Abstract
Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) promotes hepatic insulin clearance and endothelial survival. However, its role in the morphology of macrovessels remains unknown. Mice lacking Ceacam1 (Cc1−/−) exhibit hyperinsulinemia, which causes insulin resistance and fatty liver. With increasing evidence of an association among hyperinsulinemia, fatty liver disease, and atherosclerosis, we investigated whether Cc1−/− exhibited vascular lesions in atherogenic-prone aortae. Histological analysis revealed impaired endothelial integrity with restricted fat deposition and aortic plaque-like lesions in Cc1−/− aortae, likely owing to their limited lipidemia. Immunohistochemical analysis indicated macrophage deposition, and in vitro studies showed increased leukocyte adhesion to aortic wall, mediated in part by elevation in vascular cell adhesion molecule 1 levels. Basal aortic eNOS protein and NO content were reduced, in parallel with reduced Akt/eNOS and Akt/Foxo1 phosphorylation. Ligand-induced vasorelaxation was compromised in aortic rings. Increased NADPH oxidase activity and plasma 8-isoprostane levels revealed oxidative stress and lipid peroxidation in Cc1−/− aortae. siRNA-mediated CEACAM1 knockdown in bovine aortic endothelial cells adversely affected insulin's stimulation of IRS-1/PI 3-kinase/Akt/eNOS activation by increasing IRS-1 binding to SHP2 phosphatase. This demonstrates that CEACAM1 regulates both endothelial cell autonomous and nonautonomous mechanisms involved in vascular morphology and NO production in aortae. Systemic factors such as hyperinsulinemia could contribute to the pathogenesis of these vascular abnormalities. Cc1−/− mice provide a first in vivo demonstration of distinct CEACAM1-dependent hepatic insulin clearance linking hepatic to macrovascular abnormalities.
Keywords: NEFA, metabolic syndrome, obesity, fatty liver disease
the carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) is a membrane glycoprotein expressed in many cell types, including hepatocytes and angiogenically activated endothelial cells, but not skeletal myocytes or adipocytes. CEACAM1 promotes vascular morphogenesis (10, 17). Consistently, mice with endothelial-specific CEACAM1 overexpression exhibit improved ensheathment of the endothelial barrier by pericytes as well as by a well-structured vascular basement membrane (11). On the other hand, mice with global deletion of Ceacam1 (Cc1−/−) develop several endothelial and vascular abnormalities, including reduced endothelial cell response to vascular endothelial growth factor (VEGF) and increased basal endothelial permeability (24).
CEACAM1 also promotes hepatic insulin clearance, as demonstrated by the development of hyperinsulinemia resulting from impairment of insulin extraction in Cc1−/− mice (8, 39). Hyperinsulinemia causes systemic insulin resistance (8, 28, 39) and increased hepatic triglyceride synthesis, leading to hepatic steatosis and substrate redistribution to white adipose tissue to cause visceral obesity (23), followed by lipolysis and elevated plasma nonesterified fatty acid (NEFA) levels (8) in addition to a rise in adipose tissue-associated macrophage recruitment and adipokine release (12). Despite increased liver triglyceride and cholesterol synthesis, plasma triglyceride (8) and total cholesterol (39) levels are unchanged. Moreover, Cc1−/− mice develop features of nonalcoholic fatty liver disease (NAFLD), with mild basal fibrosis and inflammatory infiltration in liver that could in part arise from increased fat deposition (3). When fed a high-fat diet deriving 45% of calories from fat, a more progressive phenotype replicating all features of human nonalcoholic steatohepatitis (NASH) develops. This includes a more robust fibrosis and inflammation (12). Given that chronic hyperinsulinemia causes an increase in ectopic fat accumulation, including in large vessels, and that CEACAM1 is markedly reduced in the liver of obese subjects with NAFLD (19), a population at high risk for atherosclerosis (2), we investigated whether Cc1−/− mice also develop lipid accumulation and vascular abnormalities in atherosclerosis-prone large vessels.
MATERIALS AND METHODS
Animals.
C57BL/6 Cc1−/− and their Cc1+/+ littermates (39) were kept on a 12:12-h dark-light cycle and fed standard chow ad libitum. Male mice at 6 mo of age (unless otherwise mentioned) were examined. All procedures were approved by the Animal Care and Utilization Committee in each participating institution. Plasma levels of insulin, triglycerides, and NEFA were determined as described (8), of thiobarbituric acid reactive substance (TBARS) biochemically (25), and of reduced glutathione (GSH) by the Bioxytech GSH-400 kit (OxisResearch).
8-Isoprostane measurement.
Plasma was purified in 4xV cold ethanol and centrifuged at 1500 g for 10 min, and the supernatant was collected and ethanol vacuum-evaporated before acidification to pH 4.0 with ∼50 μl of 30% acetic acid followed by purification on preactivated SPE cartridges (C-18) (Item no. 400020, Cayman Chemical, Ann Arbor, MI). 8-Isoprostane was eluted at 4°C with 5 ml of ethyl acetate containing 1% methanol, vacuum-dried, reconstituted in 200 μl of EIA buffer, and assayed (50 μl) in triplicates using the 8-Isoprostane EIA Kit (Item no. 516351, Cayman Chemical). At the end of the incubation period with 8-isoprostane tracer and 8-isoprostane EIA antiserum at 4°C for 18 h, samples were rinsed five times with buffer, and Ellman's Reagent was added in the dark at room temperature for 120 min. Absorbance was read at 420 nm and data wereplotted as %B/B0 vs. log concentration using a four-parameter logistic fit.
Lipoprotein analysis.
Lipoproteins (VLDL, intermediate-density lipoprotein plus LDL, and HDL) were separated by sequential density ultracentrifugation of plasma in a TLA100 rotor, and their cholesterol content was determined by colorimetric assays and measurement on the SpectraMax 250 system (13).
Plasma fatty acid analysis.
Fatty acid distribution in whole plasma was assayed as described (31). Briefly, each sample was subjected to direct transesterification and injected into a gas chromatograph by using a (90 m × 0.32 mm) WCOT-fused silica capillary column VF-23ms coated with 0.25 mm film thickness (Varian, Canada).
Transfection of endothelial cells.
Bovine aortic endothelial (BAE) cells were maintained in MCDB-131 medium (Vec Technologies, Rensselaer, NY). Cells were transfected with 100 pmol of scrambled or CEACAM1-specific siRNA, using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) as previously described (24).
Nitric oxide release analysis in cell media.
Nitric oxide (NO) level was assessed in 20 μl of medium using a Nitrate/Nitrite Fluorometric Assay Kit (catalog no. 780051, Cayman Chemical), per the manufacturer's instructions. Fluorescence was read using the Synergy H1 Hybrid Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT) at 360 nm excitation and 430 nm emission wavelengths.
Analysis of NO production in isolated aortic rings.
Thoracic aorta segments were removed and cut into four rings (2.5 mm each) before concentration-response studies of vasorelaxation stimulated by acetylcholine and sodium nitroprusside were performed (29).
NADPH oxidase activity.
Aortic tissue was homogenized in lysis buffer (20 mmol/l KH2PO4, 1 mmol/l EGTA, and protease inhibitors, pH 7.4) and subjected to a lucigenin-derived luminescence assay in the presence of NADPH (0.1 mM) (33). Luminescence was measured every 1.8 s for 5 min in a luminometer (Veritas Microplate Luminometer; Turner Biosystems, Sunnyvale, CA).
Toluidine blue staining and histological examination by light microscopy.
Aortic arch (3 mice/group) was serially sectioned (4–5 μm thick), and every 10th section was H&E stained. To identify plaque area, the internal elastic membrane of the aortic wall marking the border between the tunica intima (endothelial layer) and the tunica media (smooth muscle layer) was used as a reference point. Additionally, the morphology of cells under the endothelial layer in relation to the following smooth muscle cells and to the internal elastic membrane was considered to determine the plaque border within the aortic wall. Measurements were done under (Keyence, BZ 9000) light microscope using the BZ-II image analysis software (Keyence, Neu-Isenburg, Germany). The automatically calculated plaque area was recalculated based on the final magnification at ×200. Measurements were performed on 15 H&E-stained sections (5 per mouse).
Aortic arch was sectioned, fixed in phosphate-buffered glutaraldehyde (5.5%) for 18 h immediately after removal, embedded in Epon 812, cut in semithin sections (0.5 μm thick), and stained with Toluidine blue before analysis with a Leica microscope equipped with a digital camera (Leica, Bennsheim, Germany) and the software Leica Application Suite v. 2.7.
Goldner trichrome staining.
Paraffin aortic arch sections of 5 μm thick were rehydrated in ethanol and treated with iron hematoxylin stain for 2 min, washed in water for 10 min, and exposed to Mason-Goldner (MG) mixture for 7 min and sequentially to a short treatment with 1% acetic acid, Phosphormolybden-Orange-G solution for 10 min, 1% acetic acid, 0.1% light green for 8–10 min, triple treatment with 100% ethanol (1 min each time) and a double treatment with Xylol (5 min each time).
Oil red O staining.
Aortic roots (n > 5 mice/group) were fixed overnight in 4% paraformaldehyde and frozen in OCT embedding medium prior to staining with Oil red O. To quantify the positive lipid-stained areas, the NIS-Elements Imaging Software 3.0 system was used.
Immmunohistochemistry.
Frozen sections of aortic roots were immunostained with MOMA-2 antibody (1:500; Abcam, Cambridge, UK) overnight at 4°C prior to incubation with secondary antibody for 1 h according to Dako IHC LSAB kit instructions. To quantify positive-stained areas, the NIS-Elements Imaging Software 3.0 system was used.
Tissue pieces of aortic arch were fixed in Bouin's solution and embedded in paraffin before immunostaining with polyclonal antibodies against endothelial NO synthase (eNOS, 1:100; R&D Systems; Minneapolis, MN). The specific staining was developed by glucose-peroxidase technique (17). Aortic fragments were cut, fixed immediately in 4% paraformaldehyde for 2 h, and whole-mounted on glass slides. Following an overnight incubation with goat polyclonal vascular endothelial (VE)-cadherin antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C, aortae were washed twice in PBS for 10 min, treated with FITC-conjugated anti-goat IgG for 60 min, washed three times, and analyzed using a Leica microscope.
Adhesion of isolated mouse leukocytes to mouse aorta in vitro.
As previously described (26), the thoracic aorta and blood-resident leukocytes were isolated from anesthetized donor littermate Cc1+/+ and Cc1−/− mice (8–9 mo of age). Following a midline thoracotomy, the aorta was quickly removed and placed in cold, oxygenated phosphate-buffered saline (PBS). After careful removal of the adventitia, aortas were cut into 4-mm segments under a dissecting microscope (PZMIV; WPI, Sarasota, FL). Aortic segments were then carefully opened longitudinally and placed in culture dishes containing 1 ml of Krebs-Henseleit solution with their luminal surface facing up.
Whole blood was obtained from anesthetized mice through a cannula inserted in the carotid artery. Leukocytes were isolated from whole blood by gradient centrifugation as previously described (29). Isolated leukocytes were then fluorescently labeled using a PKH26GL staining kit (Sigma-Aldrich, St. Louis, MO). Briefly, a suspension of leukocytes was incubated with 4 μM PKH26GL for 5 min prior to adding PBS containing 10% fetal calf serum. Leukocytes were then incubated with the aortic segments at a concentration of 105 cells/aortic segment in incubation wells for 60 min at 37°C in an orbital shaker platform. The aortic segments were then removed, gently washed in fresh Krebs-Henseleit buffer, and placed lumen side up on microscope slides and treated with a drop of immersion oil followed by a glass coverslip. The number of leukocyte adhering to the endothelial surface was counted in 20 separate microscopic fields under epifluorescent microscopy at a magnification of ×200. Results are expressed as total number of cells per microscopic field.
Western blot analysis.
Cells were serum starved overnight prior to insulin treatment (100 nM) for 5–30 min and protein analysis by 4–12% SDS-PAGE (Invitrogen, Carlsbad, CA), followed by Western blot with antibodies against phospho-Ser1177 eNOS, eNOS, phospho-Ser473 Akt, Akt A (Cell Signaling Technologies, Beverly, MA), and CEACAM1 (Ab 3759, a custom-made rabbit polyclonal). Proteins were detected using LiCOR secondary antibodies per manufacturer's instructions (LiCOR Biosciences, Lincoln, NE). Aortae were perfused with 10 ml of PBS after opening of the left cardiac chamber. Fragments of the aortic arch were homogenized using RIPA buffer on ice for 15 min. Proteins were analyzed by 8% reducing SDS-PAGE and Western blot using 1:100 vascular endothelial growth factor receptor (VEGFR)-2 (Santa Cruz Biotechnology) and eNOS antibodies followed by β-actin monoclonal antibody (1:50, Sigma-Aldrich) for protein loading. Proteins were detected by enhanced chemiluminescence (ECL; Amersham Biosciences, Sunnyvale, CA) and imaged using Image Reader LAS-3000 (Fujifilm), and the intensity was determined by comparable densitometric analyses via MultiGauge software (Fujifilm).
Semiquantitative real-time PCR analysis.
Total RNA was prepared using a PerfectPure RNA Tissue kit (5Prime, Gaithersburg, MD) following the manufacturer's instructions. cDNA was synthesized with oligo(dT) primers and Improm II Reverse Transcriptase (Promega), using 1 μg of total RNA and primers (Table 1). cDNA was evaluated with semiquantitative real-time PCR (qRT-PCR) using the StepOne Plus Real Time PCR system (Applied Biosystems, Foster City, CA). The relative amount of mRNA was normalized relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Results are expressed as means ± SE relative to controls.
Table 1.
Forward and reverse primers used for quantitative real-time PCR analysis
| Gene | Primer Sequence 5′ to 3′ |
|---|---|
| CTGF | F: AATGTCAGTGCGCAGCCGAAGCA |
| R: AGGGGTCACGCTCCGTACACAG | |
| Fibronectin | F: ACGGTGTCAACTACAAGATCG |
| R: GTCTTCCCATCGTCATAGCAC | |
| TGF-β | F: AGCAACATGTGGAACTCTACC |
| R: GTATTCCGTCTCCTTGGTTCAG | |
| Nox4 | F: TCCAAGCTCATTTCCCACAG |
| R: CGGAGTTCCATTACATCAGAGG | |
| F4/80 | F: CTTTGGCTATGGGCTTCCAGTC |
| R: CAAGGAGGACAGAGTTTATCGTG | |
| TNFα | F: ACGGCATGGATCTCAAAGAC |
| R: CGGACTCCGCAAAGTCTAAG | |
| VCAM-1 | F: ATTTTCTGGGGCAGGAAGTT |
| R: ACGTCAGAACAACCGAATCC | |
| CD3 | F: AGAGCAGCTGGCAAAGGTGGT |
| R: CAGCCATGGTGCCCGAGTCTAGC | |
| CD4 | F: TCACCTGGAAGTTCTCTGACC |
| R: GGAATCAAAACGATCAAACTGCG | |
| CD8 | F: CTCTGGCTGGTCTTCAGTATGA |
| R: TCTTTGCCGTATGGTTGGTTT | |
| IL-6 | F: GGCCTTCCCTACTTCACAAG |
| R: ATTTCCACGATTTCCCAGAG | |
| IFNγ | F: ATGAACGCTACACACTGCATC |
| R: CCATCCTTTTGCCAGTTCCTC | |
| MCP-1 | F: CTTCTGGGCCTGCTGTTCA |
| R: CCAGCCTACTCATTGGGATCA | |
| TLR2 | F: TTGCTGGGCTGACTTCTCTCA |
| R: GAAGAGTCAGGTGATGGATGTCG | |
| TLR4 | F: TCAGAACTTCAGTGGCTGGATT |
| R: AACTCTGGATAGGGTTTCCTGTCA | |
| NPC-1 | F: GGGGCATCAGTTACAATGCT |
| R: AAACACCGCACTTCCCATAG | |
| Ang-1 | F: CTCGTCAGACATTCATCATCCA |
| R: CACCTTCTTTAGTGCAAAGGCT | |
| Ang-2 | F: CAGCCACGGTCAACAACTC |
| R: CTTCTTTACGGATAGCAACCGAG | |
| VEGF-A | F: GCACATAGAGAGAATGAGCTTC |
| R: CTCCGCTCTGAACAAGGCT | |
| VEGF-C | F: GTGAGGTGTGTATAGATGTGGG |
| R: GTCTTGCTGAGGTAACCTGTG | |
| VEGF-D | F: TCACGCTCAGCATCCCATC |
| R: ACTTCTACGCATGTCTCTCTAGG | |
| VEGFR-1 | F: TGGCTCTACGACCTTAGACTG |
| R: CAGGTTTGACTTGTCTGAGGTT | |
| VEGFR-2 | F: TTTGGCAAATACAACCCTTCAG |
| R: GCAGAAGATACTGTCACCACC | |
| VE-cadherin | F: CACTGCTTTGGGAGCCTTC |
| R: GGGGCAGCGATTCATTTTTCT | |
| β-Catenin | F: TCCCTGAGACGCTAGATGAGG |
| R: CGTTTAGCAGTTTTGTCAGCTC | |
| Gp91 phox | F: TATGCTGATCCTGCTGCCAGT |
| R: TGTCTTCGAATCCTTGTCGAGC | |
| Gapdh | F: CCAGGTTGTCTCCTGCGACT |
| R: ATACCAGGAAATGAGCTTGACAAAG |
See text for definitions.
Statistical analysis.
Statistical analysis was determined by unpaired two-tailed Student's t-test with GraphPad Prism software. Statistical significance was set at 0.01–5%.
RESULTS
Development of morphological vascular abnormalities in Cc1−/− aortae.
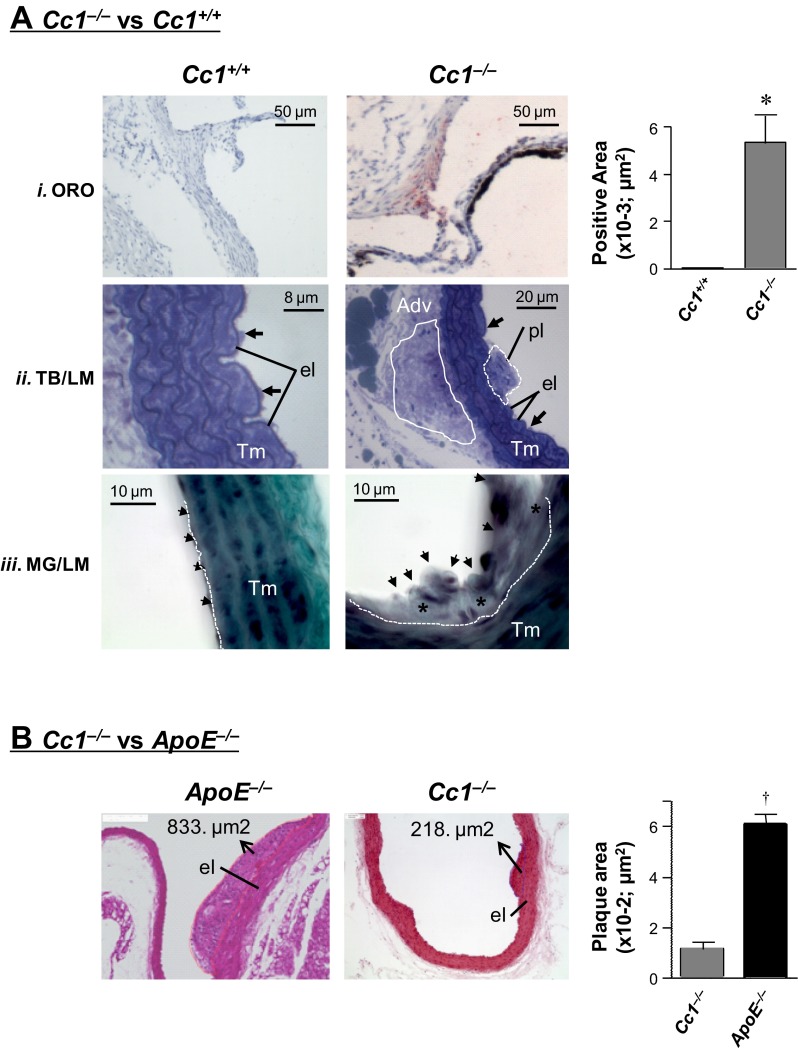
Histological analysis on H&E-stained sections from 6-mo-old mice revealed small plaque-like lesions in the aortae of Cc1−/− mice (aortic arch, ascending, thoracic, and abdominal parts; Fig. 1B). High-power light microscopic (LM) analysis on Toluidine blue (TB)-stained semithin sections demonstrated formation of lesions in the aortic intima with thickening and marked cell accumulation in the opposite aortic adventitia of Cc1−/− mice (Fig. 1A.ii). MG trichrome staining of aortic arch areas with lesions revealed subendothelial accumulation of fibrotic materials (Fig. 1A.iii, *green stains). Consistently, aortic mRNA levels of fibrosis markers, transforming growth factor-β (Tgfβ) connective tissue growth factor (Ctgf), and fibronectin were also elevated (Table 2). Oil red O (ORO) staining of cross-sections of aortic roots showed evidence of lipid deposition in the intima of Cc1−/− mice (Fig. 1A.i and accompanying graph).
Fig. 1.
Morphological analysis of aortic lesions. A: staining of aortae from 6-mo-old male mice (n > 5 per group). A.i: cross-sections of aortic root stained with Oil red O (ORO) depicted lipid deposition in the intima of carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1)-null (Cc1−/−) but not Cc1+/+ mice. Quantification of stained areas is represented in the side graph, *P < 0.05 vs. Cc1+/+. A.ii: light microscopic (LM) evaluation of Toluidine blue (TB)-stained semithin sections from aortic arch and ascending aorta revealed plaques in aortic intima of Cc1−/− aorta (dotted white line, pl) with increased cell density of aortic adventitia (solid white line, Adv) at the opposite site. Black arrows, Endothelial lining cells; Tm, tunica media. A.iii: LM evaluation of aortic arch sections stained by Masson-Goldner (MG) technique revealed a small atherosclerotic lesion with increased subendothelial deposition of fibrotic materials (*) in Cc1−/− aorta. Arrows, endothelial lining of aorta; white dotted line, border between the aortic intima and Tm. B: histological analyses of aortae from 8-mo-old mice (n = 3 per group), sectioned and H&E stained for plaque area measurement in 5 sections per mouse. el, Internal elastic membrane layer. Values are presented as means ± SE in ApoE−/− relative to Cc1−/− (†P < 0.01).
Table 2.
mRNA Levels of markers of fibrosis and oxidative stress in aortae of 6 mo-old mice
| Cc1+/+ | Ccl−/− | |
|---|---|---|
| Fibrosis | ||
| Ctgf | 4.86 ± 0.62 | 7.78 ± 1.25* |
| Fibronectin | 0.50 ± 0.06 | 0.75 ± 0.08* |
| Tgfβ | 0.87 ± 0.09 | 1.16 ± 0.09* |
| Oxidative stress | ||
| Nox4 | 1.11 ± 0.10 | 1.55 ± 0.16* |
Values are expressed as means ± SE. Aortic arch was isolated from 6-mo-old male mice (n ≥ 6) to measure mRNA content by qRT-PCR (normalized to Gapdh).
P < 0.05 vs. Cc1+/+.
Absence of proatherogenic hypercholesterolemia in Cc1−/− mice.
The area of aortic lesions ranged from 33.7 to 218.3 μm2, with a mean value approximately sixfold smaller than the mean plaque area in age-matched apolipoprotein E-deficient mice (ApoE−/−), a genetic model of atherosclerosis (Fig. 1B and accompanying graph). Unlike ApoE−/− mice, Cc1−/− mice exhibited normal total and LDL-cholesterol levels in addition to human-like HDL-cholesterol compared with wild-type littermates (Table 3). As previously observed (8, 39), these mice also exhibited normal plasma total and VLDL-triglyceride levels despite increased hepatic lipid production and mildly elevated plasma apoB-48/100 (8), consistent with increased lipid redistribution from liver to white adipose tissue (WAT) and elevated NEFA levels (Table 3) (8). However, this did not translate into changes in the relative composition of fatty acids that are commonly associated with macrovascular abnormalities (21, 37), such as saturated and transsaturated fat, and in the ratio of omega 6 to omega 3 polyunsaturated fatty acids (Table 3). Because wild-type mice resist atherosclerosis due to their low circulating apoB-containing lipoproteins, it is likely that limited circulating cholesterol and triglyceride levels, together with unfavorable changes in fatty acid composition, restricted vascular lesions in Cc1−/− mice.
Table 3.
Plasma and tissue biochemistry
| Cc1+/+ | Ccl−/− | |
|---|---|---|
| Plasma insulin (ng/ml) | 0.5 ± 0.1 | 1.2 ± 0.2* |
| Plasma cholesterol | ||
| Total (mg/dl) | 61.0 ± 6.8 | 61.0 ± 7.0 |
| VLDL (mg/dl) | 1.8 ± 0.6 | 3.2 ± 0.8 |
| LDL (mg/dl) | 14.0 ± 1.4 | 14.0 ± 2.3 |
| HDL (mg/dl) | 46.0 ± 5.7 | 47.0 ± 5.2 |
| Plasma triacylglycerol (TG) | ||
| Total (mg/dl) | 44.0 ± 8.2 | 39.0 ± 5.0 |
| VLDL (mg/dl) | 22.0 ± 4.5 | 22.0 ± 4.1 |
| LDL (mg/dl) | 15.0 ± 2.5 | 11.0 ± 1.5 |
| HDL (mg/dl) | 10.0 ± 2.2 | 10.0 ± 1.4 |
| Hepatic TG (μg/mg protein) | 59.0 ± 4.2 | 91.0 ± 6.3* |
| Total plasma NEFA (mEq/l) | 0.6 ± 0.1 | 1.0 ± 0.1* |
| Fatty acid composition (%) | ||
| Saturated FA | 37.7 ± 0.41 | 38.6 ± 0.33 |
| Trans-FA | 0.37 ± 0.02 | 0.36 ± 0.02 |
| Monounsaturated FA | 17.1 ± 0.44 | 19.0 ± 0.47* |
| Polyunsaturated FA | 44.9 ± 0.53 | 42.0 ± 0.58* |
| ω6-PUFA | 37.2 ± 0.46 | 34.6 ± 0.55* |
| ω3-PUFA | 7.43 ± 0.16 | 7.17 ± 0.16 |
Values are expressed as means ± SE. All triglycerides and cholesterol measurements were performed in plasma from 6 mo-old mice (n ≥ 5). Fatty acid composition was performed on n > 9–11 mice per group.
P < 0.05 vs. Cc1+/+.
Elevated proinflammatory state in Cc1−/− mice.
Consistent with increased inflammation in visceral obesity, mRNA levels of WAT-associated F4/80 and the proinflammatory interleukin-6 (IL-6) and TNFα cytokines, in addition to monocyte chemotactic protein-1 (MCP-1) and interferon (IFN)γ chemokines were elevated (∼2-fold or more) in Cc1−/− mice (Table 4). Because CEACAM1 is not expressed in adipocytes, the rise in WAT-associated inflammation likely results from a hyperinsulinemia-driven increase in lipid production in liver and redistribution to WAT for storage (8).
Table 4.
mRNA levels of macrophage and inflammatory markers in aorta and white adipose tissue of 6-mo-old mice
| Cc1+/+ | Ccl−/− | |
|---|---|---|
| Aorta | ||
| VCAM-1 | 0.03 ± 0.01 | 0.06 ± 0.01† |
| F4/80 | 0.04 ± 0.01 | 0.11 ± 0.01† |
| TNFα | 1.08 ± 0.31 | 4.78 ± 0.36† |
| CD3 | 0.02 ± 0.00 | 0.99 ± 0.01‡ |
| CD4 | 0.01 ± 0.01 | 0.23 ± 0.01‡ |
| CD8 | 0.02 ± 0.01 | 0.38 ± 0.00‡ |
| TLR2 | 0.53 ± 0.06 | 1.07 ± 0.11‡ |
| TLR4 | 0.15 ± 0.01 | 0.28 ± 0.04† |
| WAT | ||
| F4/80 | 1.18 ± 0.16 | 1.67 ± 0.16* |
| TNFα | 0.03 ± 0.01 | 0.05 ± 0.01‡ |
| IL-6 | 0.14 ± 0.04 | 0.40 ± 0.12* |
| IFNγ | 0.04 ± 0.01 | 0.06 ± 0.01† |
| MCP-1 | 2.83 ± 0.16 | 4.79 ± 0.50† |
Values are expressed as means ± SE. See text for definitions. Aortic arch was isolated from 6-mo-old male mice (n ≥ 6) to measure mRNA content by qRT-PCR (normalized to Gapdh).
P < 0.05,
P < 0.01,
P < 0.001 vs. Cc1+/+.
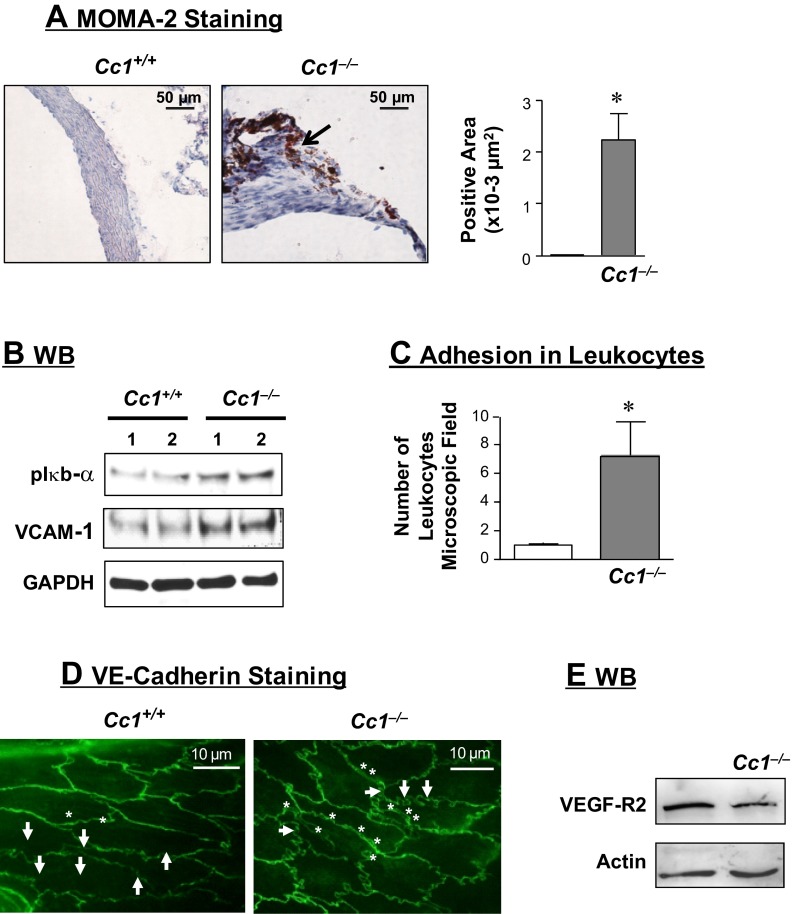
Immunostaining of cross-sections of aortic roots with monocyte and macrophage antibody 2 (MOMA-2) showed inflammatory infiltration in the aortic wall of Cc1−/− mice at 6–12 mo of age (Fig. 2A and graph). qRT-PCR analysis of Cc1−/− aortae revealed increased mRNA levels of F4/80 (a macrophage marker), T cell markers (CD3+, CD4+, and CD8+T), and Toll-like receptor 2 and 4 (TLR-2 and -4) with their target gene, the TNFα inflammatory cytokine (Table 4).
Fig. 2.
Vascular inflammation and leukocyte adhesion. A: immunostaining cross-sections of aortic root with monocyte and macrophage antibody 2 (MOMA-2; n > 5 of 6-mo-old mice per group). Stained area was measured and presented in the accompanying graph. Values are presented as means ± SE. *P < 0.05 vs. Cc1+/+. B: Western blot (WB) analysis of phospho-Iκbα and vascular cell adhesion molecule-1 (VCAM-1) content in aortic lysates from 6-mo-old mice, normalized against GAPDH. Gel represents 4 mice per group, but only 2 mice were included. C: aortic segments from 8-mo-old mice (n > 5) were incubated with isolated leukocytes for in vitro measurement of leukocyte adhesion. Values are presented as means ± SE. *P < 0.05 vs. Cc1+/+. D: en face VE-cadherin analysis of aortae (n > 5 of 6-mo-old mice per group). E: WB analysis of vascular endothelial growth factor receptor 2 (VEGFR-2) content in aortic lysates derived from 6-mo-old mice. Gel represents 3 mice per group.
Elevated Iκbα phosphorylation (Fig. 2B) indicates activation of the NF-κB inflammatory pathway (20), which induces the transcription of TNFα and the vascular cell adhesion molecule-1 (VCAM-1), as shown by increased mRNA (Table 4) and protein levels (Fig. 2B) in aortae derived from 6-mo-old Cc1−/− mice. Consistent with VCAM-1 playing an important role in attracting leukocytes to endothelium (6, 7), leukocyte adhesion to aortic wall was elevated in Cc1−/− mice (Fig. 2C).
Impaired endothelial membrane integrity in Cc1−/− aortae.
We then examined the level of VE-cadherin, a major transmembrane adhesion molecule of the endothelial adherent junctions that preserves endothelial integrity and regulates leukocyte adhesion (1). En face staining revealed elongated endothelial cells that were oriented longitudinally to the aortic axis in Cc1+/+ mice, as opposed to Cc1−/− aortae, in which the majority of endothelial cells were mostly round and with a cell border exhibiting more meander (Fig. 2D, arrows). Cc1−/− aortae also exhibited increased number and size of areas with separation of the VE-cadherin barrier and altered junction between neighboring endothelial cells (Fig. 2D, *). Because CEACAM1 regulates the activity of β-catenin (16), which in turn regulates VE-cadherin (18), it is possible that the decrease in β-catenin mRNA levels (Table 5) contributes to the aberrant intercellular junctions seen in Cc1−/− aortae.
Table 5.
mRNA levels of vascular markers in aortae of 6-mo-old mice
| Cc1+/+ | Ccl−/− | |
|---|---|---|
| Ang-1 | 0.98 ± 0.24 | 0.25 ± 0.01* |
| Ang-2 | 0.37 ± 0.05 | 0.10 ± 0.02† |
| VEGF-A | 9.53 ± 1.38 | 3.40 ± 0.41† |
| VEGF-C | 0.14 ± 0.02 | 0.10 ± 0.01 |
| VEGF-D | 0.62 ± 0.08 | 0.72 ± 0.10 |
| VEGFR-1 | 1.91 ± 0.22 | 0.60 ± 0.10‡ |
| VEGFR-2 | 1.31 ± 0.13 | 0.50 ± 0.06‡ |
| β-Catenin | 8.22 ± 0.73 | 4.83 ± 0.36† |
Values are expressed as means ± SE. Ang, angiopoietin; VEGF, vascular endothelial growth factor. Aortic arch was isolated from 6-mo-old male mice (n ≥ 6) to measure mRNA content by qRT-PCR (normalized to Gapdh).
P < 0.01,
P < 0.001,
P < 0.0001 vs. Cc1+/+.
In support of a role for CEACAM1 in regulating the expression of several factors relevant in endothelial barrier and vascular integrity in cultured endothelial cells (17), Cc1−/− aortae showed a more than threefold decrease in the mRNA (Table 5) of VEGF-A, its receptors VEGFR-1 and -2, and angiopoietins (Ang-1 and -2). Western blot analysis demonstrated an ∼40% reduction of aortic VEGFR-2 (Fig. 2E). In contrast, mRNA levels of VEGF-C and VEGF-D, which are mainly involved in the formation of lymphatic vessels, were not altered (Table 5).
Reduced endothelial relaxation in Cc1−/− aortae.
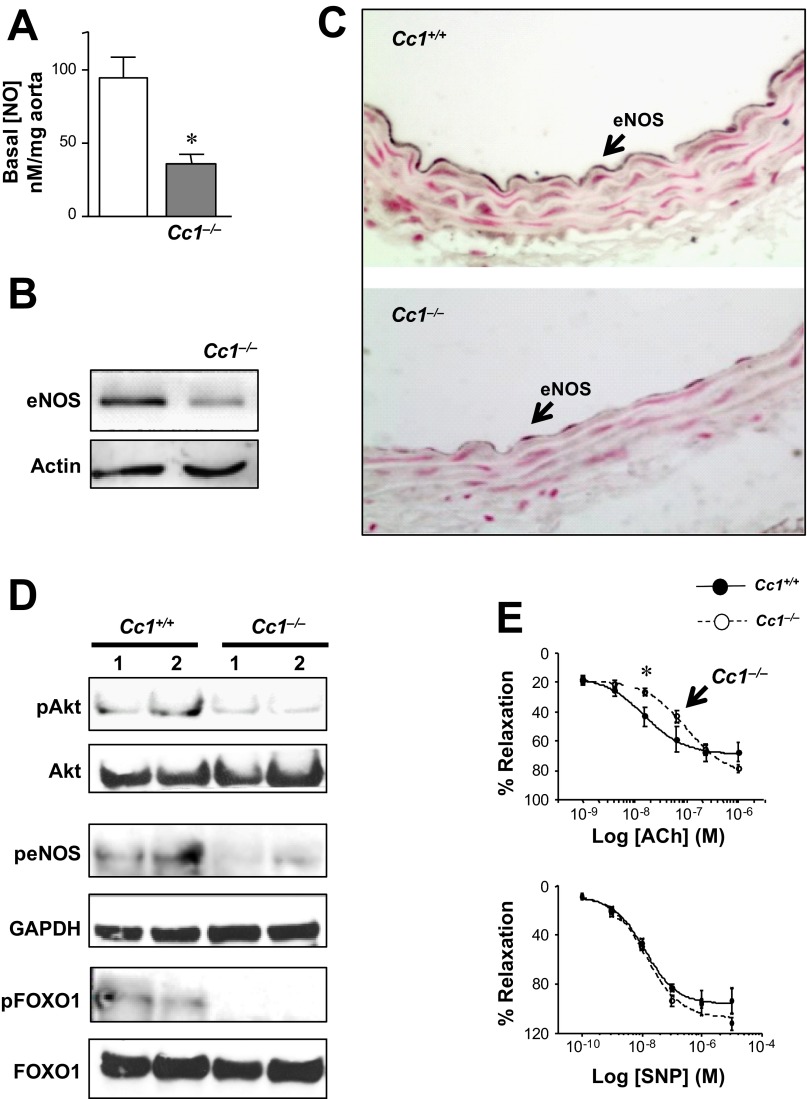
Because loss of NO initiates the development of proatherogenic risk factors, including increased vascular permeability, VCAM-1 levels and chronic inflammation of the arterial wall (15), we next examined NO levels in Cc1−/− aortae. Basal endothelial NO content was lower (by ∼60%) in the aortae of 6-mo-old Cc1−/− mice (Fig. 3A). This could in part result from a reduced eNOS level, as shown by Western (Fig. 3B) and immunohistochemical (Fig. 3C) analyses, and eNOS activation (phosphorylation) by Akt-dependent pathways (Fig. 3D). Reduction of eNOS levels in turn could result, in part, from decreased transcription by activated (dephosphorylated) Foxo1 (Fig. 3D) (27, 34) in these insulin-resistant mice (8).
Fig. 3.
Basal and ligand-stimulated aortic NO level. A–D: mice were 6 mo of age. A: basal release of endothelial NO (nM/g tissue) in segments of aortae isolated from 6-mo-old mice (n = 4, 2–3 segments per mouse). Values are presented as means ± SE. *P < 0.05 vs. Cc1+/+. B: WB analysis of eNOS content. Gel represents 3 mice per group. C: immunostaining cross-sections of the aortic arch (n = 5 per group); arrows point to the endothelial cell layer. D: WB analysis of pAkt, peNOS, and pFoxo1 in aortic lysates to measure activity. peNOS was normalized against GAPDH because total eNOS is reduced (B), to examine activation of eNOS independently of the decrease in its expression. Gels represent 4 mice per group, but only 2 mice were included. E: ACh and sodium nitroprusside (SNP)-induced vasorelaxation in thoracic aorta segments (n > 5 of 9-mo-old mice/group). Values are presented as means ± SE. *P < 0.05 vs. Cc1+/+.
Functional studies in aortic rings revealed an approximately twofold increase in the EC50 of endothelial NO-dependent relaxation in response to acetylcholine (0.170 ± 0.023 vs. 0.085 ± 0.003 μM in Cc1+/+, P < 0.05) but not in the EC50 of endothelial-independent relaxation in response to nitroprusside (P > 0.05) in Cc1−/− mice (Fig. 3E). This demonstrates a moderate decrease in basal and ligand-stimulated relaxation in Cc1−/− aortae.
Elevated lipid peroxidation and oxidative stress in Cc1−/− mice.
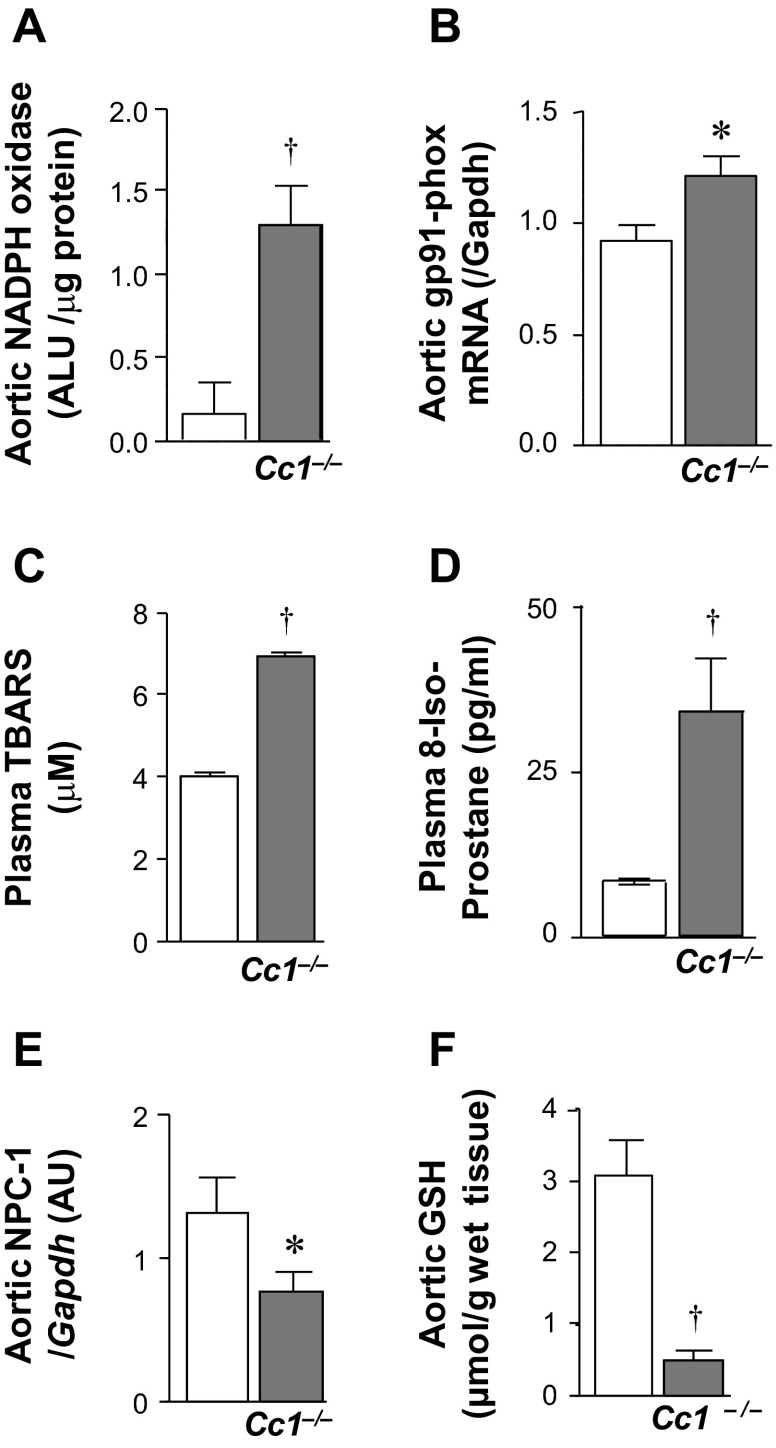
In addition to lower eNOS activation, increased catalytic NO consumption by a high oxidative environment could reduce NO levels, and elevation of NADPH oxidase activity contributes to increased macrophage adherence to the artery wall and lipid peroxidation. Thus, we examined whether Cc1−/− aortae develop oxidative stress. NADPH oxidase activity and mRNA of gp91-phox (Fig. 4, A and B) and Nox4 (Table 2) were elevated in aortic lysates derived from 6-mo-old Cc1−/− mice. Together with elevated concentrations of plasma TBARS and 8-isoprostane (Fig. 4, C and D), this suggests that Cc1−/− mice developed oxidative stress and lipid peroxidation.
Fig. 4.
Oxidative stress and nitroso-redox evaluation. Aortae were removed from 6-mo-old mice (n > 5 per group) and analyzed for: A: NADPH oxidase activity; B: gp91 phox mRNA levels (by qRT-PCR); E: Niemann-Pick disease, type c1 (NPC-1) mRNA levels; and F: GSH content. C and D: plasma lipid peroxidation levels by thiobarbituric acid-reactive substances (TBARS; n ≥ 8 each age group) and 8-isoprostane. Values are presented as means ± SE. *P < 0.05 and †P < 0.01 vs. Cc1+/+.
Moreover, reduction in Niemann-Pick type C1 protein (NPC-1) represses GSH, an important mitochondrial defence system against the cytotoxic effect of TNFα. qRT-PCR analysis revealed a significant (∼2-fold) reduction in NPC-1 mRNA levels in Cc1−/− aortae (Fig. 4E) in association with a marked (∼6-fold) reduction in GSH (Fig. 4F). The decrease in GSH could have caused a robust response to the TNFα cytotoxic effect.
Potential underlying endothelial cell autonomous mechanisms.
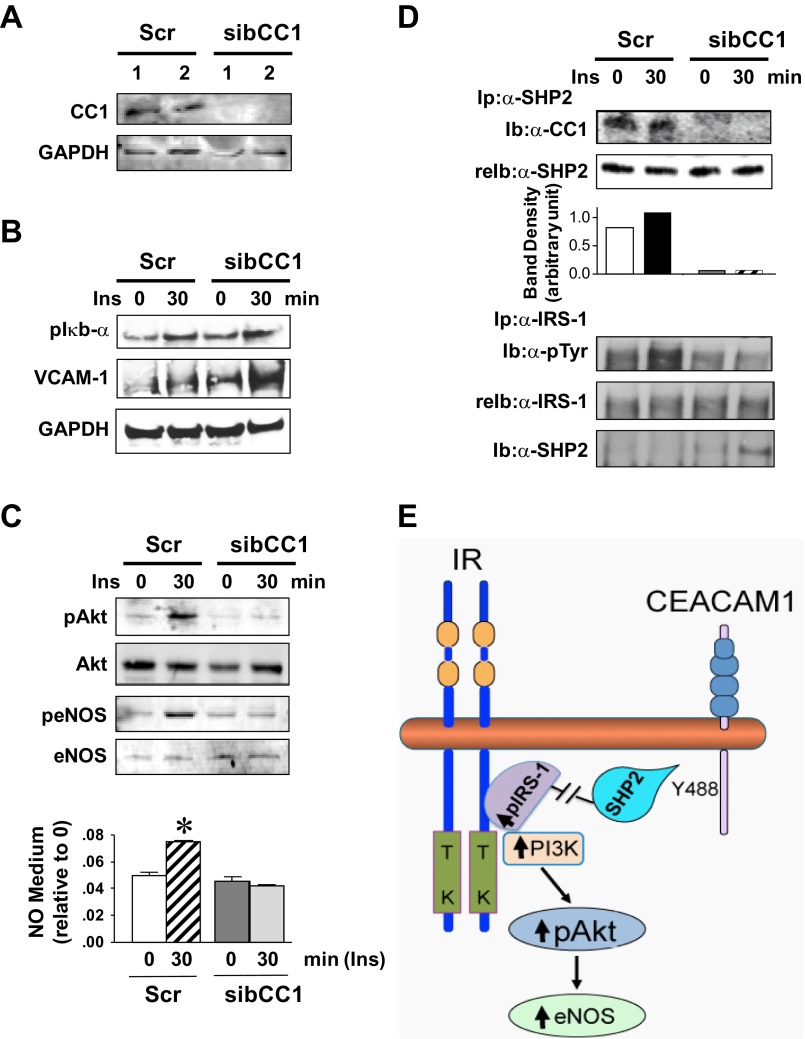
Next, siRNA-reduction of CEACAM1 in bovine aortic endothelial cells (BAEC) was employed to determine the endothelial cell autonomous mechanisms underlying changes in VCAM-1 and NO levels in Cc1−/− aortae. Decreasing CEACAM1 protein levels by >90% (Fig. 5A) induced the transcriptional activity of NF-κB, as inferred from elevated Iκb-α phosphorylation (Fig. 5B), to elevate VCAM-1 protein levels about twofold.
Fig. 5.
Cell autonomous effect of Ceacam1 deletion on Akt/eNOS activation in cultured bovine aortic endothelial (BAE) cells. A: BAE cells were transfected with scrambled (Scr) or bovine CEACAM1-specific siRNA (sibCC1) prior to WB analysis of lysates with anti-CEACAM1 antibody to assess loss of CEACAM1. B: cells were serum starved overnight and treated with insulin (100 nM) for 0–30 min and their lysates analyzed for p-Iκbα and VCAM-1 content. C: lysates were analyzed for Akt/eNOS phosphorylation and cell media assayed for NO level. Gels represent 3 different experiments, and NO data are represented as means ± SE of ≥3 experiments. *P < 0.05 vs. time 0 per group. D: some lysates were immunoprecipitated (Ip) with α-SHP2 (Src homology tyrosine phosphatase 2) prior to being immunoblotted (Ib) with α-CC1 (top) or with α-IRS-1 (bottom) prior to being immunoblotted sequentially with α-SHP2 to assess binding, and with α-phosphotyrosine (α-p-Tyr) antibody to assess phosphorylation. Gels represent 3 different experiments. E: working model summarizing the mechanisms underlying the cell autonomous effect of CEACAM1 on Akt/eNOS activation.
Because acetylcholine-mediated relaxation requires eNOS phosphorylation, which is regulated, among other things, by insulin (41), we next investigated whether Ceacam1 deletion in endothelial cells adversely affects insulin's regulation of eNOS phosphorylation and NO production. As previously reported (24), Western blot analysis revealed that siRNA knockdown of CEACAM1 (sibCC1) in BAEC did not significantly affect basal phosphorylation (activation) of the Akt/eNOS pathway and, consequently, basal NO level relative to control scrambled RNA cells (Scr) (Fig. 5C). However, it impeded the ability of insulin to activate the Akt/eNOS pathway and stimulate NO production (Fig. 5C). Coimmunoprecipitation experiments revealed binding of CEACAM1 to the Src homology tyrosine phosphatase-2 (SHP2) in Scr cells, as previously shown (14), but not in sibCC1 cells (Fig. 5D). This caused a decrease in SHP2 binding to insulin receptor substrate-1 (IRS-1) in response to insulin and, consequently, an increase in insulin-stimulated tyrosine phosphorylation of IRS-1 in Scr but not sibCC1 cells, in which insulin induced an approximately twofold higher IRS-1 binding to SHP2 than in Scr cells (Fig. 5D). As summarized in Fig. 5E, CEACAM1 binding to SHP2 caused its sequestration, reciprocally limiting IRS-1 binding to SHP2 and its dephosphorylation. This led to a higher activation of IRS-1 and the downstream PI3K/Akt/eNOS pathway in response to insulin. Thus, CEACAM1 appears to play an important role in insulin's regulation of NO production via the Akt/eNOS pathway in aortic endothelial cells.
DISCUSSION
Mice with global Ceacam1 deletion (Cc1−/−) display impaired endothelial barrier resulting from increased basal endothelial permeability (24). They also exhibit hyperinsulinemia at 2 mo of age resulting from impaired insulin clearance (8, 28, 39) in addition to liver steatosis and fibrosis with predisposition to diet-induced nonalcoholic steatohepatitis (12). Because hyperinsulinemia drives global ectopic fat accumulation (4), we investigated whether Ceacam1 deletion also causes fat deposition in large vessels together with an associated rise in inflammatory infiltration (3). We herein present a first in vivo demonstration linking global Ceacam1 deletion to the formation of small intimal plaque-like lesions exhibiting subendothelial deposition of lipids and fibrotic materials with increased inflammatory infiltration and leukocyte adhesion, and elevated oxidative stress in large vessels in the absence of proatherogenic hypercholesterolemia. Eventually, these vascular derangements are accompanied by a reduced endothelial NO-dependent relaxation in aortic rings.
The vascular abnormalities in Cc1−/− aortae are associated with reduced basal NO level, which could be caused by multiple mechanisms, including lower eNOS levels (likely resulting from Foxo1-dependent downregulation of eNOS transcription) (27, 34) and eNOS inactivation via an Akt-dependent pathway. Both events could, in turn, be caused, at least partly, by the systemic insulin resistance state resulting from impaired hepatic insulin clearance in these mice (8). Hyperinsulinemia and elevated plasma NEFA could contribute to systemic regulation of eNOS level/activation, although the relative composition of NEFA in these mice is not commonly associated with marked vascular anomalies (21, 37). For instance, elevated plasma NEFA could reduce eNOS mRNA stability by increasing TLR-2/4-mediated TNFα transcription (30, 40), which is elevated in Cc1−/− aortae. On the other hand, reduction in basal Akt activation in parallel to that of eNOS precludes a significant role for a NEFA-induced ROS-mediated (9), Akt-independent (32) pathway regulating NO production in Cc1−/− aortae.
Normal basal eNOS activation and NO level in BAEC with siRNA-mediated reduction of CEACAM1 supports a role for a negative systemic effect on endothelial NO production. However, impediment of insulin-induced Akt/eNOS-mediated NO production in these cells demonstrated an additional cell autonomous negative effect of CEACAM1 deletion on insulin signaling. This appears to be mediated by reduction in CEACAM1 sequestration of SHP2 phosphatase and a reciprocal increase in its binding to IRS-1, causing IRS-1 dephosphorylation and deactivation of downstream Akt signaling pathways. Because CEACAM1 deletion caused a similar negative effect on Akt/eNOS-mediated NO production in response to VEGF-A in BAEC (24), CEACAM1, a common substrate of insulin receptor and VEGFR-2 (22, 24), could link insulin to VEGF actions with regard to Akt2/eNOS or Akt1/eNOS activation, respectively (32), in an endothelial cell autonomous manner.
Global Ceacam1 deletion induced leukocyte adhesion to the aortic wall, which could be partly mediated by the elevated level of the VCAM-1 leukocyte adhesion molecule. Several endothelial cell autonomous and nonautonomous (systemic) factors could contribute to this molecular event. For instance, siRNA reduction of CEACAM1 induced VCAM-1 expression in BAEC by activating NF-κB-mediated transcriptional activity. Systemic factors such as hyperinsulinemia could also be involved in VCAM-1 regulation. For instance, hyperinsulinemia induces fat production, which alters the inflammatory milieu (3), causing macrophage recruitment and a rise in TNFα and other proinflammatory cytokines. TNFα induces expression of VCAM-1 directly (36, 42) and indirectly, by reducing the repressive effect of NO (7). In the presence of low GSH, the response to TNFα activation of IκKβ/NF-κB-dependent oxidative stress and inflammatory pathways is more robust (5). Given that oxidative stress and inflammation were also detected in the liver of Cc1−/− mice (12), we postulate that hyperinsulinemia, caused by impaired hepatic insulin clearance, links lipid accumulation, inflammation, and oxidative stress to global Ceacam1 deletion.
Despite developing a constellation of proatherogenic factors, such as hyperinsulinemia, oxidative stress, reduced NO production, and endothelial NO-dependent relaxation, in addition to increased vascular permeability and chronic inflammation, Cc1−/− mice exhibited restricted plaque-like lesions. These morphological abnormalities were limited to the formation of small subintimal foci of lipid accumulation and macrophage infiltration, likely owing to the absence of proatherogenic cholesterolemia (38) as well as to Foxo1 activation in aortae, which has been associated with restricted atheroma (35).
In summary, the present studies provide the first in vivo evidence that Ceacam1 deletion causes the formation of small plaque-like intimal lesions accompanied by adventitial reactions in large vessels and that decreased insulin-stimulated NO production in aortic endothelial cells could contribute to altered ligand-induced endothelial cell-dependent relaxation along the large vessel wall. Given the early onset of morphological lesions in Cc1−/− aortae, further studies using tissue-targeted Ceacam1 deletion along the liver-endothelial cell axis are needed to understand whether these are caused by deletion of Ceacam1 in endothelial cells or are related to hyperinsulinemia caused by impaired insulin clearance in liver. Nonetheless, the similarity of this vascular phenotype to that in the liver (fatty liver, inflammation, oxidative stress, and fibrosis) and progression of the hepatic phenotype to advanced NASH in response to high fat intake (12) predict a progression of vascular abnormalities to more robust vascular lesions of earlier onset than their wild-type animals, if mice were fed an atherogenic diet. However, this would not promote a role for hyperinsulinemia distinct from hypercholesterolemia in the pathogenesis of early vascular abnormalities, which is the most remarkable attribute of the current findings. Thus, the Cc1−/− mouse provides a unique in vivo demonstration of distinct CEACAM1-dependent hepatic insulin clearance linking hepatic to early macrovascular abnormalities.
GRANTS
This work was supported by grants from the National Institutes of Health: R01 DK-054254, R01 DK-083850, and R01 HL-112248 (S. M. Najjar), P01 HL-36573 (S. V. Pierre and S. M. Najjar), R01 HL-111877 (G. Vazquez), R01 HL-45095 (I. J. Goldberg), and R01 DK-064344 (R. Scalia); American Heart Association-Great Rivers Affiliate (075100B, G. Heinrich); US Department of Agriculture (USDA 38903-19826, S. M. Najjar); Canadian Institutes of Health Research (CIHR MOP-86582, N. Beauchemin), and Deutsche Forschungsgemeinschaft (DFG ER 276 4-4 and DFG TI 690 2-1 ER 276 7-1) (S. Ergün).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M.N., G.H., S.V.P., R.B., A.A.J., G.V., R.S., and S.E. conception and design of research; S.M.N., K.J.L., L.R., S.V.P., R.B., A.A.J., E.L., G.V., I.J.G., R.S., and S.E. analyzed data; S.M.N., K.J.L., S.L.A., G.V., R.S., and S.E. interpreted results of experiments; S.M.N., S.L.A., A.P., L.R., and S.E. prepared figures; S.M.N., K.J.L., S.L.A., and S.E. drafted manuscript; S.M.N., G.V., R.S., and S.E. edited and revised manuscript; S.M.N., K.J.L., S.L.A., A.P., L.R., M.K.K., S.R., H.T.M., C.K.R., S.G.L., G.H., S.V.P., R.B., V.K., A.A.J., E.L., G.V., I.J.G., N.B., R.S., and S.E. approved final version of manuscript; K.J.L., S.L.A., A.P., L.R., M.K.K., S.R., H.T.M., C.K.R., S.G.L., G.H., V.K., E.L., I.J.G., N.B., and R.S. performed experiments.
ACKNOWLEDGMENTS
We thank M. Kopfman and J. Kalisz (Najjar laboratory) as well as D. Schünke (Institute of Anatomy, University Hospital Essen), and G. Landesberg (Temple University) for excellent technical assistance. We also thank A.-L. Nouvion (Beauchemin laboratory), K. Preston (Scalia laboratory), and H. Jastrow and B. B. Singer (University Hospital Essen) for their technical assistance and scientific discussions. We also thank Dr. Z. A. Shah (University of Toledo College of Pharmacy) for the use of the Synergy H1 Hybrid Multi-Mode Microplate Reader, and D. Accili (Columbia University) for helpful scientific discussions.
REFERENCES
- 1. Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW. Endothelial-dependent mechanisms regulate leukocyte transmigration: a process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J Exp Med 186: 517–527, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bieghs V, Rensen PC, Hofker MH, Shiri-Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis 220: 287–293, 2012 [DOI] [PubMed] [Google Scholar]
- 3. Bigorgne AE, Bouchet-Delbos L, Naveau S, Dagher I, Prevot S, Durand-Gasselin I, Couderc J, Valet P, Emilie D, Perlemuter G. Obesity-induced lymphocyte hyperresponsiveness to chemokines: a new mechanism of Fatty liver inflammation in obese mice. Gastroenterology 134: 1459–1469, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7: 95–96, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Crespo J, Cayon A, Fernandez-Gil P, Hernandez-Guerra M, Mayorga M, Dominguez-Diez A, Fernandez-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 34: 1158–1163, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 107: 1255–1262, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 96: 60–68, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeAngelis AM, Heinrich G, Dai T, Bowman TA, Patel PR, Lee SJ, Hong EG, Jung DY, Assmann A, Kulkarni RN, Kim JK, Najjar SM. Carcinoembryonic antigen-related cell adhesion molecule 1: a link between insulin and lipid metabolism. Diabetes 57: 2296–2303, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edirisinghe I, McCormick Hallam K, Kappagoda CT. Effect of fatty acids on endothelium-dependent relaxation in the rabbit aorta. Clin Sci (Lond) 111: 145–151, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Ergun S, Kilik N, Ziegeler G, Hansen A, Nollau P, Gotze J, Wurmbach JH, Horst A, Weil J, Fernando M, Wagener C. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell 5: 311–320, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Gerstel D, Wegwitz F, Jannasch K, Ludewig P, Scheike K, Alves F, Beauchemin N, Deppert W, Wagener C, Horst AK. CEACAM1 creates a pro-angiogenic tumor microenvironment that supports tumor vessel maturation. Oncogene 30: 4275–4288, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Ghosh S, Kaw M, Patel PR, Ledford KJ, Bowman TA, McLnerney MF, Erickson SK, Bourey RE, Najjar SM. Mice with null mutation of Ceacam I develop nonalcoholic steatohepatitis. Hepat Med: Res Evidence 2010: 69–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldberg IJ, Hu Y, Noh HL, Wei J, Huggins LA, Rackmill MG, Hamai H, Reid BN, Blaner WS, Huang LS. Decreased lipoprotein clearance is responsible for increased cholesterol in LDL receptor knockout mice with streptozotocin-induced diabetes. Diabetes 57: 1674–1682, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem 274: 335–344, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Jimenez A, Arriero MM, Lopez-Blaya A, Gonzalez-Fernandez F, Garcia R, Fortes J, Millas I, Velasco S, Sanchez De Miguel L, Rico L, Farre J, Casado S, Lopez-Farre A. Regulation of endothelial nitric oxide synthase expression in the vascular wall and in mononuclear cells from hypercholesterolemic rabbits. Circulation 104: 1822–1830, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Jin L, Li Y, Chen CJ, Sherman MA, Le K, Shively JE. Direct interaction of tumor suppressor CEACAM1 with beta catenin: identification of key residues in the long cytoplasmic domain. Exp Biol Med (Maywood) 233: 849–859, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kilic N, Oliveira-Ferrer L, Wurmbach JH, Loges S, Chalajour F, Neshat-Vahid S, Weil J, Fernando M, Ergun S. Pro-angiogenic signaling by the endothelial presence of CEACAM1. J Biol Chem 280: 2361–2369, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129: 203–217, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee W. The CEACAM1 expression is decreased in the liver of severely obese patients with or without diabetes. Diagn Pathol 6: 40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today 19: 80–88, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Merchant AT, Kelemen LE, de Koning L, Lonn E, Vuksan V, Jacobs R, Davis B, Teo KK, Yusuf S, Anand SS. Interrelation of saturated fat, trans fat, alcohol intake, and subclinical atherosclerosis. Am J Clin Nutr 87: 168–174, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Najjar SM, Philippe N, Suzuki Y, Ignacio GA, Formisano P, Accili D, Taylor SI. Insulin-stimulated phosphorylation of recombinant pp120/HA4, an endogenous substrate of the insulin receptor tyrosine kinase. Biochemistry 34: 9341–9349, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Najjar SM, Yang Y, Fernstrom MA, Lee SJ, Deangelis AM, Rjaily GA, Al-Share QY, Dai T, Miller TA, Ratnam S, Ruch RJ, Smith S, Lin SH, Beauchemin N, Oyarce AM. Insulin acutely decreases hepatic fatty acid synthase activity. Cell Metab 2: 43–53, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Nouvion AL, Oubaha M, Leblanc S, Davis EC, Jastrow H, Kammerer R, Breton V, Turbide C, Ergun S, Gratton JP, Beauchemin N. CEACAM1: a key regulator of vascular permeability. J Cell Sci 123: 4221–4230, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Otogawa K, Kinoshita K, Fujii H, Sakabe M, Shiga R, Nakatani K, Ikeda K, Nakajima Y, Ikura Y, Ueda M, Arakawa T, Hato F, Kawada N. Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: implications for the pathogenesis of human nonalcoholic steatohepatitis. Am J Pathol 170: 967–980, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ouedraogo R, Gong Y, Berzins B, Wu X, Mahadev K, Hough K, Chan L, Goldstein BJ, Scalia R. Adiponectin deficiency increases leukocyte-endothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest 117: 1718–1726, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potente M, Urbich C, Sasaki KI, Hofmann WK, Heeschen C, Aicher A, Kollipara R, Depinho RA, Zeiher AM, Dimmeler S. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest 115: 2382–2392, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poy MN, Yang Y, Rezaei K, Fernstrom MA, Lee AD, Kido Y, Erickson SK, Najjar SM. CEACAM1 regulates insulin clearance in liver. Nat Genet 30: 270–276, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall'Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab 11: 379–389, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cersosimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 57: 2595–2602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Spahis S, Vanasse M, Belanger SA, Ghadirian P, Grenier E, Levy E. Lipid profile, fatty acid composition and pro- and anti-oxidant status in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids 79: 47–53, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104: 1085–1094, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tajima S, Ikeda Y, Sawada K, Yamano N, Horinouchi Y, Kihira Y, Ishizawa K, Izawa-Ishizawa Y, Kawazoe K, Tomita S, Minakuchi K, Tsuchiya K, Tamaki T. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. Am J Physiol Endocrinol Metab 302: E77–E86, 2012 [DOI] [PubMed] [Google Scholar]
- 34. Tanaka J, Qiang L, Banks AS, Welch CL, Matsumoto M, Kitamura T, Ido-Kitamura Y, DePinho RA, Accili D. Foxo1 links hyperglycemia to LDL oxidation and endothelial nitric oxide synthase dysfunction in vascular endothelial cells. Diabetes 58: 2344–2354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab 15: 372–381, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walker T, Wendel HP, Tetzloff L, Raabe C, Heidenreich O, Simon P, Scheule AM, Ziemer G. Inhibition of adhesion molecule expression on human venous endothelial cells by non-viral siRNA transfection. J Cell Mol Med 11: 139–147, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wan JB, Huang LL, Rong R, Tan R, Wang J, Kang JX. Endogenously decreasing tissue n-6/n-3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E-deficient mice by inhibiting systemic and vascular inflammation. Arterioscler Thromb Vasc Biol 30: 2487–2494, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med 5: 91–102, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Xu E, Dubois MJ, Leung N, Charbonneau A, Turbide C, Avramoglu RK, DeMarte L, Elchebly M, Streichert T, Levy E, Beauchemin N, Marette A. Targeted disruption of carcinoembryonic antigen-related cell adhesion molecule 1 promotes diet-induced hepatic steatosis and insulin resistance. Endocrinology 150: 3503–3512, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Yan G, You B, Chen SP, Liao JK, Sun J. Tumor necrosis factor-alpha downregulates endothelial nitric oxide synthase mRNA stability via translation elongation factor 1-alpha 1. Circ Res 103: 591–597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101: 1539–1545, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Zhang J, Alcaide P, Liu L, Sun J, He A, Luscinskas FW, Shi GP. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One 6: e14525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]