, they are more obesogenic (9, 24). Furthermore, saturated fatty acids have a strong diabetogenic effect, whereas unsaturated fatty acids are protective and can even reverse obesity-induced insulin resistance (31, 40). Similarly, medium-chain (C6–C12) saturated fatty acids (MCSFAs) have been reported to induce higher energy expenditure and fatty acid oxidation compared with long-chain saturated fatty acids (LCFAs), and this is associated with lower adipose mass (38, 39). However, despite different effects on fat accumulation, both MCSFAs and LCFAs have been shown to induce insulin resistance to a similar extent (19). The exact mechanism by which saturated fatty acids cause insulin resistance is unclear. It has been shown that saturated fatty acids serve as ligands for Toll-like receptor (TLR)-2 and TLR-4, resulting in NF-κB and JNK activation (29, 36). Moreover, it has been suggested that saturated fatty acid incorporation reduces membrane fluidity, resulting in ER stress and subsequent JNK activation (30). In addition, it has been shown that saturated fatty acids cause partitioning and activation of c-Src within membrane subdomains, leading to JNK activation (20). Previously, we identified MLK3 as mediator of saturated fatty acid-induced JNK activation in vitro (21, 35); however, the exact mechanism for diet-induced JNK activation in vivo is not known. MLK3 is a ubiquitously expressed member of mixed-lineage kinases (MLK1–4), a family of serine/threonine kinases that have been implicated in the regulation of multiple MAPK pathways, including the extracellular signal-regulated kinase (ERK), JNK, and the p38 MAPK pathways (15). MLK3-deficient mice are viable and have no overt metabolic phenotype on regular chow diet (21). MLK3 deficiency has no effect on obesity or glucose homeostasis in a lard-based high-fat diet model (21). However, lard contains a mixture of saturated and mono- and polyunsaturated fatty acids, and it has been demonstrated that mono- and polyunsaturated fatty acids inhibit saturated fatty acid-induced ER stress and JNK activation (2, 10). To test the role of MLK3 in a saturated fatty acid-rich diet model, we have used hydrogenated coconut oil in this study. Coconut oil is highly enriched in saturated fatty acids (>90%), although the vast majority are MCSFAs rather than LCFAs. Interestingly, MCSFAs, through activation of GPR84, have been suggested to provide a link between inflammation and metabolic disorders (41). Furthermore, although LCFAs such as palmitate are the most potent activators of JNK, MCSFAs such as lauric acid have been shown to induce JNK activation, albeit more moderately (37). MLK3-knockout (KO) mice fed a high-fat diet enriched in MCSFA for 16 wk had attenuated JNK activation in visceral adipose tissue, decreased adiposity, and reduced adipose tissue inflammation accompanied by improved glucose tolerance and decreased hepatic steatosis. Together, these data demonstrate that MLK3 promotes saturated fatty acid-induced JNK activation in vivo as well as obesity, adipose tissue inflammation, insulin resistance, and steatosis.
MATERIALS AND METHODS
Mice.
Mlk3−/− mice were back-crossed 10 generations to the C57BL/6J strain (Jackson Laboratories) and have been described previously (5). Mlk3−/− mice and C57BL/6J mice were obtained from homozygous crosses and were housed in a facility accredited by the American Association for Laboratory Animal Care. The animal studies were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. Starting at 10 wk of age, male mice were fed a high-fat diet ad libitum (Diet D12331: 16% protein, 58% saturated fat, and 26% carbohydrate; Research Diets) or a standard diet for 16 wk, and their body mass was recorded weekly. Food intake, energy expenditure, respiratory exchange ratio, and physical activity were measured using metabolic cages (TSE System). Mice were acclimated to the metabolic cages for 24 h and analyzed for 72 h. Data were collected every 10 min and represented an average of three consecutive light and dark phases. Whole body composition (fat and lean mass) was measured by MRI (EchoMRI-100; Echo Medical Systems, Houston, TX). Body temperature was measured once, using a rectal probe (KE OP-TS). Glucose tolerance tests were performed after 16 wk on a high-fat diet after a 12-h overnight fast by intraperitoneal injection of 1.5 g/kg glucose, as described previously (21). Tissues were removed and rapidly frozen in liquid nitrogen for biochemical analysis.
Measurement of blood glucose, insulin, cytokine, and catecholamine levels.
Following a 12-h overnight fast, plasma was collected to measure blood glucose using a FreeStyle Lite glucometer (Abott) and insulin concentrations [measured by ELISA (Millipore) by the Mouse Metabolic Phenotype Center at the University of Cincinnati]. Monocyte chemoattractant protein-1 (MCP-1) and IL-6 were measured by ELISA (R & D Systems) according to the manufacturer's instructions. Adrenaline and noradrenaline were measured by ELISA (Rocky Mountains Diagnostics) according to the manufacturer's instructions.
Analysis of tissue sections.
Histology was performed using tissue fixed in 10% formalin, dehydrated, and embedded in paraffin. Sections were stained with hematoxylin and eosin. For quantification of crown-like structures (CLS), paraffin sections were stained with antibody to F4/80 (Abcam), treated with a biotinylated secondary antibody followed by incubation with avidin-biotin complex (Vectastain Elite ABC; Vector Laboratories), and visualized with ImmPACT DAB peroxidase substrate. Slides were counterstained with hematoxylin. Images of three representative fields per slide were taken in a blinded fashion. F4/80-positive CLS were counted, and the percentage of CLS compared with total adipocyte number was used as quantification of adipose tissue macrophage content.
Immunoblot analysis.
Tissue extracts were prepared as described previously (21). Extracts were examined by immunoblot analysis using antibodies to JNK and phospho-JNK (Cell Signaling Technology). Quantifications were performed using NIH Image J.
Bone marrow macrophages.
Bone marrow-derived macrophages were prepared as described (46) and cultured in DMEM-F-12 supplemented with 10% fetal bovine serum and 20% L929 supernatant. Polarization was induced after 7 days in culture using 20 ng/ml INF-γ and 10 ng/ml LPS or 50 ng/ml IL-4. For some experiments, macrophages were treated with 1 mM palmitate complexed with BSA as described (21).
RNA analysis.
RNA was prepared using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA was reverse transcribed using the iScript cDNA synthesis kit from Bio-Rad (Bio-Rad Life Science Research, Hercules, CA). TNFα (ATCCGCGACGTGGAACTG and ACCGCCTGGAGTTCTGGAA), MCP-1 (CCTCCTCCACCACCATGCA and CCAGCCGGCAACTGTGA), F4/80 (TGTCTGACAATTGGGATCTGCCCT and ATACGTTCCGAGAGTGTTGTGGCA), inducible nitric oxide synthase (iNOS; TTCACCCAGTTGTGCATCGACCTA and TCCATGGTCACCTCCAACACAAGA), arginase (ACCTGGCCTTTGTTGATGTCCCTA and AGAGATGCTTCCAACTGCCAGACT), PPARγ (TGTGGGGATAAAGCATCAGGC and CCGGCAGTTAAGATCACACCTA), and fibroblast growth factor 21 (FGF21; CTACCAAGCATACCCCATCC and GCCTACCACTGTTCCATCCT) gene expression was determined by quantitative RT-PCR analysis using the Bio-Rad iCycler iQ real-time PCR Detection System and normalized to the expression of cyclophilin (TCATGTGCCAGGGTGGTGAC) and (CCATTCAGTCTTGGCAGTGC). Taqman assays were used to quantitate stearoyl-CoA desaturase-1(Scd1; Mm00772290_m1) and carnitine palmitoyltransferase I (CPT I) (Mm00487200_1m) and normalized to the expression of actin (Mm00607939_s1) using Taqman assays (Applied Biosystems).
Adipose tissue explants.
After 16 wk on the diet, epididymal tissues were surgically removed, finely minced in PBS containing 5 mM glucose and 100 nM N6-phenylisopropyladenosine, and washed three times through a 100-μm nylon mesh. Tissue pieces (50 mg) were incubated in 1 ml of M199 medium containing 0.2% BSA and 7 nM insulin. Media was collected after 24 h for cytokine measurements by ELISA (R & D Systems).
Statistical analysis.
Statistical analysis was performed using the Student t-test or by two-way ANOVA followed by Bonferroni post hoc analysis. All data are expressed as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Effects of MLK3 deficiency on adiposity and insulin resistance induced by saturated fatty acid-enriched diet.
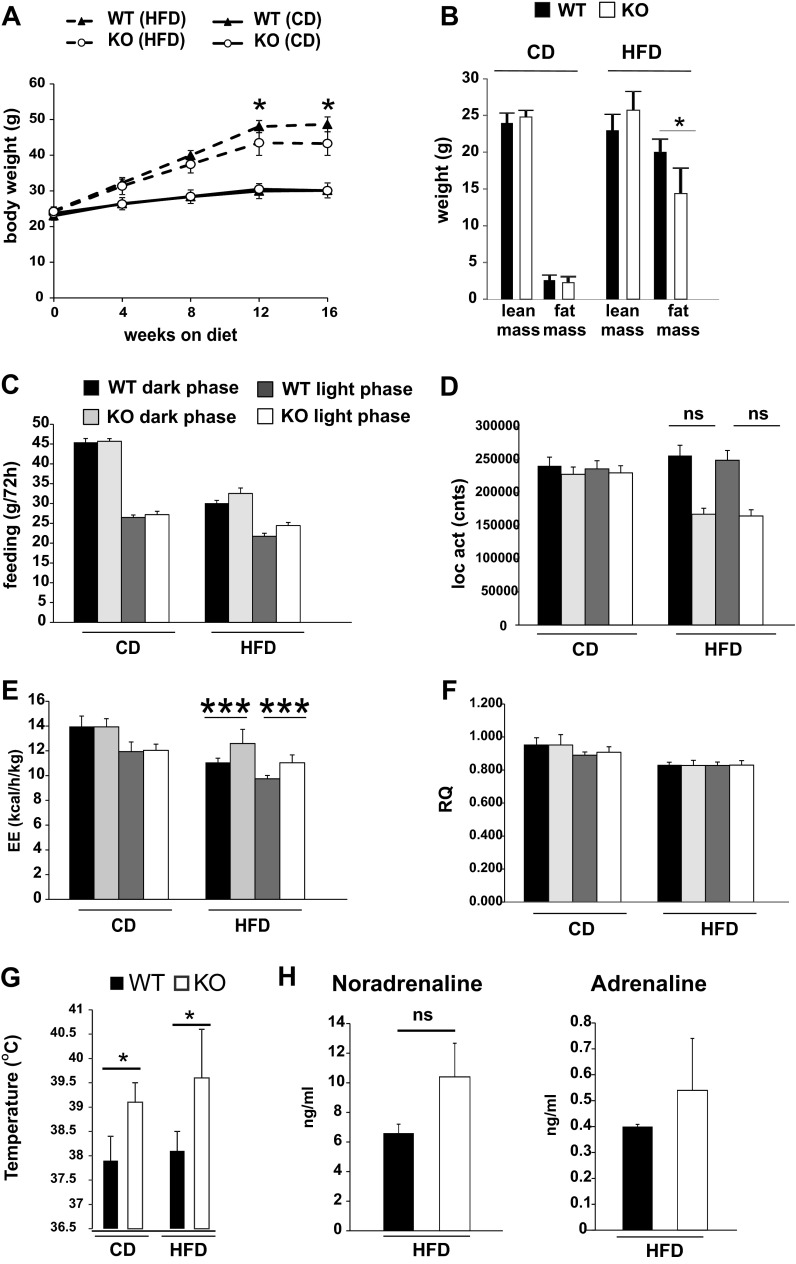
To test the role of MLK3 in metabolic signaling in vivo, we fed MLK3-KO mice a high-fat diet enriched in MCSFAs for 16 wk. We observed that MCSFA-induced weight gain in MLK3-KO mice compared with WT mice was moderately decreased (Fig. 1A). Body composition analysis indicated that this decrease was due to reduced accumulation of adipose tissue without a change in lean mass (Fig. 1B). In contrast, body composition of WT and KO animals on the control diet was comparable (Fig. 1B). No differences in adipocyte size were observed in WT or Mlk3−/− mice (Fig. 4A and data not shown), suggesting that the difference in fat mass was accounted for primarily by cell number.
Fig. 1.
Loss of mixed-lineage kinase 3 (MLK3) reduces diet-induced body weight gain and adiposity. A: wild-type (WT) and MLK3-knockout (KO) mice were maintained on chow (CD) or high-fat diet (HFD) for 16 wk. Body weight was measured monthly. B: after 16 wk on diet, lean and fat mass were determined by MRI. Data are means ± SE; n = 8. *P < 0.05. C–F: CD and HFD mice were monitored for 3 days to measure food consumption (C), physical activity (D), energy expenditure (E), and respiratory quotient (F). Data are means ± SE; n = 8. G: body temperature of WT and MLK3-KO mice is presented. Data are means ± SE; n = 8. H: plasma noradrenaline and adrenaline concentrations were measured on HFD. Data are means ± SE; n = 8. *P < 0.05; ***P < 0.001. NS, not significant.
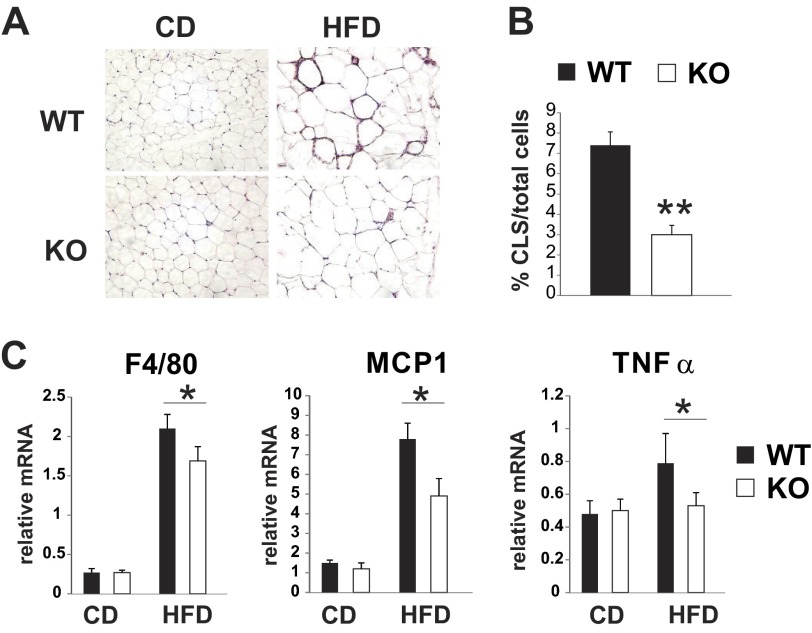
Fig. 4.
MLK3 deficiency reduces macrophage infiltration in visceral adipose tissue. A: representative images of hematoxylin and eosin-stained epididymal fat sections from WT and MLK3-KO mice. B: no. of crown-like structures (CLS) in epididymal fat sections from HFD-fed mice was calculated as %total cells. More than 1,000 cells/section were counted. Data are means ± SD; n = 8 mice. C: expression of F4/80, MCP-1, and TNFα was measured by quantitative RT-PCR analysis normalized to the expression of cyclophilin. Data are means ± SE; n = 8. **P < 0.01; *P < 0.05.
The decreased adiposity of MLK3-deficient mice may be due to decreased food consumption or increased energy expenditure. To determine the mechanism of altered energy balance in MLK3-KO mice, we performed metabolic cage analysis. Food intake was comparable in WT and Mlk3−/− mice (Fig. 1C), whereas there was a trend toward decreased locomotor activity in MLK3-deficient mice fed a high-calorie diet (Fig. 1D). The phenotype of decreased weight gain despite normal food intake in MLK3-deficient mice suggests that there must be an increase in whole body energy expenditure. To explore this possibility, we examined the metabolic rate by indirect calorimetry. The analysis of the data using two-way ANOVA revealed a significant diet-genotype interaction in the high-fat diet group (diet, P < 0.0001; genotype, P = 0.0059; diet-genotype interaction, P = 0.0081). The post hoc test detected a significant difference between WT and MLK-deficient mice only in the high-fat diet-fed group (Fig. 1A). When the data from the high-fat diet-fed groups were analyzed considering the light-dark period, we detected a significant increase in energy expenditure in MLK3-deficient mice compared with WT mice during both the light and dark phases (light-dark phase, P < 0.0001; genotype, P = 0.0016 by ANOVA). P values remained significant after Bonferroni correction for multiple comparisons (Fig. 1E). Metabolic fuel source analyzed by respiratory quotient was comparable between the genotypes (Fig. 1F). In addition, body temperature was increased significantly in MLK3-deficient mice compared with WT mice independent of the diet (Fig. 1G). The sympathetic nervous system, including the sympathoadrenal system, plays a critical role in the regulation of thermogenesis (23). Therefore, we measured circulating levels of noradrenaline and adrenaline, the main effectors of the sympathetic nervous system and adrenal medulla. We found a moderate, albeit not significant, increase of both catecholamines in plasma of MLK3-deficient mice compared with WT mice (Fig. 1H).
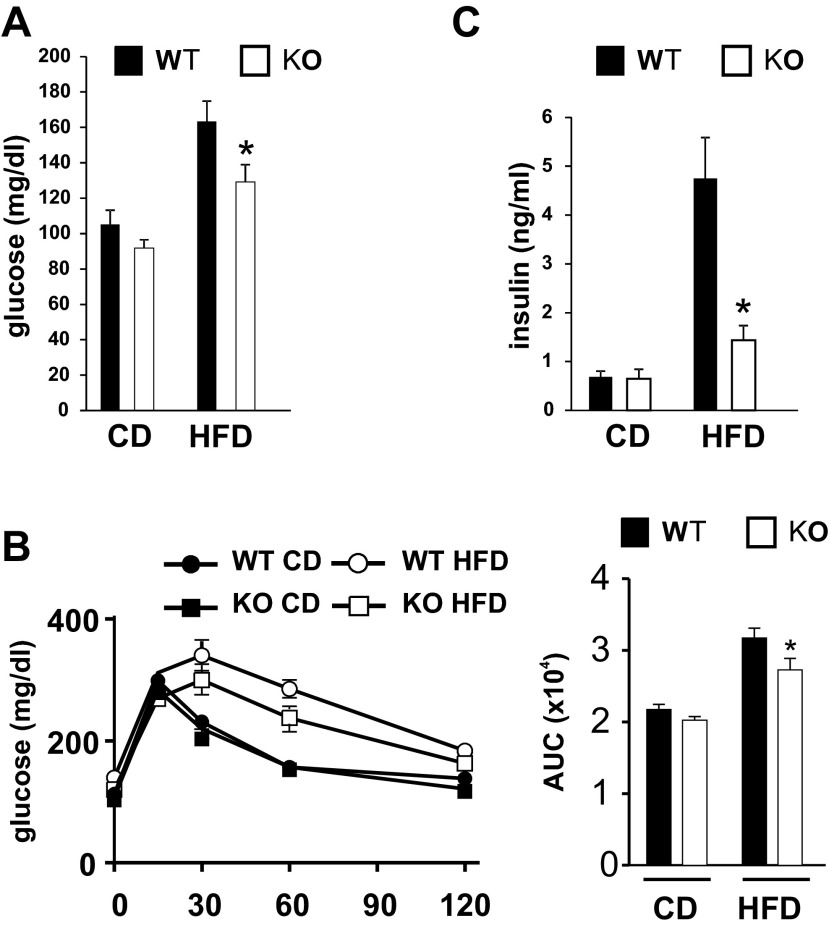
Obesity is often associated with insulin resistance, and the JNK pathway has been causally linked to the development of insulin resistance (18). Therefore, we hypothesized that MLK3 deficiency results in improved glucose homeostasis. Indeed, we found that fasting blood concentrations were reduced significantly in obese Mlk3−/− mice compared with WT control animals (Fig. 2A). Consistent with these observations, MCSFA-fed MLK3-KO mice were more glucose tolerant compared with WT mice (Fig. 2B), as measured by glucose tolerance test. In addition, hyperinsulinemia caused by MCSFA feeding was significantly reduced in Mlk3−/− mice (Fig. 2C). Together, these data indicate that MLK3 promotes the development of diet-induced insulin resistance.
Fig. 2.
Loss of MLK3 improves diet-induced insulin resistance. A: blood glucose concentration of WT and MLK3-KO mice on CD and HFD were measured after an overnight fast. B: glucose tolerance tests were performed by measuring blood glucose concentrations following intraperitoneal injection of glucose (1.5 g/kg). Area under the curve (AUC) was calculated (right). C: insulin concentration of WT and MLK3-KO mice on CD and HFD were measured after an overnight fast. Data are means ± SE; n = 8. *P < 0.05.
Effect of MLK3 deficiency on inflammation.
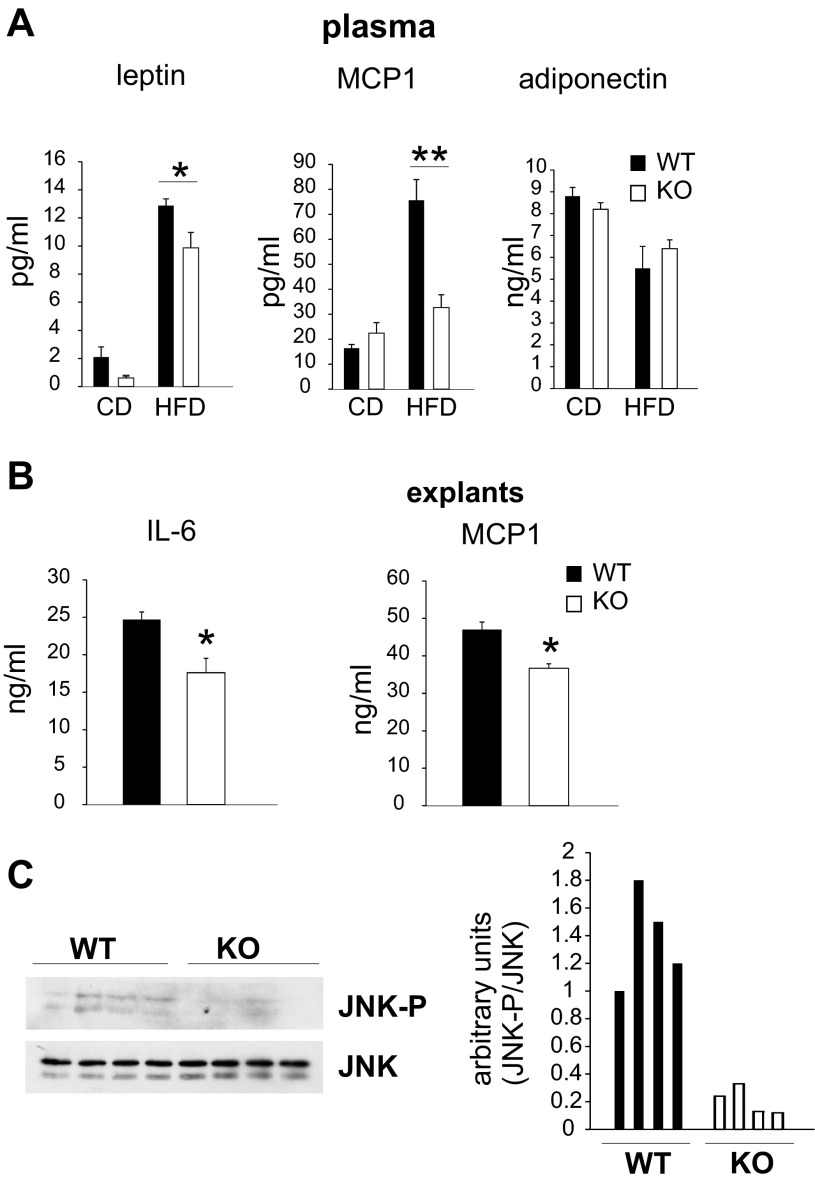
One factor that is commonly associated with adiposity and insulin resistance is low-grade systemic inflammation (43). Therefore, we measured circulating inflammatory markers and adipose tissue-derived adipokines with known roles in obesity and insulin action. Plasma leptin levels were significantly decreased in MCSFA-fed MLK3-KO compared with WT mice (Fig. 3A, left), and MCP-1 levels were increased in WT mice fed a MCSFA-enriched diet but significantly attenuated in MLK3-deficient mice (Fig. 3A, middle). In contrast, MLK3 deficiency did not affect blood concentrations of adiponectin (Fig. 3A, right), an adipokine shown to reverse insulin resistance (4, 45). It has been shown that expression of MCP-1 is regulated by IL-6 (12). Since plasma concentration of the proinflammatory cytokines IL-6 and TNFα were below detection level in our model, and adipocytes play a primary role in obesity-induced IL-6 expression (28), we isolated explants of epididymal adipose tissue from WT and Mlk3−/− mice. We found that fat explants from MLK3-deficient mice released significantly less IL-6 and MCP-1 than explants from WT mice (Fig. 3B). Expression of JNK1 in adipocytes has been linked to regulation of IL-6 secretion by adipose tissue (34). To determine the mechanism of reduced IL-6 secretion, we evaluated JNK phosphorylation in epididymal adipose tissue by immunoblot analysis. Indeed, we found that JNK phosphorylation was significantly decreased in MCSFA-fed MLK3-KO mice compared with WT mice (Fig. 3C).
Fig. 3.
MLK3 deficiency decreases secretion of inflammatory markers. A: plasma concentrations of leptin, monocyte chemoattractant protein-1 (MCP-1), and adiponectin were measured in CD and HFD mice. Data are means ± SE; n = 8. B: IL-6 and MCP-1 in conditioned medium were collected for 24 h from visceral fat explants. Data are means ± SE (n = 4) from 2 different experiments. C: phosphorylation and expression of JNK in epididymal fat pads was examined by immunoblot analysis. Quantification of immunoblot data is shown at right. *P < 0.05; **P < 0.01.
Since diet-induced inflammatory cytokines originate to a substantial degree from visceral adipose tissue, we next analyzed the inflammatory status of this fat depot in WT and MLK3-KO mice. Histological evaluation of epididymal fat revealed that MLK3-deficient mice fed a MCSFA-enriched diet had significantly decreased CLS compared with WT mice (Fig. 4, A and B). In addition, we found reduced expression of the macrophage-specific marker F4/80 (Fig. 4C), indicating that MLK3 expression modulates infiltration of macrophages into adipose tissue. Because low-grade inflammation associated with obesity is characterized by conversion of alternatively activated M2 to classically activated M1 macrophages (26), we analyzed adipose tissue gene expression. We observed that expression of markers associated with M1 polarization (MCP-1 and TNFα) was reduced (Fig. 4C), suggesting that M1/2 polarization of tissue macrophages is altered by MLK3 deficiency.
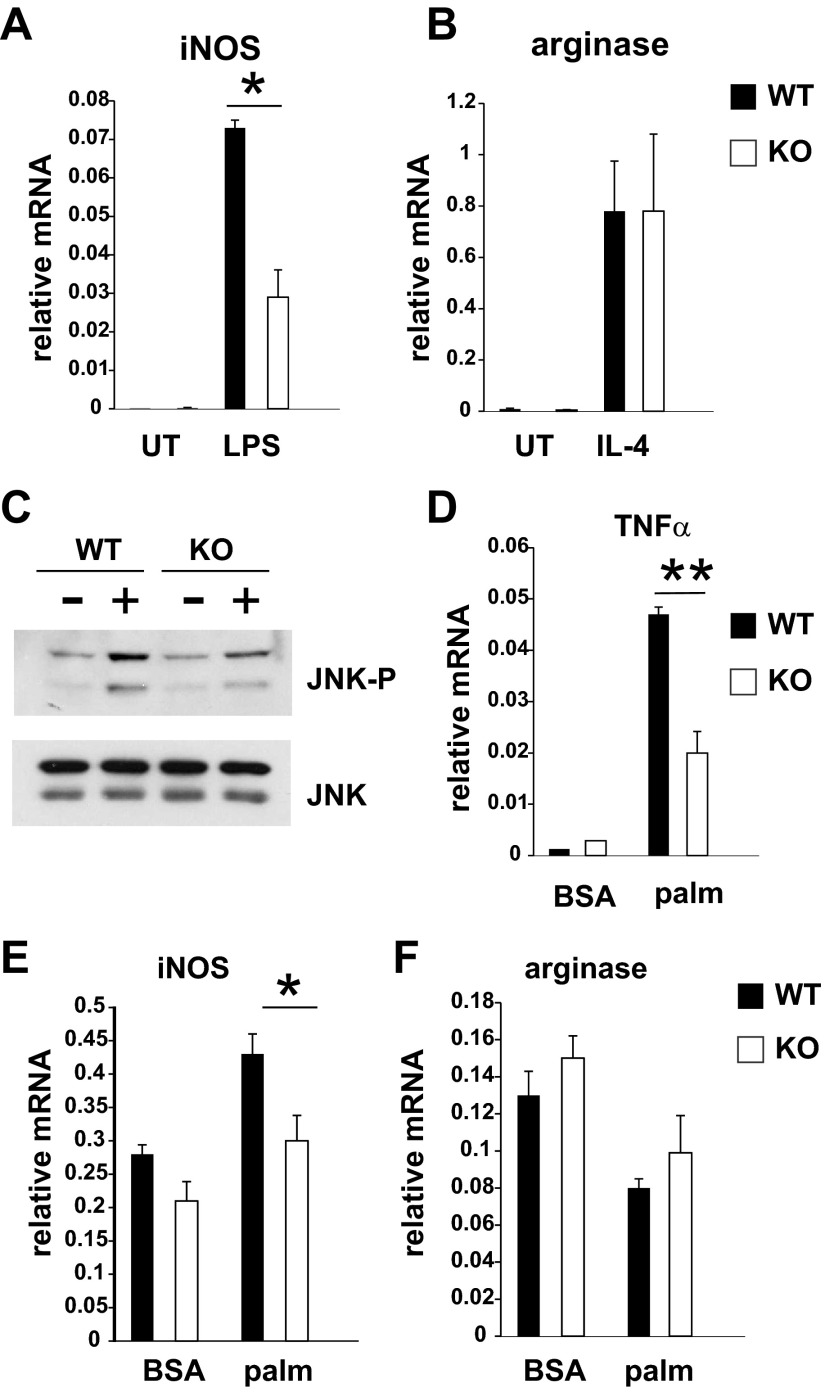
Altered adipose tissue macrophage polarization could be due to changes in adipocytes or to intrinsic defects in macrophages. To test the role of MLK3 for macrophage polarization in vitro, we isolated bone marrow-derived macrophages (BMDM) from WT and MLK3-deficient mice. Macrophages treated with lipopolysaccharide (LPS) polarize to an M1 phenotype characterized by high expression of iNOS and production of proinflammatory cytokines such as TNFα, IL-1β, and MCP-1, whereas macrophages treated with IL-4 polarize to an M2 phenotype characterized by increased expression of arginase (27). We found that loss of MLK3 decreased iNOS expression in LPS-stimulated macrophages without affecting arginase expression in IL-4-treated BMDM (Fig. 5, A and B). Previously, it was demonstrated that JNK is required for proinflammatory macrophage polarization. To determine the mechanism for altered macrophage polarization in MLK3 deficiency, we treated BMDM with palmitate. JNK phosphorylation (Fig. 5C), expression of TNFα (Fig. 5D), and iNOS (Fig. 5E), but not arginase (Fig. 5F), was attenuated in Mlk3−/− BMDM but not completely inhibited. Together these data indicate that MLK3 participates in saturated fatty acid-induced JNK activation and M1 polarization in macrophages. However, other MLK isoforms may also contribute to JNK activation and polarization in BMDM.
Fig. 5.
MLK3 deficiency alters macrophage polarization. A: bone marrow-derived macrophages (BMDM) were left untreated (UT) or were treated with 10 ng/ml lipopolysaccharide (LPS) for 24 h. Inducible nitric oxide synthase (iNOS) expression was measured by quantitative RT-PCR analysis normalized to the expression of cyclophilin. B: BMDM were left UT or were treated with 50 ng/ml IL-4 for 24 h. Arginase expression was measured by quantitative RT-PCR analysis normalized to the expression of cyclophilin. C: BMDM were treated with BSA or 1 mM palmitate (palm) for 6 h. A representative immunoblot of JNK phosphorylation (JNK-P) and expression from 3 independent experiments is shown. D–F: BMDM were treated with 1 mM palmitate for 24 h, and expression of TNFα, iNOS, and arginase was measured by quantitative RT-PCR analysis normalized to the expression of cyclophilin. Data are means ± SE from 3 independent experiments. *P < 0.05; **P < 0.01.
Effect of MLK3 deficiency on hepatic steatosis.
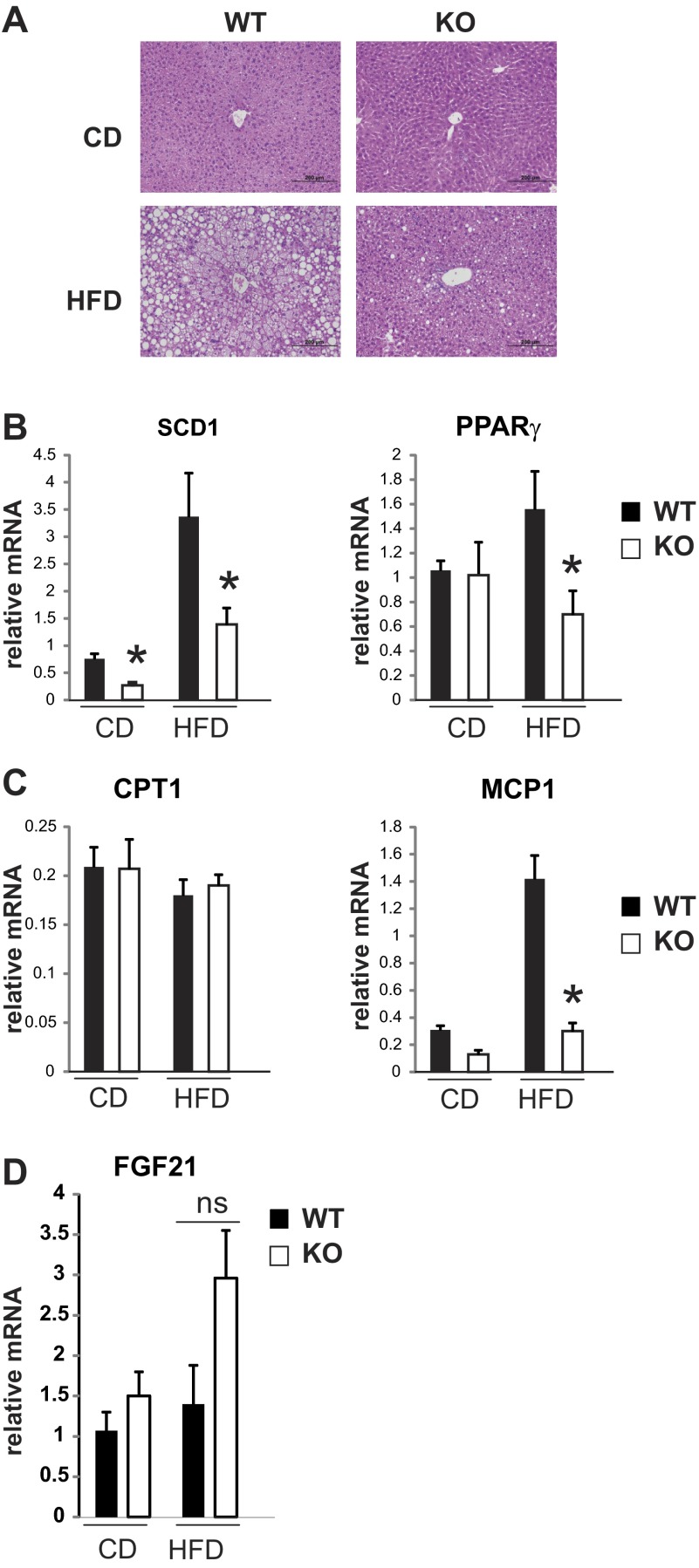
Interestingly, MCP-1 has been associated not only with the development of insulin resistance but also with the development of hepatic steatosis. Previously, it was shown that transgenic expression of MCP-1 in adipose tissue increased, whereas loss or inhibition of MCP-1 reduced hepatic steatosis (22). In addition, it was demonstrated that MCP-1 is capable of inducing triglyceride accumulation in hepatocytes in vitro in a time- and dose-dependent manner (6). Indeed, we found that diet-induced steatosis was significantly reduced in MLK3-deficient mice (Fig. 6A), which was associated with decreased expression of genes that promote lipogenesis, such as PPARγ and SCD1 (Fig. 6B). In contrast, genes that promote fatty acid oxidation, such as CPT I, were not different between WT and MLK3-KO mice (Fig. 6C).
Fig. 6.
MLK3 deficiency improves hepatic steatosis. A: hematoxylin and eosin stain of representative sections of WT and MLK3-KO livers fed HFD for 16 wk. B–D: expression of SCD1, PPARγ, MCP1, carnitine palmitoyltransferase I (CPT I), and fibroblast growth factor 21 (FGF21) was measured by quantitative RT-PCR analysis normalized to the expression of cyclophilin. Data are means ± SE; n = 8. *P < 0.05.
FGF21 has been identified recently as a metabolic hormone expressed predominantly in liver. Pharmacological administration of FGF21 or overexpression in liver of diet-induced obese mice reduced plasma glucose and insulin concentrations, improved insulin sensitivity, and decreased hepatic triglycerides (7, 44). Therefore, we measured FGF21 mRNA in liver. We observed a trend toward increased FGF21 expression in high-fat-fed MLK3-deficient mice; however, the data did not reach statistical significance (Fig. 6D). Thus, the decrease in diet-induced steatosis in MLK3-deficient mice may be due in part to decreased lipogenesis and MCP-1 expression.
DISCUSSION
Here, we used a MLK3-deficient mouse model to investigate the role of MLK3 in diet-induced metabolic stress signaling. We showed that MLK3-deficient mice have attenuated JNK activation in adipose tissue, decreased adiposity, improved insulin sensitivity, and decreased steatosis when fed a high-fat diet enriched in saturated fatty acids. In addition, we found that palmitate-induced JNK activation and expression of TNFα were decreased in Mlk3−/− BMDM. These observations are in contrast to our previous findings that showed that MLK3 deficiency did not affect obesity and glucose homeostasis in a high-fat diet model with similar fat content (60%) but different fatty acid composition (21). Interestingly, it has been demonstrated that the content of saturated fat, independently of total fat, profoundly affects adiposity, inflammation, macrophage behavior, and metabolic dysfunction (11). The diet used in this study contained hydrogenated coconut oil as primary fat, which contains more than 90% saturated fatty acids, whereas the diet used in our previous study was lard based, which contains about 40% saturated and 60% mono- and polyunsaturated fatty acids. Since we observed that mono- or polyunsaturated fatty acids inhibit saturated fatty acid-induced JNK activation in a dose-dependent manner (data not shown), it is possible that the differences in fatty acid composition may account for the different results. Nevertheless, our observations strongly support our hypothesis that MLK3 contributes to JNK activation in response to saturated fatty acids.
JNK1 deficiency or tissue-specific ablation of JNK1 in the central nervous system suppresses diet-induced obesity through decreased food intake, increased body temperature, increased physical activity, and increased energy expenditure that is associated with the activation of the hypothalamic-pituitary-thyroid axis (3, 32). In contrast, we did not observe increased thyroid activity (data not shown), decreased food intake, or increased locomotor activity (Fig. 1, C and D) in MLK3-deficient mice, suggesting no or a redundant function for MLK3 in regulating JNK in the central nervous system. Indeed, MLK1 and MLK2 are highly expressed in brain and may compensate for MLK3 deficiency. The mechanism by which MLK3 modulates energy expenditure is currently unclear. We found a trend toward higher circulating levels of adrenaline and noradrenaline in MLK3-deficient mice compared with WT mice. This may contribute to the increased energy expenditure and body temperature observed in MLK3-deficient mice. However, other factors such as secretion of FGF21 may also contribute to the phenotype of MLK3-deficient mice. Future studies will be required to address the exact mechanism by which MLK3 modulates body temperature and energy expenditure.
JNK activation has been associated with the development of insulin resistance by phosphorylating IRS-1 at an inhibitory site that can block signal transduction by the insulin receptor (1) and by regulating cytokine secretion (34). In agreement with MLK3 as mediator of saturated fatty acid-induced JNK activation, we found that MLK3 deficiency attenuates diet-induced JNK activation in adipose tissue (Fig. 3C) and decreases diet-induced insulin resistance (Fig. 2).
Inflammatory mediators originating from adipocytes and macrophages infiltrating the expanding adipose tissue are crucial in the development of insulin resistance. Recently, it was demonstrated that JNK in macrophages regulates adipose tissue macrophage infiltration and macrophage polarization toward an inflammatory M1 phenotype (17). Interestingly, we observed reduced macrophage infiltration into adipose tissue (Fig. 4), altered M1/2 macrophage polarization in high-fat diet-fed MLK3-deficient mice (Fig. 4C), and LPS- and palmitate-stimulated BMDM (Fig. 5), suggesting a role for MLK3 in macrophage polarization. Palmitate-induced JNK activation was significantly attenuated (Fig. 5C) but not completely abolished in MLK3-deficient macrophages, suggesting that other MLK3 isoforms may play a role in MCSFA-induced JNK activation. Previous studies have established a role for adipocyte JNK1 in IL-6 secretion (34), and although plasma levels of IL-6 were below detection level, we found that adipose tissue explants from MLK3-KO mice secreted less IL-6 than fat explants from WT mice, suggesting that MLK3 in adipocytes regulates JNK activity and subsequent IL-6 secretion. Since IL-6 is a regulator of MCP-1 expression (12), it is not surprising that we also observed decreased secretion of the chemokine MCP-1 in the plasma of MLK3-deficient mice (Fig. 3). Together these data indicate that MLK3 contributes to diet-induced insulin resistance by regulating JNK activity and cytokine secretion in adipocytes and macrophages.
MCP-1 has been associated with the development of steatosis. Thus, it has been demonstrated that loss of MCP-1 reduced diet-induced hepatic steatosis, whereas overexpression of MCP1 or incubation of hepatocytes with MCP-1 increased triglyceride accumulation (22). In addition, it was demonstrated that MCP-1, but not IL-6 or IL-8, is capable of inducing triglyceride accumulation in hepatocytes in vitro in a time- and dose-dependent manner (6). Consistent with decreased MCP-1 expression, we observed decreased diet-induced steatosis in MLK3-KO mice (Fig. 6A). It is currently unclear whether the protection of MLK3-deficient mice against diet-induced steatosis is due to MLK3 expression in adipocytes and macrophages or in hepatocytes. Previously, we have demonstrated that MLK3 mediates saturated fatty acid-induced JNK activation in hepatocytes (21); however, it has been shown that JNK1 inhibits hepatic steatosis (33). Since MLK3 regulates activation of both JNK isoforms expressed in liver, JNK1, and JNK2, it is possible that the phenotype observed in liver-specific JNK1-KO mice is due to compensatory signaling from JNK2. Alternatively, reduced steatosis in MLK3-KO mice may be due to regulation of JNK activation in adipocytes and macrophages and subsequent MCP1 secretion independent of hepatic JNK activation by MLK3. Future studies using bone marrow transplants will be required to address MLK3 function in the hematopoetic and nonhematopoetic compartments.
In summary, we show that MLK3 promotes MCSFA diet-induced JNK activation as well as diet-induced adiposity, adipose tissue inflammation, insulin resistance, and steatosis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-084310 (to R. Kohli), DK-077975 (to D. Perez-Tilve), and DK-082583 (to A. Jaeschke). R. Kohli is supported in part by Ethicon Endo Surgery.
DISCLOSURES
The authors have no potential conflicts of interest, financial or otherwise, in regard to this article.
AUTHOR CONTRIBUTIONS
V.G., A.M., D.P.-T., and A.J. performed the experiments; V.G., D.P.-T., and A.J. analyzed the data; V.G. and A.J. interpreted the results of the experiments; V.G., D.P.-T., and A.J. prepared the figures; V.G., R.K., D.Y.H., D.P.-T., and A.J. edited and revised the manuscript; R.K., D.Y.H., D.P.-T., and A.J. contributed to the conception and design of the research; A.J. drafted the manuscript; A.J. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Christine Raver, Jazzminn Hembree, Nikki Ottaway, Rosa Salazar, James Cash, Colleen Goodin, Eddy Konaniah, Ling Shen, and Manju Sharma for providing excellent technical support.
REFERENCES
- 1.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J Biol Chem 275: 9047–9054, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Akazawa Y, Cazanave S, Mott JL, Elmi N, Bronk SF, Kohno S, Charlton MR, Gores GJ. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol 52: 586–593, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belgardt BF, Mauer J, Wunderlich FT, Ernst MB, Pal M, Spohn G, Brönneke HS, Brodesser S, Hampel B, Schauss AC, Brüning JC. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proc Natl Acad Sci USA 107: 6028–6033, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7: 947–953, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol 25: 3670–3681, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, Pittet-Cuenod B, Meier CA, Negro F. Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology 48: 799–807, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149: 6018–6027, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252, 2000 [DOI] [PubMed] [Google Scholar]
- 9.DeLany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr 72: 905–911, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Diakogiannaki E, Morgan NG. Differential regulation of the ER stress response by long-chain fatty acids in the pancreatic beta-cell. Biochem Soc Trans 36: 959–962, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Enos RT, Davis JM, Velazquez KT, McClellan JL, Day SD, Carnevale KA, Murphy EA. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J Lipid Res 54: 152–163, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasshauer M, Klein J, Kralisch S, Klier M, Lossner U, Bluher M, Paschke R. Monocyte chemoattractant protein 1 expression is stimulated by growth hormone and interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 317: 598–604, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA 298: 2028–2037, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28: 1769–1778, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3: 663–672, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 339: 218–222, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Hoeks J, Mensink M, Hesselink MK, Ekroos K, Schrauwen P. Long- and medium-chain fatty acids induce insulin resistance to a similar extent in humans despite marked differences in muscle fat accumulation. J Clin Endocrinol Metab 97: 208–216, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 147: 173–184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell 27: 498–508, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landsberg L, Saville ME, Young JB. Sympathoadrenal system and regulation of thermogenesis. Am J Physiol Endocrinol Metab 247: E181–E189, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Leyton J, Drury PJ, Crawford MA. Differential oxidation of saturated and unsaturated fatty acids in vivo in the rat. Br J Nutr 57: 383–393, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Lottenberg AM, Afonso Mda S, Lavrador MS, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem 23: 1027–1040, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 13: 453–461, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab 82: 4196–4200, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Robinson LE, Buchholz AC, Mazurak VC. Inflammation, obesity, and fatty acid metabolism: influence of n-3 polyunsaturated fatty acids on factors contributing to metabolic syndrome. Appl Physiol Nutr Metab 32: 1008–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Sabio G, Cavanagh-Kyros J, Barrett T, Jung DY, Ko HJ, Ong H, Morel C, Mora A, Reilly J, Kim JK, Davis RJ. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev 24: 256–264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, Barrett T, Mora A, Kim JK, Davis RJ. Prevention of steatosis by hepatic JNK1. Cell Metab 10: 491–498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322: 1539–1543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma M, Urano F, Jaeschke A. Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol 56: 192–198, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci USA 103: 16454–16459, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.St-Onge MP, Bourque C, Jones PJ, Ross R, Parsons WE. Medium- versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord 27: 95–102, 2003 [DOI] [PubMed] [Google Scholar]
- 39.St-Onge MP, Ross R, Parsons WD, Jones PJ. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res 11: 395–402, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 237: 885–888, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Suzuki M, Takaishi S, Nagasaki M, Onozawa Y, Iino I, Maeda H, Komai T, Oda T. Medium-chain fatty acid-sensing receptor, GPR84, is a proinflammatory receptor. J Biol Chem 288: 10684–10691, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Veniant MM. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 58: 250–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol Chapter 14: Unit 14.1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]