Abstract
We hypothesized that insulin alters plasma free fatty acid (FFA) trafficking into intramyocellular (im) long-chain acylcarnitines (imLCAC) and triglycerides (imTG). Overnight-fasted adults (n = 41) received intravenous infusions of [U-13C]palmitate (0400–0900 h) and [U-13C]oleate (0800–1400 h) to label imTG and imLCAC. A euglycemic-hyperinsulinemic (1.0 mU·kg fat-free mass−1·min−1) clamp (0800–1400 h) and two muscle biopsies (0900 h, 1400 h) were performed. The patterns of [U-13C]palmitate incorporation into imTG-palmitate and palmitoylcarnitine were similar to those we reported in overnight postabsorptive adults (saline control); the intramyocellular palmitoylcarnitine enrichment was not different from and correlated with imTG-palmitate enrichment for both the morning (r = 0.38, P = 0.02) and afternoon (r = 0.44, P = 0.006) biopsy samples. Plasma FFA concentrations, flux, and the incorporation of plasma oleate into imTG-oleate during hyperinsulinemia were ∼1/10th of that observed in the previous saline control studies (P < 0.001). At the time of the second biopsy, the enrichment in oleoylcarnitine was <25% of that in imTG-oleate and was not correlated with imTG-oleate enrichment. The intramyocellular nonesterified fatty acid-palmitate-to-imTG-palmitate enrichment ratio was greater (P < 0.05) in women than men, suggesting that sex differences in intramyocellular palmitate trafficking may occur under hyperinsulinemic conditions. We conclude that plasma FFA trafficking into imTG during hyperinsulinemia is markedly suppressed, and these newly incorporated FFA fatty acids do not readily enter the LCAC preoxidative pools. Hyperinsulinemia does not seem to inhibit the entry of fatty acids from imTG pools that were labeled under fasting conditions, possibly reflecting the presence of two distinct imTG pools that are differentially regulated by insulin.
Keywords: nonesterified fatty acids, isotopic tracers, long-chain acylcarnitines
considerable efforts have been made to understand the role of fatty acids (FA) and intramyocellular lipid accumulation (9, 22, 24) as they relate to energy storage, utilization (7), and skeletal muscle insulin resistance. The greater intramyocellular triglyceride (imTG) content in obese, insulin-resistant individuals compared with lean, sedentary, insulin-sensitive individuals (24) suggests a possible direct role of imTG in insulin resistance. However, endurance-trained, insulin-sensitive adults also have greater imTG concentrations, the so called athlete's paradox (9).
In the postabsorptive state, adipose tissue lipolysis is the major contributor of free fatty acids (FFA) that serve as oxidative substrate for lean tissues (25, 28). It was believed that plasma FFA are directly shuttled to intramyocellular long-chain acylcarnitines (imLCAC) for oxidation. However, our earlier results from the combination of isotope dilution techniques and muscle biopsies indicated that imLCAC are likely derived from imTG-FA rather than directly from plasma FFA (18), consistent with an early model of Dagenais et al. (6). We reported that, in resting, overnight postabsorptive humans who received intravenous infusions of [13C]FFA, the enrichment of FA in imTG and imLCAC was not different, highly correlated, and much less than plasma FFA enrichment. This suggested to us that imTG are an obligate precursor pool for the imLCAC (18).
Under hyperinsulinemic conditions, FFA release from adipose tissue and FA oxidation are suppressed, but the extent to which this alters muscle FFA trafficking is unknown. Sidossis et al. (28) and Rasmussen et al. (25) employed the hyperglycemic-hyperinsulinemic clamp technique to demonstrate that glucose and/or insulin regulate FA oxidation, presumably by increasing malonyl-CoA, thereby inhibiting carnitine palmitoyltransferase 1 (CPT-1). This would inhibit the synthesis of imLCAC and thereby limit the rate of long-chain FA entry into the mitochondria. The implication is that FA taken up under these circumstances are redirected to other pools. We found no studies, however, that examined the effects of insulin on how hyperinsulinemia alters the trafficking of plasma FFA, imTG-FA, and imLCAC. We employed a pulse-chase FFA tracer design to study the effects of hyperinsulinemia on prelabeled imTG-FA and plasma FFA incorporation into imTG and the relationship(s) between imLCAC, imTG, and plasma FFA. We hypothesized that hyperinsulinemia would inhibit the delivery of imTG-FA to the imLCAC pool. Surprisingly, our findings under hyperinsulinemic conditions suggest the existence of at least two imTG pools that store plasma FFA and release FA to the imLCAC pools differently.
METHODS
Experimental design and subjects.
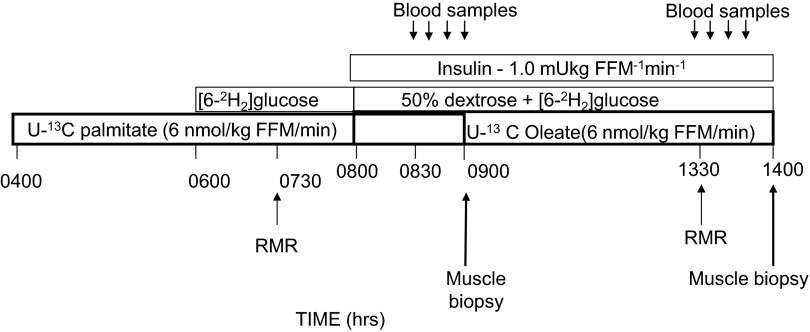
This report describes part two of a coordinated study of muscle FA metabolism, the saline portion of which has been previously reported (18). The experimental design of the tracer infusion/muscle biopsy/insulin clamp study is provided in Fig. 1. These volunteers underwent euglycemic-hyperinsulinemic clamp studies contemporaneously with those reported for the saline control condition (18). Briefly, during an overnight fast, the volunteers received an intravenous infusion of [U-13C]palmitate beginning at 0400 h to label imTG and LCAC pools under fasting (euinsulinemic) conditions. They then subsequently underwent a euglycemic-hyperinsulinemic clamp while receiving an infusion of [U-13C]oleate. Blood samples and muscle biopsies were collected using a pulse chase design (10). This approach prelabels imTG (pulse) with one FA tracer, which is assessed with the first biopsy, after which the [U-13C]palmitate is discontinued and the [U-13C]oleate continued until a second muscle biopsy. Our study design was intended to measure the effect of hyperinsulinemia on the trafficking of [U-13C]oleate into imTG, intramyocellular nonesterified fatty acids (imNEFA), and imLCAC, as well as to assess whether hyperinsulinemia alters the relationships between the [U-13C]palmitate tracer in imTG and imLCAC pools.
Fig. 1.
Schematic of the experimental design. Time is presented as clock time. RMR, resting metabolic rate using indirect calorimetry; FFM, fat-free mass.
Forty-one volunteers underwent the euglycemic-hyperinsulinemic clamp experiments (21 men and 20 premenopausal women). Participants were recruited across a range of body weight (body mass index: mean 27.3, range 18–38 kg/m2), fitness status [mean peak V̇o2: 50 ± 1.5 ml·kg fat-free mass (FFM)−1·min−1: range 34–71 ml·kg FFM−1·min−1], and an age range of 21–50 yr. None of the volunteers was taking medications known to affect FA metabolism, including oral contraceptives, and all participants signed an informed consent before participation, approved by the Mayo Institutional Review Board.
Body composition and aerobic capacity testing.
As previously described, body composition and aerobic capacity were measured during subject screening. Peak aerobic capacity was determined using a modified Bruce protocol (18), and 10-lead EKG was used to monitor heart rate. Body composition was assessed using a dual-energy X-ray absorptiometry (Lunar Radiation, Madison, WI) to measure both total body fat and FFM. Computerized tomography scan was employed to capture a single slice scan at L2–3 interspace for the determination of visceral and abdominal subcutaneous fat area (17). For 20 of the men and 18 of the women we were able to collect a fasting blood sample for plasma glucose and insulin concentrations.
Procedures.
For 5 days before the study the volunteers consumed a standard, weight-maintenance diet prepared in the Mayo General Clinical Research Center (GCRC) metabolic kitchen, providing 55% carbohydrate, 30% fat, and 15% protein. The volunteers were admitted to the GCRC the afternoon before the study and provided with an evening meal. An intravenous catheter was placed before the tracer infusions in a forearm vein and kept patent with an infusion of 0.45% NaCl throughout the study. At 0400 h an infusion of [U-13C]palmitate (6 nmol·kg FFM−1·min−1) was initiated and continued until 0900 h; at 0800 h an infusion of [U-13C]oleate (6 nmol·kg FFM−1·min−1) was started. The “pulse” of [U-13C]palmitate tracer (10) labels the imTG pool, which was assessed with the first muscle biopsy, whereas the second ([U-13C]oleate “chase”) infusion was continued until the second muscle biopsy.
To assess glucose metabolism, a primed (6 mg/kg FFM) continuous (0.06 mg·kg FFM−1·min−1) infusion of [6-2H2]glucose was initiated at 0600 h, and the 6-h euglycemic-hyperinsulinemic clamp (insulin dose of 1.0 mU·kg FFM−1·min−1) was started at 0800 h using a variable, [6-2H2]glucose-labeled 50% dextrose solution to maintain glucose concentrations in the range of 5.0–5.2 mmol/l. Blood was sampled every 10 min to allow for adjustment of the glucose infusion rate to maintain stable blood glucose concentrations. Between 0800–0830 h and 1330–1400 h, energy expenditure was measured with indirect calorimetry using a DeltaTrac Metabolic Cart (Yorba Linda, CA) (18).
Blood samples were collected from an intravenous catheter placed in a retrograde fashion in a heated hand vein contralateral to the infusion site. A blood sample taken before the initiation of tracer infusions was used to measure the background glucose and FA enrichments; blood samples were also collected at 10-min intervals between 1300 and 1400 h for FFA palmitate, oleate, and plasma glucose enrichment. Muscle biopsies were taken from the vastus lateralis at 0900 and 1400 h (18). The volunteers remained in bed throughout the study but were instructed to move their legs at 15-min intervals to avoid complete immobility.
Muscle tissue (∼300 mg) collected from the biopsies was immediately washed of blood with ice-cold 0.9% saline, dissected of visible adipose tissue, rinsed of lipid droplets, and saved in liquid nitrogen. Samples were stored at −80°C until analysis.
We were unable to collect all of the necessary samples to perform all of the planned analyses from some volunteers. For outcomes where we did not have sufficient data to present results for all 41 volunteers, the number of complete observations is annotated.
Materials.
[U-13C]palmitate, [U-13C]oleate, [6-2H2]glucose, and custom-synthesized standards for [U-13C]palmitoyl- and oleoylcarnitine were obtained from Isotec (Miamisburg, OH). The palmitate and oleate tracer infusions consisted of a solution of 0.3% albumin in 0.9% NaCl.
Assays.
A Beckman Instrument (Fullerton, CA) was used to measure plasma glucose. Plasma total FFA, oleate, and palmitate concentrations were measured using HPLC (23), and enrichment was measured using gas chromatography/combustion/isotope ratio mass spectrometry (12). Muscle and plasma TG concentrations were measured by a microflourometric method (15). Muscle lipids were measured after the frozen samples were dissected free of extramyocellular adipocytes while kept at 0°C on dry ice (11). The muscle was then pulverized into a fine powder by using a stainless steel mortar and pestle on dry ice (11). Intramyocellular palmitoyl- and oleoylcarnitine concentrations and enrichments were measured by liquid chromatography/tandem mass spectrometry as previously described (13) with a few modifications. Briefly, palmitoyl- and oleoylcarnitines were separated on a Varian C8 100 × 2.0 mm column with 3 μm particle (Palo Alto, CA) at 0.25 ml/min flow rate via Cohesive TX2 liquid chromatography system-LC (Franklin, MA). All ions were monitored in multireaction mode on an Applied Biosystem API5000 mass spectrometer-MS (Foster City, CA). Palmitoylcarnitine, [13C16]palmitoylcarnitine, oleoylcarnitine, and [13C18]oleoylcarnitine were monitored as 402/343, 416/357, 428/369, and 444/385, respectively. The interassay coefficients of variation (CVs) for palmitoyl- and oleoylcarnitine concentrations were 4 and 9%, respectively, whereas the interassay CVs for palmitoyl- and oleoylcarnitine enrichments were 2.8 and 3.5%, respectively.
Calculations.
Similar to our previous report (18), we used steady-state formulas to calculate oleate flux (μmol/min) during the final 30 min of the insulin clamp; oleate was used to extrapolate to total FFA kinetics. We used the change in imTG-oleate enrichment between 0900 and 1400 h during the oleate tracer infusion to calculate the storage of FFA into imTG using previously described formulas, assuming the plasma oleate enrichment was equal to that observed during the afternoon sampling interval for the time between the first and second biopsy (16). Substrate oxidation was calculated as per Frayn (8), and nonoxidative glucose disposal was calculated as glucose rate of disappearance (Rd, using the [6-2H2]glucose tracers) − glucose oxidation (indirect calorimetry and urinary nitrogen excretion rates).
Statistics.
Descriptive statistics were conducted using an unpaired t-test to compare the descriptive variables between men and women. A 2 (time: morning vs. afternoon) × 2 (sex: male vs. female) ANOVA with repeated measures was used to establish differences between the primary outcome variables. A 2 (time: morning vs. afternoon) × 2 (condition: saline vs. clamp day) ANOVA with repeated measures was used to compare the results of this study with our previous findings under saline conditions. A Pearson's product correlation was used to establish associations between visceral fat, glucose disposal, and FA concentrations. The statistical program SPSS was employed for data analysis (version 19, Chicago, IL). All data are reported as means ± SE, and an alpha level of 0.05 was used.
RESULTS
Subject characteristics (Table 1).
Table 1.
Subject characteristics
| Males (n = 21) | Females (n = 20) | |
|---|---|---|
| Age, yr | 34 ± 2 | 39 ± 2* |
| Weight, kg | 90.2 ± 3.2 | 72.6 ± 3.1* |
| BMI, kg/m2 | 28.0 ± 1.0 | 27.9 ± 1.2 |
| Fat-free mass, kg | 63.4 ± 0.8 | 45.0 ± 1.0* |
| Body fat, % | 27 ± 2 | 36 ± 2* |
| Visceral fat area, cm2 | 156 ± 23 | 71 ± 12* |
| Abdominal subcutaneous fat, cm2 | 178 ± 24 | 179 ± 25 |
| Waist circumference, cm | 98 ± 4 | 91 ± 3 |
| Resting energy expenditure (morning), kcal/day | 1,944 ± 37* | 1,511 ± 43 |
| RER (morning) | 0.81 ± 0.01 | 0.81 ± 0.01 |
| Glucose disposal (afternoon), μmol · kg FFM−1 · min−1 | 37.5 ± 2.8 | 41.8 ± 3.0 |
| Peak V̇o2, ml · kg FFM−1 · min−1 | 51.9 ± 2.0 | 49.5 ± 2.1 |
| Peak V̇o2, ml/min | 3,296 ± 134 | 2,211 ± 104* |
Values are means ± SE; n, no. of subjects. BMI, body mass index; RER, respiratory exchange ratio.
P < 0.05 between groups.
In this cohort of subjects (n = 41), the men were heavier and had more FFM and visceral fat area while the women were ∼5 yr older and, as expected, had more body fat (P < 0.05). Abdominal subcutaneous fat and waist circumference were similar between groups. Peak V̇o2 adjusted for FFM was not different between men and women. The fasting plasma glucose and insulin concentrations from the screening exam averaged 93 ± 2 mg/dl and 7.0 ± 1.4 μU/ml in men and 93 ± 3 and 7.0 ± 1.2 μU/ml in women, respectively. Consistent with their greater FFM, resting energy expenditure (morning) was greater in men than women (P < 0.05). Afternoon (steady-state insulin clamp) insulin concentrations averaged 45.5 ± 2.8 and 33.9 ± 2.1 μU/ml in men and women, respectively. Plasma glucose concentrations during the clamp averaged 92 ± 2 and 94 ± 3 mg/dl in men and women; glucose disposal rates were similar in men and women. In response to hyperinsulinemia, the respiratory exchange ratio increased from the morning (0.81 ± 0.01) to the afternoon (0.87 ± 0.01, P < 0.0001) in both men and women. Substrate oxidation rates and nonoxidative glucose disposal rates just before the first and second muscle biopsies are provided in Table 2.
Table 2.
Substrate oxidation and nonoxidative glucose disposal
| Males |
Females |
|||
|---|---|---|---|---|
| Morning | Afternoon | Morning | Afternoon | |
| FA oxidation, μmol/min* | 236 ± 22 | 135 ± 21 | 164 ± 22 | 102 ± 20 |
| CHO oxidation* | 791 ± 87 | 1,161 ± 92 | 670 ± 87 | 882 ± 93 |
| Nonoxidative CHO disposal* | −3 ± 246 | 1,110 ± 148 | −32 ± 246 | 941 ± 148 |
Values are means ± SE. FA, fatty acid; CHO, carbohydrate (glucose).
P < 0.001, main effect of time. There was no significant effect of sex on these variables.
Muscle and plasma concentrations of FAs and FA-containing lipids.
imTG concentrations (μmol/g wet wt) in the morning biopsy and 5 h later (afternoon biopsy) were ∼50% greater in women than in men (P < 0.05) and decreased by ∼18% (P < 0.05) from the morning to the afternoon biopsy in women but did not change significantly (P = 0.77) in men. The intramyocellular palmitoyl- and oleoylcarnitine concentrations were not significantly different between the morning and the afternoon or between men and women. The slight decreases in concentrations from morning to afternoon in response to the insulin clamp did not reach statistical significance for men (P = 0.06) or women (P = 0.36) (Table 3). The range of observed values for palmitoylcarnitine or oleoylcarnitine concentrations was 0.02–0.15 and 0.06–0.60 μmol/g, respectively. The same patterns were observed for the combined palmitoyl + oleoylcarnitine concentrations. Of interest, the palmitoylcarnitine + oleoylcarnitine concentrations were significantly correlated with glucose Rd/plasma insulin concentrations (r = 0.61, P < 0.001), indicating that the most insulin-sensitive participants had greater imLCAC concentrations.
Table 3.
Muscle concentrations of lipid compounds containing palmitate and oleate
| Males (n = 21) |
Females (n = 20) |
|||
|---|---|---|---|---|
| Morning | Afternoon | Morning | Afternoon | |
| Intramyocellular acyl-carnitine-fatty acid concentration, μmol/g wet wt | ||||
| Palmitoyl | 0.068 ± 0.006 | 0.066 ± 0.006 | 0.069 ± 0.007 | 0.068 ± 0.006 |
| Oleoyl | 0.247 ± 0.030 | 0.224 ± 0.032 | 0.267 ± 0.028 | 0.251 ± 0.030 |
| Palmitoyl + oleoyl | 0.307 ± 0.031 | 0.277 ± 0.030 | 0.338 ± 0.039 | 0.319 ± 0.035 |
| imTG concentration, μmol/g wet wt | ||||
| 1.12 ± 0.19† | 1.09 ± 0.21† | 2.77 ± 0.37 | 2.36 ± 0.31* | |
Values are means ± SE; n, no. of subjects. For women the afternoon time interval n = 19 for long-chain acyl-carnitine concentrations and for men the morning and afternoon time interval n = 20 for long-chain acyl-carnitine concentrations. imTG, intramyocellular triglyceride.
P < 0.05 between A.M. and P.M. sample;
P < 0.05, sex differences.
Plasma palmitate, oleate, and total FFA concentrations during the final 30 min of the insulin clamp were 14 ± 2, 15 ± 2, and 56 ± 7 μmol/l in men and 16 ± 5, 18 ± 6, and 60 ± 22 μmol/l in women [P = not significant (NS) between men and women]. The average oleate enrichments used to calculate steady-state oleate/FFA flux during the last 30 min of the insulin clamp are provided in Table 4; oleate and FFA concentrations for men and women were 37 ± 26/147 ± 104 and 34 ± 30/133 ± 122 μmol/min, respectively.
Table 4.
Palmitate and oleate enrichments in the morning and afternoon biopsy in both males and females
| Males |
Females |
|||
|---|---|---|---|---|
| Morning | Afternoon | Morning | Afternoon | |
| Plasma free fatty acid 13C enrichment, MPE | ||||
| Oleate | 0.182 ± 0.021 (n = 21) | 0.196 ± 0.024 (n = 20) | ||
| imTG-fatty acid 13C enrichment, MPE | ||||
| Palmitate | 0.021 ± 0.002 (n = 20) | 0.020 ± 0.001 (n = 20) | 0.017 ± 0.002 (n = 21) | 0.015 ± 0.002 (n = 21) |
| Oleate | 0.010 ± 0.000** (n = 20) | 0.012 ± 0.001 (n = 20) | 0.008 ± 0.001* (n = 19) | 0.009 ± 0.001 (n = 19) |
| im Long chain acyl-carnitine fatty acid 13C enrichment, MPE | ||||
| Palmitoyl | 0.016 ± 0.002 (n = 20) | 0.017 ± 0.002 (n = 20) | 0.014 ± 0.002 (n = 19) | 0.015 ± 0.003 (n = 19) |
| Oleoyl | 0.001 ± 0.001* (n = 20) | 0.002 ± 0.001 (n = 20) | 0.001 ± 0.000* (n = 19) | 0.002 ± 0.001 (n = 19) |
| imNEFA fatty acid 13C enrichment, MPE | ||||
| Palmitate | 0.020 ± 0.003* (n = 13) | 0.018 ± 0.002 (n = 13) | 0.022 ± 0.002* (n = 19) | 0.020 ± 0.002 (n = 19) |
| Oleate | 0.008 ± 0.001** (n = 13) | 0.012 ± 0.002 (n = 13) | 0.005 ± 0.001** (n = 19) | 0.008 ± 0.002 (n = 19) |
Values are means ± SE; n, no. of subjects. The plasma oleate enrichments are those from the final 30 min of the insulin clamp. MPE, moles percent enrichment; im, intramyocellular.
P < 0.05 between A.M. and P.M. sample;
P < 0.01 between morning and afternoon samples.
FA enrichments.
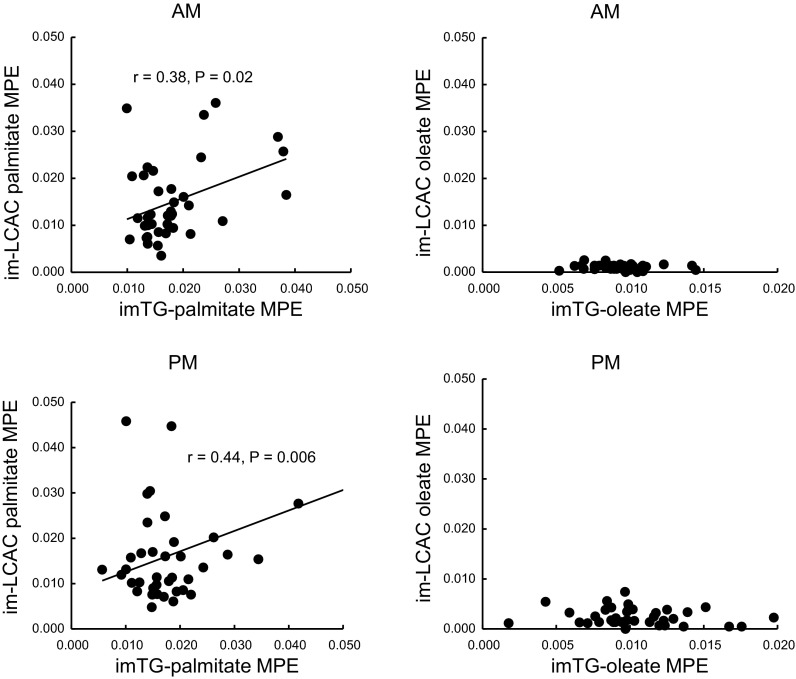
Despite initiating the [U-13C]oleate infusion only 1 h before the morning muscle biopsy, there was readily quantifiable enrichment in both imTG and imNEFA oleate in these samples. However, the enrichment in oleoylcarnitine was well below that of the oleate in imTG and imNEFA, essentially at the lower limit of detection. The enrichments in imTG-oleate increased from the morning to the afternoon biopsy (males: 0.010 ± 0.000 → 0.012 ± 0.001; females: 0.008 ± 0.001 → 0.009 ± 0.001 mole percent enrichment, respectively, P < 0.05) (Table 4), reflecting the incorporation of plasma FFA oleate into imTG (see below). There were slight but statistically significant increases in oleoylcarnitine enrichment between the morning and afternoon muscle samples (Table 4); however, the enrichment in oleoylcarnitine was less than one-fourth the enrichment in imTG-oleate (P < 0.0001). There was no correlation between the enrichment in imTG-oleate and oleoylcarnitine (Fig. 2, right). The enrichment in imTG-oleate was greater than imNEFA-oleate for the morning biopsy in men (P = 0.058) and women (P = 0.0001), perhaps indicating that the tracer that had been incorporated into imTG-oleate had yet to equilibrate with imNEFA-oleate after 1 h of tracer infusion. However, the enrichments in the imTG-oleate and imNEFA-oleate were not significantly different at the time of the afternoon biopsy in either men or women. We examined the relationship between the enrichments in these two pools to get an indication whether imTG might be the precursor pool to imNEFA. For males, the afternoon imNEFA-oleate enrichment was correlated with afternoon imTG-oleate enrichment (r = 0.56, P = 0.008), whereas in females there was no association between these variables (r = −0.02, P = NS).
Fig. 2.
Relationships between the [U-13C]palmitate and [U-13C]oleate moiety enrichments in intramyocellular triglyceride (imTG) and intramyocellular long-chain acylcarnitine (imLCAC; palmitoylcarnitine and oleoylcarnitine) from the first (AM, top) and second (PM, bottom) biopsies. Correlation coefficients and P values are provided for the statistically significant relationships. MPE, mole %enrichment.
In contrast to the oleate enrichment patterns, the imTG-palmitate enrichment did not change significantly from the morning to the afternoon, whereas imNEFA enrichment decreased (P < 0.05) in both men and women. Intramyocellular palmitoylcarnitine enrichment was stable between the morning and afternoon samples. The enrichment in intramyocellular palmitoylcarnitine was not different from the enrichment in imTG-palmitate for men or women for either the morning or afternoon samples. For the combined group of men and women, the enrichments in these two pools were correlated both for the morning (r = 0.38, P = 0.02, Fig. 2, top left) and afternoon (r = 0.44, P = 0.006, Fig. 2, bottom left) samples. This is similar to what we observed under saline control conditions (18).
Rates of plasma FFA-oleate incorporation into imTG-oleate.
Using the change in imTG-[U-13C]oleate pool size combined with steady-state plasma oleate enrichment data, we calculated the incorporation rates of plasma FFA-oleate into imTG-oleate during hyperinsulinemia. The mean incorporation of plasma oleate into imTG-oleate was the same in men and women (0.002 ± 0.002 μmol·g wet wt−1·h−1). Oleate storage rates into imTG were not associated with plasma total FFA concentrations in men or women. Because aerobic capacity, as measured by peak V̇o2, was associated with plasma FFA storage into imTG under saline control conditions in men (18), we tested whether the same would be observed under hyperinsulinemic conditions. We found a positive relationship (r = 0.41) between peak V̇o2 and plasma FFA oleate storage in imTG in men that did not reach statistical significance (P = 0.07); there was no relationship for these two variables for women. V̇o2 peak was associated with insulin-stimulated glucose disposal (r = 0.645, P = 0.0001).
Comparison of results with saline control conditions.
Under saline control conditions, plasma FFA-oleate storage averaged 0.049 ± 0.029 and 0.035 ± 0.035 μmol·g muscle−1·h−1 in women and men (18), respectively, an order of magnitude greater than we observed under insulin clamp conditions. We extrapolated these values from the quadriceps muscle to total body skeletal muscle under saline control conditions (18) and estimated that 11 ± 6 and 10 ± 9% of systemic FFA were trafficked to imTG in women and men, respectively. Using a similar approach with this dataset, we estimated that only 1 ± 4 and 4 ± 8% of systemic FFA were trafficked to imTG in women and men (P < 0.001 vs. saline control).
To understand how hyperinsulinemia may have altered the relationships between the various intramyocellular FA pools, we examined the ratios between enrichments in imLCAC-FA and imTG-FA as well as the ratios between imNEFA-FA and imTG-FA in this study compared with our previous saline control study (18). Results from the previous study (18) suggested to us that imTG-FA was well-equilibrated with the imLCAC-FA and imNEFA-FA pools (ratios not different from 1 and positive correlations between enrichments in the imTG vs. imLCAC and imNEFA pools). Table 5 presents the ratios between the enrichments of FAs in these pools for both the insulin clamp and saline control studies (18). The relationships between imTG-palmitate and intramyocellular palmitoylcarnitine as well as between imTG-palmitate and imNEFA-palmitate did not differ between saline control and insulin clamp conditions. However, significant differences between the insulin clamp and saline control studies were apparent for the [U-13C]oleate enrichments (chase tracer). The oleoylcarnitine-to-imTG-oleate enrichment ratios were ∼75% less (at least P < 0.01) under insulin clamp than saline control conditions for both men and women. In addition, the imNEFA-oleate-to-imTG-oleate enrichment ratios were significantly less under insulin clamp than saline control conditions for both women and men (P < 0.01). The only noticeable sex difference was a greater imNEFA-palmitate-to-imTG-palmitate enrichment ratio in women than men for both time points in the insulin clamp studies (P < 0.05).
Table 5.
Ratios of fatty acid enrichment in imTG fatty acids, intramyocellular long-chain acyl-carnitine, and intramyocellular nonesterified fatty acids–saline control vs. insulin clamp conditions
| LCAC-Palmitate-imTG Palmitate | LCAC-Oleate-imTG Oleate | NEFA-Palmitate-imTG Palmitate | NEFA-Oleate-imTG Oleate | |
|---|---|---|---|---|
| Saline | ||||
| Men (n = 31) | 0.81 ± 0.09 | 1.20 ± 0.17 | 1.01 ± 0.13 | 1.53 ± 0.18 |
| Women (n = 30) | 0.95 ± 0.10 | 1.22 ± 0.16 | 1.20 ± 0.14 | 1.60 ± 0.14 |
| Insulin clamp | ||||
| Men (n = 11) | 0.82 ± 0.14 | 0.21 ± 0.03* | 0.68 ± 0.16 | 0.87 ± 0.12* |
| Women (n = 10) | 1.11 ± 0.22 | 0.35 ± 0.08* | 1.23 ± 0.16‡ | 0.98 ± 0.17* |
Values are means ± SE of the ratios of the enrichments in palmitoyl-carnitine-imTG-palmitate or oleoyl-carnitine-imTG-oleate or intramyocellular nonesterified fatty acid (imNEFA)-palmitate-imTG-palmitate and imNEFA-oleate-imTG-oleate; n, no. of subjects. The ratios of enrichments from the second biopsy of the saline control condition vs. the second biopsy (taken at the end of a 6-h insulin clamp) are contrasted. LCAC, long-chain acyl-carnitine.
P < 0.01 vs. saline;
P < 0.05 vs. men.
Insulin sensitivity with respect to glucose metabolism.
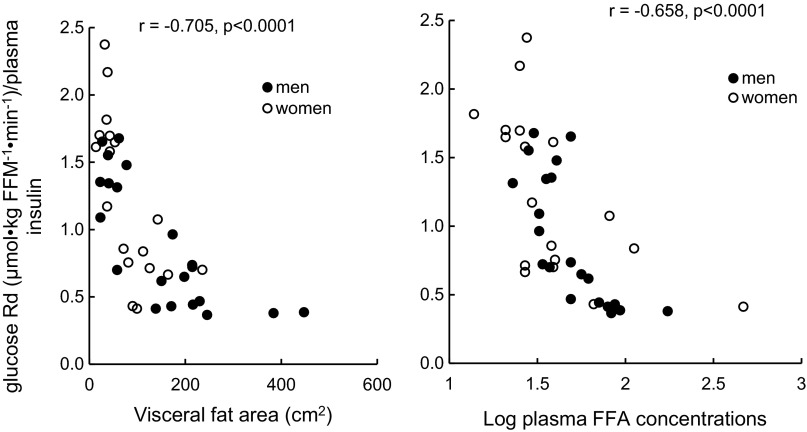
In these participants, insulin sensitivity was inversely correlated with visceral fat (Fig. 3, left, r = 0.70, P < 0.0001) and showed a curvilinear relationship with afternoon plasma total FFA concentrations (Fig. 3, right, r = 0.66, P < 0.0001 using log-transformed plasma FFA). No correlation existed between afternoon glucose Rd and intramyocellular acylcarnitine concentrations in either men or women.
Fig. 3.
Relationships between glucose rate of disappearance during the final 30 min of the insulin clamp and visceral fat area (left) and the average plasma free fatty acid (FFA) concentrations (right) during that same time interval.
DISCUSSION
This study extends our previous observations (18) by examining the relationship between plasma FFA and intramyocellular FA metabolism under hyperinsulinemic conditions. During hyperinsulinemia the rate of incorporation of plasma FFA into imTG was markedly less than in the postabsorptive state (18). In addition, the plasma FFA that were incorporated into imTG during hyperinsulinemia did not enter the imLCAC pool as readily as we saw under saline control conditions, as reflected by the much lower enrichment of the imLCAC-oleate moiety than imTG-oleate. We also found the ratio of enrichments in imNEFA-oleate-imTG-oleate was less under insulin clamp than saline control conditions, implying that hyperinsulinemia also affects this relationship. Of interest, the plasma palmitate tracer that was incorporated into imTG during the early morning infusion was present in both the LCAC and NEFA pools in a pattern similar to that observed under postabsorptive conditions, both before and after 6 h of hyperinsulinemia. These findings imply that elevated insulin concentrations alter the trafficking of newly arrived plasma FFA into intramyocellular FA pools.
We propose that the relationships between the enrichments in palmitoylcarnitine and imTG-palmitate reflect the contribution of imTG to imLCAC. If the enrichment in the FA moieties of these two lipids is not different and the enrichments correlate, this suggests that imTG is the precursor of LCACs. In these experiments, similar to the saline control studies (18), both morning and afternoon enrichments in palmitoylcarnitine and imTG-palmitate were not different and were significantly correlated. Recalling that the palmitate tracer was infused largely under saline conditions in the current experiments, these relationships between imTG-palmitate and palmitoylcarnitine imply that plasma FFA palmitate tracer incorporated into imTG during the pulse is released into the imLCAC and imNEFA pools. In our saline control experiments (18), some plasma FFA-oleate tracer had accumulated in imTG-oleate at the time of the first biopsy (1 h of tracer infusion), but the enrichment in oleoylcarnitine enrichment was only ∼30–40% of imTG-oleate. After an additional 5 h of oleate tracer infusion under saline control conditions, considerably more tracer accumulation in imTG was found, and the oleoylcarnitine and imTG-oleate enrichments were not different and highly correlated (18).
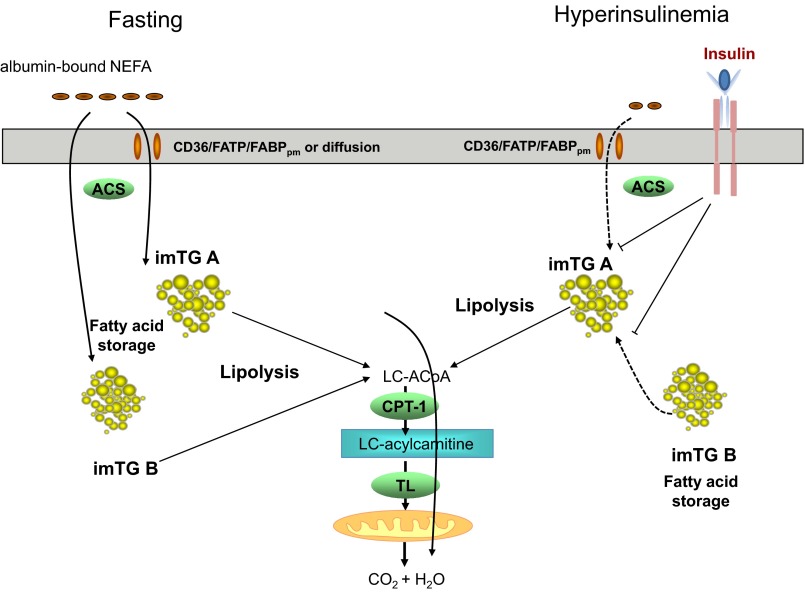
In contrast, under hyperinsulinemic conditions we describe herein, oleoylcarnitine enrichments averaged ∼25% of imTG-oleate (P < 0.0001 vs. ∼100% for saline; Table 4) at the time of the second biopsy, despite 6 h of oleate tracer infusion during hyperinsulinemia. This indicates that the plasma FFA tracer incorporated into imTG during hyperinsulinemia was not entering the oleoylcarnitine pool as readily as under saline control conditions. One possible explanation for the apparent discrepancies between the palmitate and oleate tracer data in the insulin clamp experiments is that there are two (or more) imTG pools, as depicted in Fig. 4. Both pools store and release FA to the LCAC pool under fasting conditions, but, under conditions of high-insulin/low-plasma FFA, one of these imTG pools (imTG pool A) continues to release FAs to the LCAC pool, whereas the other (imTG pool B) does not. If this is the case, then under hyperinsulinemic conditions imTG pool A does not readily store FFA from plasma. Circumstantial evidence for this is the observation that the oleoylcarnitine enrichments did not increase to equal imTG-oleate enrichment as it did in the saline control experiments, and imTG-palmitate enrichment did not decrease as a result of incorporation of unlabeled FFA. Our hypothetical imTG pool B appears to store some plasma FFA under hyperinsulinemic conditions and does not readily release them to the LCAC pool.
Fig. 4.
Proposed trafficking of plasma FFA in skeletal muscle under fasting (left) and hyperinsulinemic (right) conditions. When the plasma palmitate tracer was infused under fasting conditions, we observed an isotopic enrichment ratio of ∼1:1 between palmitate in imTG and long-chain acylcarnitines (LCAC), which are generated via carnitine palmitoyltransferase 1 (CPT-1) and subsequently enter the mitochondria via a translocase (TL). This suggests that, once inside the cell, plasma FFA likely are shunted into complex lipid synthesis, including imTG, which then feeds the LCAC pool, even in the face of subsequent hyperinsulinemia. In contrast, when plasma FFA enter muscle during hyperinsulinemia, the much lower enrichment of oleate in LCAC than imTG pools suggests these newly arrived fatty acids enter an imTG pool that does not equilibrate with the LCAC pool. These observations suggest that there may be two imTG pools that are differentially affected by insulin; in one case, newly arrived plasma FFA are blocked from entering the imTG, but imTG delivery to LCAC is not completely inhibited. The other imTG pool appears to store newly arrived plasma FFA but is inhibited from delivering them to the LCAC pool. FABPpm, fatty acid-binding protein-plasma membrane; NEFA, nonesterified fatty acid; LC, long chain; FATP/CD36, FA translocase CD36; ACS, acetyl-CoA synthetase; LC-ACoA, long-chain acyl-CoA.
Recent data suggest that subsarcolemmal and intramyofibrillar mitochondria (21) and their associated imTGs (27) are different. Possibly, these morphologically different imTG pools represent our kinetically distinct pools. Alternatively, our findings could reflect differences in how slow-twitch (type I) vs. fast-twitch (type IIa) fibers handle FA; quadriceps is a mixed slow-twitch/fast-twitch muscle, and we could not separate the samples by fiber type. It is also conceivable that the stable imLCAC concentrations as well as stable imLCAC-to-imTG palmitate enrichment ratios during hyperinsulinemia reflect simultaneous and equal inhibition of imTG hydrolysis, CPT-1, and acylcarnitine translocase. Inhibition of CPT-1 by hyperinsulinemia-induced malonyl-CoA accumulation could also explain the low enrichment ratios between LCAC-oleate-imTG-oleate. However, if that were the case, we would expect that, because of the ongoing accumulation of FFA in imTG (as manifested by rates of oleate incorporation), the imTG concentrations should have increased by ∼0.35 and ∼0.17 μmol/g in men and women, respectively, over the 5 h. Instead, we observed decreases in imTG concentrations. To the extent that FA oxidation is the predominant pathway for net loss of FAs from imTG, these observations suggest that muscle FA oxidation was not completely inhibited during the hyperinsulinemic clamp. We cannot exclude the possibility that differences between these ratios during the clamp may also reflect different trafficking and kinetics of the different FFA species (palmitate vs. oleate). Further studies will be needed to determine whether one of these hypotheses or another explanation entirely explains our so-called “pool A” and “pool B” of imTG and to establish if the kinetics of palmitate and oleate differ under these hyperinsulinemic conditions.
We were initially surprised the imLCAC concentrations did not decrease significantly from the morning to the afternoon muscle biopsy despite over 5 h of hyperinsulinemia (average insulin concentration of ∼40 μU/ml). Under hyperinsulinemic conditions, muscle glucose uptake is increased (19) as well as malonyl-CoA (25) synthesis, which should inhibit CPT-1 and thereby reduce LCAC production. Unless inward mitochondrial transport of LCAC is simultaneously inhibited, we would expect LCAC concentrations to decrease. Rasmussen et al. (25) demonstrated reduced long-chain FA oxidation and increased intramyocellular malonyl-CoA concentrations during hyperglycemia-hyperinsulinemia but did not measure imLCAC concentrations. Kelley et al. (19) demonstrated that hyperinsulinemia does not readily suppress leg muscle FA oxidation in obese adults; if our overweight/obese participants (∼50% of the volunteers) likewise continued to hydrolyze imTG and deliver the FA to the imLCAC pool for oxidation despite hyperinsulinemia, this would reduce our statistical power to detect changes in imLCAC concentrations. Alternatively, imLCAC concentrations may not rapidly reflect muscle long-chain FA oxidation potential. One reason the imLCAC pool may not change rapidly at rest relates to the metabolic flux. The oxygen uptake of resting leg muscle is ∼1.5 ml·kg−1·min−1 (16), and only ∼50% of that is directed toward FA oxidation (19). This amount of oxygen consumption would equate to only 1.3 μmol·kg−1·min−1 of FA oxidation (8). Given the concentrations of palmitoyl- and oleoylcarnitine in our volunteers, we estimate the total leg muscle LCAC concentration was ∼600 μmol/kg. Thus, the fractional turnover of LCAC would be ∼13%/h (less if FA oxidation decreased with hyperinsulinemia); detecting changes in imLCAC concentrations in resting humans over the time interval we used may be difficult.
As measured by the plasma oleate tracer, the incorporation of plasma FFA into imTG during hyperinsulinemia was ∼1/10th of the incorporation that we found during saline control conditions (15), which is in keeping with plasma FFA concentrations in the current study being an average of 1/10th those we found under postabsorptive conditions (15). We might have expected that hyperinsulinemia, by recruiting FA transport proteins to the plasma membrane (4), would have “rescued” some of the suppression of FFA incorporation into imTG. However, given the disruption of FFA trafficking within muscle that we observed, it is possible that uptake was not as suppressed as imTG synthesis from FFA and that FFA were deposited in other FA-containing compounds (e.g., long-chain acyl-CoAs, ceramides, diacylglycerols) rather than imTG.
We and others have reported sex differences in muscle FA trafficking (18, 20, 26). The results of the current study are consistent with previous reports of greater imTG content in women compared with men. In addition, imTG content decreased substantially during the clamp while no significant change was seen in men. In this study, the incorporation rates of plasma FFA-oleate into imTG were similar between men and women, and the sex differences in predictors of FFA storage into imTG that we observed under postabsorptive conditions were not seen under hyperinsulinemic conditions. The only sex difference we noted in the present study was a greater imNEFA-palmitate-to-imTG-palmitate enrichment ratio in women. This may suggest that women may traffic the palmitate FFA species differently than men to imNEFA under hyperinsulinemic conditions. To the extent that NEFA represent a product of imTG hydrolysis, data from the current study would suggest hyperinsulinemia reduces the rate at which FA from imTG enter the imNEFA pool. Given the differences in imNEFA-to-imTG and imLCAC-to-imTG enrichment ratios under insulin clamp (but not saline control) conditions (Table 5), this suggests a partial inhibition of imTG hydrolysis in the “A” pool. Unlike the LCAC, the imNEFA-oleate enrichment approaches parity with imTG-oleate, indicating a disconnect between the NEFA generated from imTG hydrolysis and the FA acted upon by CPT-1. The finding that the imNEFA and imLCAC pools are probably not tightly connected is a possibility we could not comment upon with only the saline control results available to us.
Limitations of this project include a lack of measurements of some of the proteins and enzymes that might play important regulatory roles in muscle FA metabolism. We would have liked to measure the plasma membrane FA translocase CD36 content (FAT/CD36) because of its known role to facilitate the uptake of FA into cells. Considerable evidence has shown that FAT/CD36 and plasma membrane-associated FA-binding protein are important in regulating the uptake of FA into skeletal muscle (14). Corpeleijn et al. (5) have shown that that, in obese humans, the FAT/CD36 protein content in skeletal muscle is dynamically regulated by insulin in vivo in the short term. It is possible that interindividual differences in plasma FFA-oleate storage in imTG-oleate were due to (unmeasured) interindividual differences in plasma membrane content of these proteins. Likewise, we did not have sufficient muscle sample remaining to measure the FFA tracer accumulation in long-chain acyl-CoAs (1), sphingolipids (2), or diacylglycerols (3) at the time we developed these assays. Knowing the distribution of plasma FFA in these species would be much more informative. Studies are underway that will take advantage of these new approaches.
In conclusion, we found that hyperinsulinemia markedly suppresses the incorporation of plasma FFA into imTG. Furthermore, our data suggest the existence of an imTG pool that, once having stored FFA under postabsorptive conditions, continues to supply the imLCAC pool with FA during hyperinsulinemia. One model that can explain our data is the existence of another kinetically distinct imTG pool that stores plasma FFA under hyperinsulinemic conditions but does not supply the imLCAC pool. If this is the case, this second pool may contribute to some of the metabolic inflexibility that is observed in insulin resistance. We conclude that the relatively simple model we initially proposed to explain our results from the study of postabsorptive humans (18) should be modified to consider the possibility of differentially regulated intramyocellular lipid pools.
GRANTS
This work is supported by National Institutes of Health Grants DK-40484, DK-50456, and RR-00585 and by the Mayo Foundation. S. Shadid was partly sponsored by NOVO/NORDISK.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.A.K., S.S., and M.T.S. performed experiments; J.A.K., S.S., Z.G., and M.D.J. analyzed data; J.A.K., S.S., M.T.S., Z.G., and M.D.J. interpreted results of experiments; J.A.K., Z.G., and M.D.J. prepared figures; J.A.K., Z.G., and M.D.J. drafted manuscript; J.A.K., S.S., M.T.S., Z.G., and M.D.J. edited and revised manuscript; J.A.K., S.S., M.T.S., Z.G., and M.D.J. approved final version of manuscript; Z.G. and M.D.J. conception and design of research.
ACKNOWLEDGMENTS
We thank Barbara Norby, Darlene Lucas, Mai Persson, and Lianzhen Zhou for technical support and Monica Davis for editorial support.
Current addresses: S. Shadid, VieCuri Medical Center, Venlo, The Netherlands; J. Kanaley, Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, MO; and M. Sheehan, Department of Endocrinology, Marshfield Clinic-Weston Center, Weston, WI 54476.
REFERENCES
- 1. Blachnio-Zabielska AU, Koutsari C, Jensen MD. Measuring long-chain acyl-coenzyme A concentrations and enrichment using liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Rapid Commun Mass Spectrom 25: 2223–2230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blachnio-Zabielska AU, Persson XMT, Koutsari C, Zabielski P, Jensen MD. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun Mass Spectrom 26: 1134–1140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blachnio-Zabielska AU, Zabielski P, Jensen MD. Intramyocellular diacylglycerol concentrations and [U-13C]palmitate isotopic enrichment measured by LC/MS/MS. J Lipid Res 54: 1705–1711, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonen A, Luiken JJFP, Liu S. Palmitate transport and fatty acid transporters in red and white muscle. Am J Physiol Endocrinol Metab 275: E471–E478, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Corpeleijn E, Pelsers MMAL, Soenen S, Mensink M, Bouwman FG, Kooi ME, Saris WHM, Glatz JFC, Blaak EE. Insulin acutely upregulates protein expression of the fatty acid transporter CD36 in human skeletal muscle in vivo. J Physiol Pharmacol 59: 77–83, 2008 [PubMed] [Google Scholar]
- 6. Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest 58: 421–431, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dyck DJ, Bonen A. Muscle contraction increases palmitate esterification and oxidation and triacylglycerol oxidation. Am J Physiol Endocrinol Metab 275: E888–E896, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983 [DOI] [PubMed] [Google Scholar]
- 9. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Guo Z, Burguera B, Jensen MD. Kinetics of intramuscular triglyceride fatty acids in exercising humans. J Appl Physiol 89: 2057–2064, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Guo Z, Mishra P, Macura S. Sampling the intramyocellular triglycerides from skeletal muscle. J Lipid Res 42: 1041–1048, 2001 [PubMed] [Google Scholar]
- 12. Guo Z, Nielsen S, Burguera B, Jensen MD. Free fatty acid turnover measured using ultralow doses of [U-13C]palmitate. J Lipid Res 38: 1888–1895, 1997 [PubMed] [Google Scholar]
- 13. Guo ZK, Yarasheski K, Jensen MD. High precision isotopic analysis of palmitoylcarnitine by liquid chromatography/electrospray ionization ion-trap tandem mass spectrometry. Rapid Commun Mass Spectrom 20: 3361–3366, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Holloway GP, Bonen A, Spriet LL. Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am J Clin Nutr 89: 455S–462S, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Humphreys SM, Fisher RM, Frayn KN. Micromethod for measurement of sub-nanomole amounts of triacylglycerol. Ann Clin Biochem 27: 597–598, 1990 [DOI] [PubMed] [Google Scholar]
- 16. Jensen MD, Johnson CM, Cryer PE, Murray MJ. Thermogenesis after a mixed meal: role of leg and splanchnic tissues in men and women. Am J Physiol Endocrinol Metab 268: E433–E438, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr 61: 274–278, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Kanaley JA, Shadid S, Sheehan MT, Guo ZK, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 587: 5939–5950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Kiens B, Roepstorff C, Glatz JFC, Bonen A, Schjerling P, Knudsen J, Nielsen JN. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol 97: 1209–1218, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol 288: C1074–C1082, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Lee JS, Pinnamaneni SK, Eo SJ, Cho IH, Pyo JH, Kim CK, Sinclair AJ, Febbraio MA, Watt MJ. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J Appl Physiol 100: 1467–1474, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Miles JM, Ellman MG, McClean KL, Jensen MD. Validation of a new method for determination of free fatty acid turnover. Am J Physiol Endocrinol Metab 252: E431–E438, 1987 [DOI] [PubMed] [Google Scholar]
- 24. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Rasmussen BB, Holmbäck UC, Volpi E, Morio-Liondore B, Paddon-Jones D, Wolfe RR. Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J Clin Invest 110: 1687–1693, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roepstorff C, Donsmark M, Thiele M, Vistisen B, Stewart G, Vissing K, Schjerling P, Hardie DG, Galbo H, Kiens B. Sex differences in hormone-sensitive lipase expression, activity, and phosphorylation in skeletal muscle at rest and during exercise. Am J Physiol Endocrinol Metab 291: E1106–E1114, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Shaw CS, Jones DA, Wagenmakers AJ. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol 129: 65–72, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Sidossis LS, Coggan AR, Gastaldelli A, Wolfe RR. Pathway of free fatty acid oxidation in human subjects. J Clin Invest 95: 278–284, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]