Abstract
Activation of angiotensin receptor type 1 (AT1) contributes to NADPH oxidase (Nox)-derived oxidative stress during metabolic syndrome. However, the specific role of AT1 in modulating redox signaling, mitochondrial function, and oxidative stress in the heart remains more elusive. To test the hypothesis that AT1 activation increases oxidative stress while impairing redox signaling and mitochondrial function in the heart during diet-induced insulin resistance in obese animals, Otsuka Long Evans Tokushima Fatty (OLETF) rats (n = 8/group) were treated with the AT1 blocker (ARB) olmesartan for 6 wk. Cardiac Nox2 protein expression increased 40% in OLETF compared with age-matched, lean, strain-control Long Evans Tokushima Otsuka (LETO) rats, while mRNA and protein expression of the H2O2-producing Nox4 increased 40–100%. ARB treatment prevented the increase in Nox2 without altering Nox4. ARB treatment also normalized the increased levels of protein and lipid oxidation (nitrotyrosine, 4-hydroxynonenal) and increased the redox-sensitive transcription factor Nrf2 by 30% and the activity of antioxidant enzymes (SOD, catalase, GPx) by 50–70%. Citrate synthase (CS) and succinate dehydrogenase (SDH) activities decreased 60–70%, whereas cardiac succinate levels decreased 35% in OLETF compared with LETO, suggesting that mitochondrial function in the heart is impaired during obesity-induced insulin resistance. ARB treatment normalized CS and SDH activities, as well as succinate levels, while increasing AMPK and normalizing Akt, suggesting that AT1 activation also impairs cellular metabolism in the diabetic heart. These data suggest that the cardiovascular complications associated with metabolic syndrome may result from AT1 receptor-mediated Nox2 activation leading to impaired redox signaling, mitochondrial activity, and dysregulation of cellular metabolism in the heart.
Keywords: NADPH oxidase, metabolic syndrome, Nrf2
insulin resistance is characterized by incomplete glucose utilization and metabolism due to decreased secretion and/or impaired insulin signaling (36), and is frequently associated with hypertension and cardiovascular disease (15, 20, 43). Activation of the angiotensin II (Ang II) type 1 receptor (AT1) contributes to the manifestation of insulin resistance (23, 40) by stimulating oxidant generation through the activation of NADPH oxidase (Nox) (6). In the heart, Nox2 and Nox4 are coexpressed but may have contrasting functions (3, 11), which may be due to differential cellular localization, the types of the oxidants produced, and the need to recruit cytosolic subunits for activation (7, 22, 45, 52). Nox2 is typically involved in cardiac hypertrophy and contractile dysfunction induced by Ang II, pressure overload, and myocardial infarction (5, 9, 11, 31, 34, 38). In contrast, Nox4 can be beneficial during cardiac remodeling, pressure overload, or myocardial infarction (42, 45, 62). Moreover, H2O2 generated by Nox4 has been implicated in activating p38 mitogen-activated protein kinase (32) alleviating cardiac stress and improving insulin resistance by activating the redox-sensitive transcription factor Nrf2 (9, 45). This is important because Nfr2 regulates the expression of antioxidant genes (24).
Besides increasing Nox activity, and thus oxidant generation, metabolic disorders, such as hyperglycemia and hyperlipidemia, can impair mitochondrial function by increasing mitochondrial oxidant production and the subsequent oxidative damage (47, 48). Moreover, increased rates of superoxide production induced by elevated Nox2 activity can also promote the uncoupling of nitric oxide synthase (NOS), further increasing oxidant generation and impairing antioxidant defenses due to the depletion of NADPH (21). Therefore, limiting the assemblage of the membrane-bound Nox2 complex by blocking AT1 during the development of the metabolic syndrome may also be effective in reducing mitochondrial oxidant generation and improving energy balance and overall cardiac function without altering Nox4-derived redox signaling (16, 17, 21).
Although the contribution of AT1 signaling to the increase in Nox2-derived oxidative stress in cardiovascular and metabolic disease is well established (25, 49, 50, 63), the associations among AT1 signaling, mitochondrial function, and redox-mediated adaptive responses (e.g., Nrf2 activation) during insulin resistance have not been examined simultaneously in the diabetic heart. Therefore, using Otsuka Long Evans Tokushima Fatty (OLETF) rats, a model of diet-induced obesity, hyperlipidemia, insulin resistance, and hypertension (26, 27, 37, 44), we tested the hypothesis that AT1 activation increases Nox2 expression and oxidative damage, and impairs redox signaling and mitochondrial function in the heart of obese, insulin-resistant rats.
METHODS
All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committees of Kagawa Medical University, Japan, and the University of California, Merced.
Animals.
Male, 9-wk-old, lean (256 ± 6 g), strain-control Long Evans Tokushima Otsuka (LETO) and age-matched, obese (351 ± 2 g), Otsuka Long Evans Tokushima Fatty (OLETF) rats (Otsuka Pharmaceutical, Tokushima, Japan) were randomly assigned to one of the following groups (n = 8/group): 1) LETO, 2) untreated OLETF, and 3) OLETF + ARB (ARB; 10 mg olmesartan·kg−1·day−1). Our decision to exclude LETO + ARB group a priori was based on the following: 1) LETOs are normotensive, and ARB treatment in this group does not reduce their SBP significantly less than normal and thus would not allow us to decipher the hypertension component beyond that in the OLETF + ARB group; 2) although we have shown that ARB treatment does significantly reduce body mass (BM) and retro and epi fat compared with untreated animals in the OLETF strain (44), ARB treatment in LETO does not significantly reduce BM or fat mass (unpublished observations), which would not provide any further information on the contribution of the obesity-related increase in SBP; and 3) because plasma Ang II in LETO is already relatively low, dampening the AT1-mediated signaling even more with ARB treatment did not seem very fruitful. Thus the LETO and LETO + ARB are essentially the same phenotype and thus would not provide new information that justified the additional use of these animals. Animals were maintained at the Kagawa Medical University vivarium and housed in groups of three or four in a specific, pathogen-free facility under controlled temperature (23°C) and humidity (55%) with a 12-h light and dark cycle for 6 wk. By 15 wk of age, OLETF rats had developed insulin resistance, hypertension, and hyperlipidemia (37, 44). Animals were given free access to water and fed ad libitum standard laboratory rat chow (MF, Oriental Yeast, Tokyo, Japan). The ARB was mixed in the food and added according to initial food consumption rates to attain the desired dosage (44).
Blood pressure and tissue collection.
Systolic blood pressure (SBP) was measured before and after 6 wk of treatment in conscious rats by tail-cuff plethysmography (BP-98A; Softron, Tokyo, Japan) and has been reported elsewhere (44). After the treatment, animals were fasted overnight before being scarified the subsequent morning. The hearts were harvested, weighed, and snap-frozen in liquid nitrogen. Frozen samples were kept at −80°C until analyzed.
Western blots.
Frozen tissue samples were homogenized in RIPA buffer (crude extracts) or using the Pierce NE-PER nuclear protein extraction kit. Mitochondrial protein fractions were prepared using a mitochondria isolation kit (Pierce, Rockford, IL). Protease and phosphatase inhibitors (Pierce) were added to extraction buffers. Total protein content was measured in crude extracts, nuclear, and mitochondrial fractions by Bradford assay (Bio-Rad, Hercules, CA). Content of the proteins of interest was semi-quantified by standard Western blot technique as described previously (37, 44, 55). Primary antibodies were purchased from Abcam (TBP, catalog no. ab818; COX IV, catalog no. ab16056; Cambridge, MA), Epitomics (Nox4, catalog no. 3174-1, Burlington, CA), Millipore (Nox2, catalog no. 07-024, Temecula, CA), Novus Biologicals (UCP2, catalog no. NB 100-59742, Littleton, CO), and Santa Cruz Biotechnology (AMPK, catalog no. sc-128; p-AMPK Thr 172, catalog no. sc-33524; Akt2, catalog no. sc-7127; p-Akt Ser 473, catalog no. sc-7985; Nrf2, catalog no. sc-13032; Santa Cruz, CA).
Cardiac enzyme activities, oxidative damage, and succinate content.
Superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) activities were measured in crude extracts using commercial kits (Cayman Chemical, Ann Arbor, MI). Aconitase activity was measured in mitochondrial protein fractions prepared by differential centrifugation using a homogenization buffer containing sodium citrate to avoid aconitase ex vivo oxidation as described previously (37). Succinate-coenzyme Q reductase (complex II; SDH) activity was measured in heart mitochondria as previously described (37). Citrate synthase (CS) activity was measured in heart mitochondria using a commercial kit (ScienCell, Carlsband, CA). Cardiac 4-hydroxynonenal (4-HNE) and nitrotyrosine (NT) concentrations were measured in crude extracts using EIA kits (Cell BioLabs, San Diego, CA). Succinate content was measured as previously described (54).
Nuclear factor erythroid-2-related factor 2 transcriptional activity.
Binding of activated Nrf2 to the electrophile-responsive element (EpRE) was measured in nuclear extracts using a TransAM Nrf2 Transcription Factor kit (Active Motif, Carlsbad, CA). Fifteen micrograms of nuclear protein were diluted in lysis buffer supplemented with protease and phosphatase inhibitors and incubated with immobilized oligonucleotides containing the EpRE consensus binding site (5′-GTCACAGTACTCAGCAGAATCTG-3′). The assay was performed following manufacturer's instructions.
Real-time quantitative PCR.
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). Genomic DNA was eliminated by digestion with DNase I (Roche, Indianapolis, IN). First-strand cDNAs were reverse-transcribed from total DNA-free RNA (1 μg) using the QuantiTec Reverse Transcription kit (Qiagen, Valencia, CA) and oligo-dT. Real-time PCR reactions were performed for Nox2, Nox4, and Nrf2, and normalized by the mRNA expression of actin using 1 μl of cDNA for each gene. Primer sequences used are shown in Table 1.
Table 1.
Primer sequences used for qualitative real-time PCR
| Primer Name | Sequence (5′-3′) |
|---|---|
| NOX2-F1 | CCAATCACTTTGCTGTGCACC |
| NOX2-R1 | CCGGAGTCAGAGTTGGAGATG |
| NOX4-F1 | CCTGACTTTGTGAACATCCAG |
| NOX4-R1 | GTCCCATATGAGTTGTTCCGG |
| Nrf2 FW | AGCACATCCAGACAGACACC |
| Nrf2 RV | CCAGAGAGCTATCGAGTGAC |
| aActinFw1 | TTCCAGCCCTCCTTCATCGG |
| aActinRv1 | CTTGATCTTCATGGTGCTGGG |
Statistical analysis.
Means ± SE were compared by ANOVA followed by Fisher's protected least-significant difference post hoc test and considered significant at P < 0.05. Kruskal-Wallis nonparametric followed by Mann-Whitney U tests were used to compare groups not showing a normal distribution. Statistical analyses were performed with the SYSTAT 11.0 software (SPSS, Richmond, CA).
RESULTS
SBP, BM, and heart mass.
Data demonstrating the development of hypertension and obesity in OLETF rats and improvement with ARB treatment have been previously published (44) but are briefly included here to substantiate the characterization of the model. SBP increased 33% in OLETF compared with LETO and was normalized by ARB treatment (Table 2). A strain effect on food intake was observed, but ARB treatment did not significantly alter intake (44). This strain effect on food intake was associated with a 27% increase in mean BM. ARB treatment decreased BM by 11% compared with OLETF (Table 2). Absolute heart mass increased 22% in OLETF compared with LETO and returned to control levels with ARB treatment (Table 2). Relative heart mass decreased 5% in OLETF compared with LETO, whereas ARB treatment reduced it an additional 4% (Table 2).
Table 2.
SBP, BM, and absolute and relative heart masses from lean LETO, obese, insulin-resistant OLETF, and OLETF + ARB rats after 6 wk of treatment
| LETO | OLETF | OLETF + ARB | |
|---|---|---|---|
| SBP, mmHg | 113 ± 4 | 146 ± 4* | 107 ± 2† |
| BM, g | 385 ± 8 | 489 ± 6* | 437 ± 6† |
| Absolute heart mass, g | 1.13 ± 0.03 | 1.38 ± 0.02* | 1.18 ± 0.02† |
| Relative heart mass, g/100 g BM | 0.294 ± 0.003 | 0.281 ± 0.005* | 0.270 ± 0.004† |
Values are means ± SE. Systolic blood pressure (SBP) and body mass (BM) taken from Ref. 39. LETO, Long Evans Tokushima Otsuka rats; OLETF, Otsuka Long Evans Tokushima Fatty rats; ARB, angiotensin receptor type 1 blocker.
Signficant difference vs. LETO (P < 0.05).
Signficant difference vs. OLETF (P < 0.05).
NADPH oxidase mRNA and protein expression.
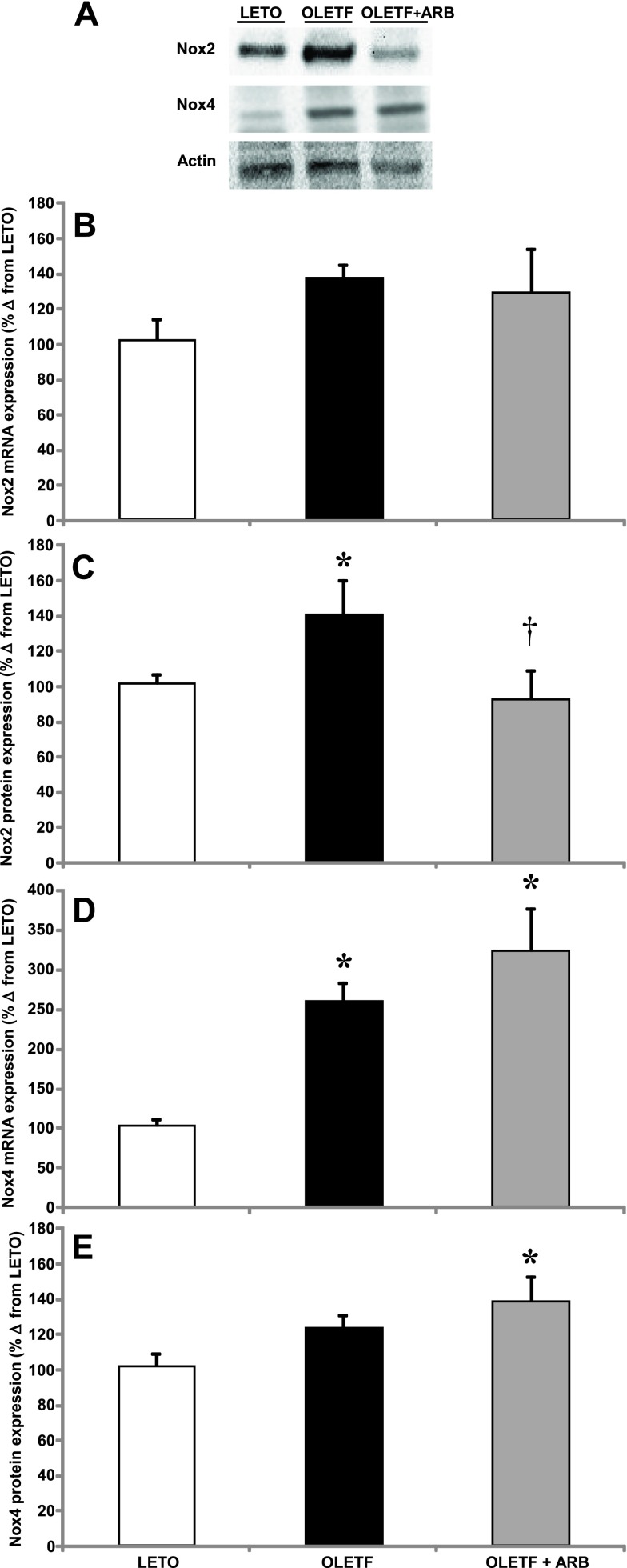
Transcript and protein expression of Nox2 and Nox4 were measured to assess the effects of AT1 activation on Nox-derived oxidant production and signaling in the heart of obese, insulin-resistant animals. Mean Nox2 mRNA and protein expression increased 40% in OLETF compared with LETO (Fig. 1, A and B). ARB treatment did not alter mean mRNA expression (Fig. 1B); however, mean protein content was normalized (Fig. 1C). Mean Nox4 mRNA levels increased nearly 2.5-fold in OLETF compared with LETO and were increased by an additional 75% with ARB treatment (Fig. 1D). The 25% increase in mean Nox4 protein expression was not significant (P < 0.10), but the 36% increase with ARB treatment was significant (Fig. 1E) compared with LETO. These results suggest that, although ARB treatment protects from potentially damaging oxidant production by decreasing Nox2, it does not interfere with oxidant signaling derived from Nox4 in the heart of obese, insulin-resistant rats.
Fig. 1.
A: representative Western blots of NADPH oxidase (Nox) 2 and Nox4 protein expression. Means ± SE values of Nox2 mRNA expression (B), Nox2 protein expression (C), Nox4 mRNA expression (D), and Nox4 protein expression levels (E) from Long Evans Tokushima Otsuka (LETO; n = 6), Otsuka Long Evans Tokushima Fatty (OLETF; n = 8), and OLETF + angiotensin receptor type 1 blocker (ARB; n = 8) rats. *Significant difference from LETO (P < 0.05). †Significant difference from OLETF (P < 0.05).
Cardiac oxidative damage: 4-hydroxynonenal and nitrotyrosine.
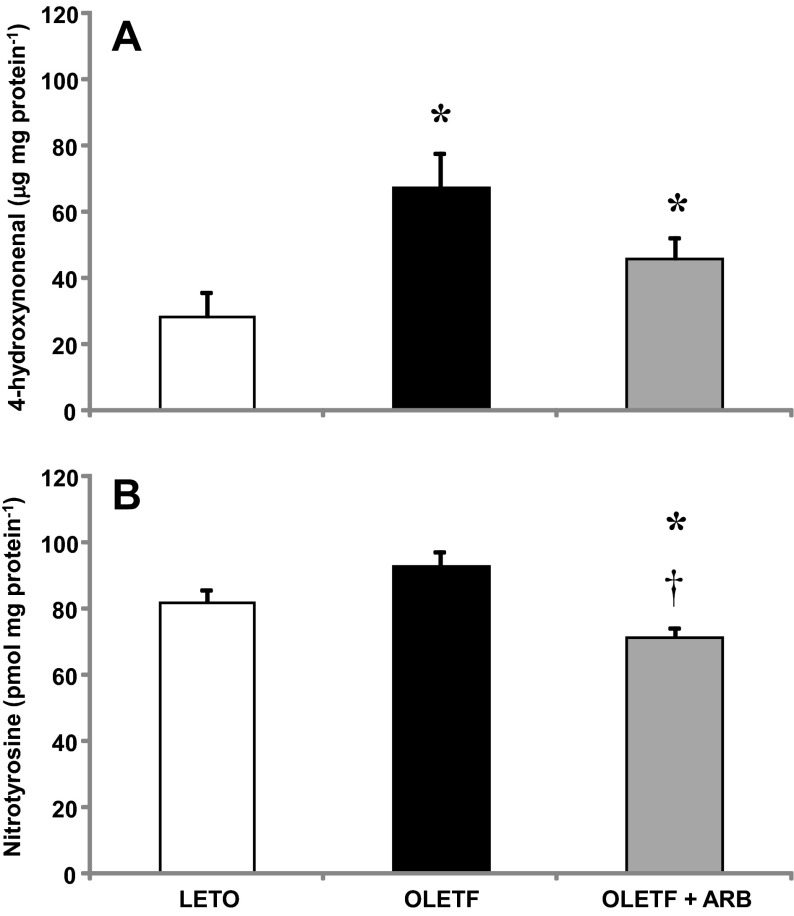
Levels of 4-HNE and nitrotyrosine (NT) were measured to assess the contribution of AT1 activation to oxidative damage in the heart of obese, insulin-resistant animals. Mean cardiac 4-HNE levels increased nearly 2.5-fold in OLETF compared with LETO, and despite the 25% decrease with ARB treatment, these levels remained higher than LETO (Fig. 2A). Although the 12% increase in mean cardiac NT levels was not significant (P < 0.10), ARB treatment reduced levels 22% to LETO levels (Fig. 2B). These data suggest that AT1 activation increases oxidative damage in the heart during insulin resistance.
Fig. 2.
Values are means ± SE. Cardiac 4-hydroxynonenal (4-HNE; A) and nitrotyrosine (NT; B) levels from LETO, OLETF, and OLETF + ARB rats. *Significant difference from LETO (P < 0.05). †Significant difference from OLETF (P < 0.05).
Nuclear factor erythroid-2-related factor 2 and antioxidant enzyme activities.
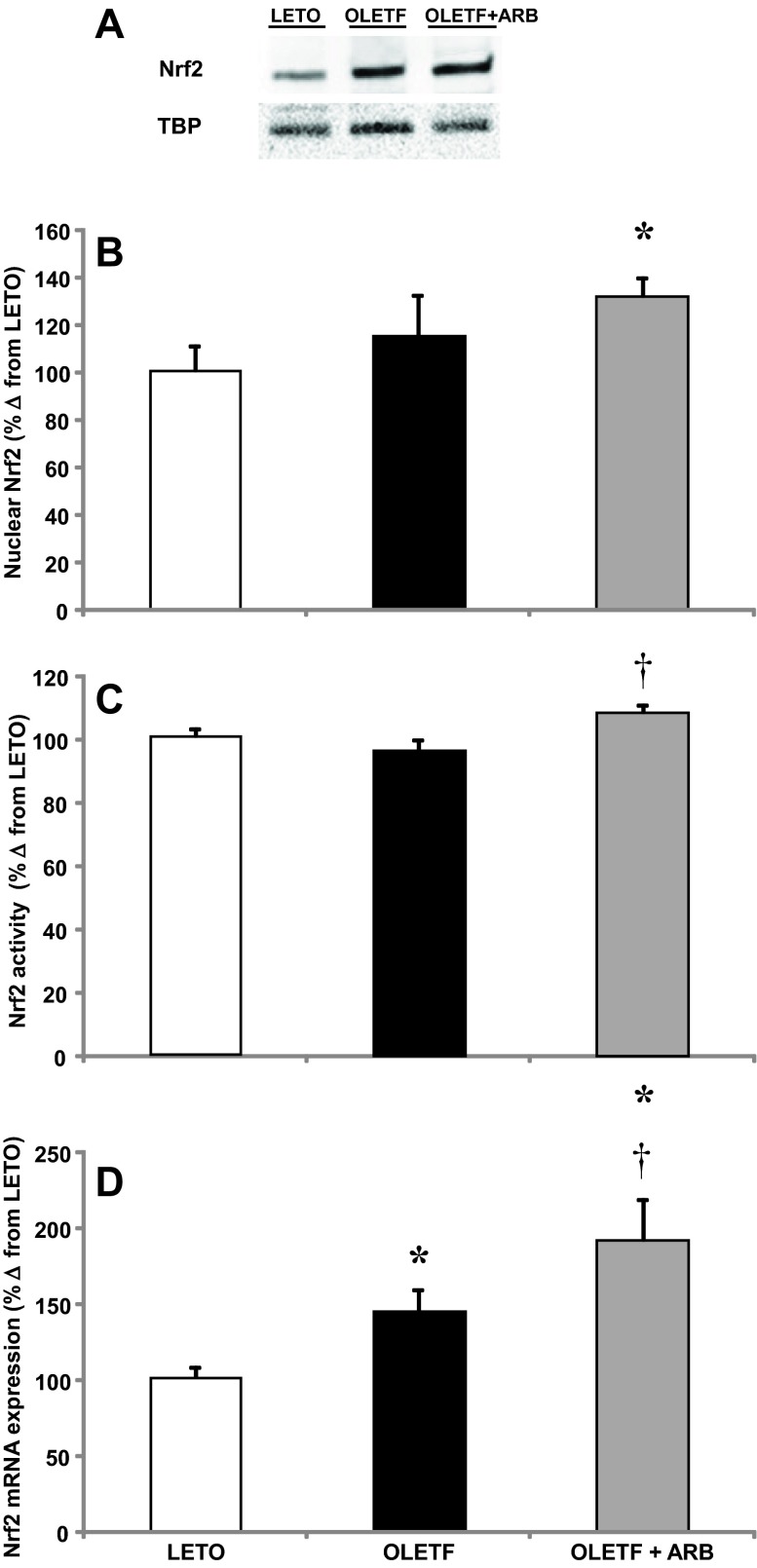
Nuclear factor erythroid-2 related factor 2 (Nrf2) mRNA, nuclear protein expression, and activity levels were measured to assess the contributions of AT1 signaling on the redox-regulated cellular antioxidant defense in the heart of obese, insulin-resistant rats. Mean Nrf2 mRNA expression increased 43% in OLETF compared with LETO, and ARB treatment further increased expression levels an additional 47% (Fig. 3D). The increase in mRNA expression did not translate into a significant increase in nuclear protein content in OLETF rats, but the increase in expression in ARB-treated rats did coincide with a 30% increase in nuclear protein content (Fig. 3, A and B). In the same way, there was no difference in Nrf2 activity between LETO and OLETF, but the increase in nuclear Nrf2 protein was associated with a 7% increase in activity in ARB-treated rats (Fig. 3C).
Fig. 3.
A: representative Western blot of nuclear Nrf2 protein content. Means ± SE values of cardiac nuclear factor erythroid 2-related factor 2 (Nrf2) mRNA expression (B), nuclear Nrf2 protein expression (C), and Nrf2 activity (D) from LETO, OLETF, and OLETF + ARB rats. *Significant difference from LETO (P < 0.05). †Significant difference from OLETF (P < 0.05).
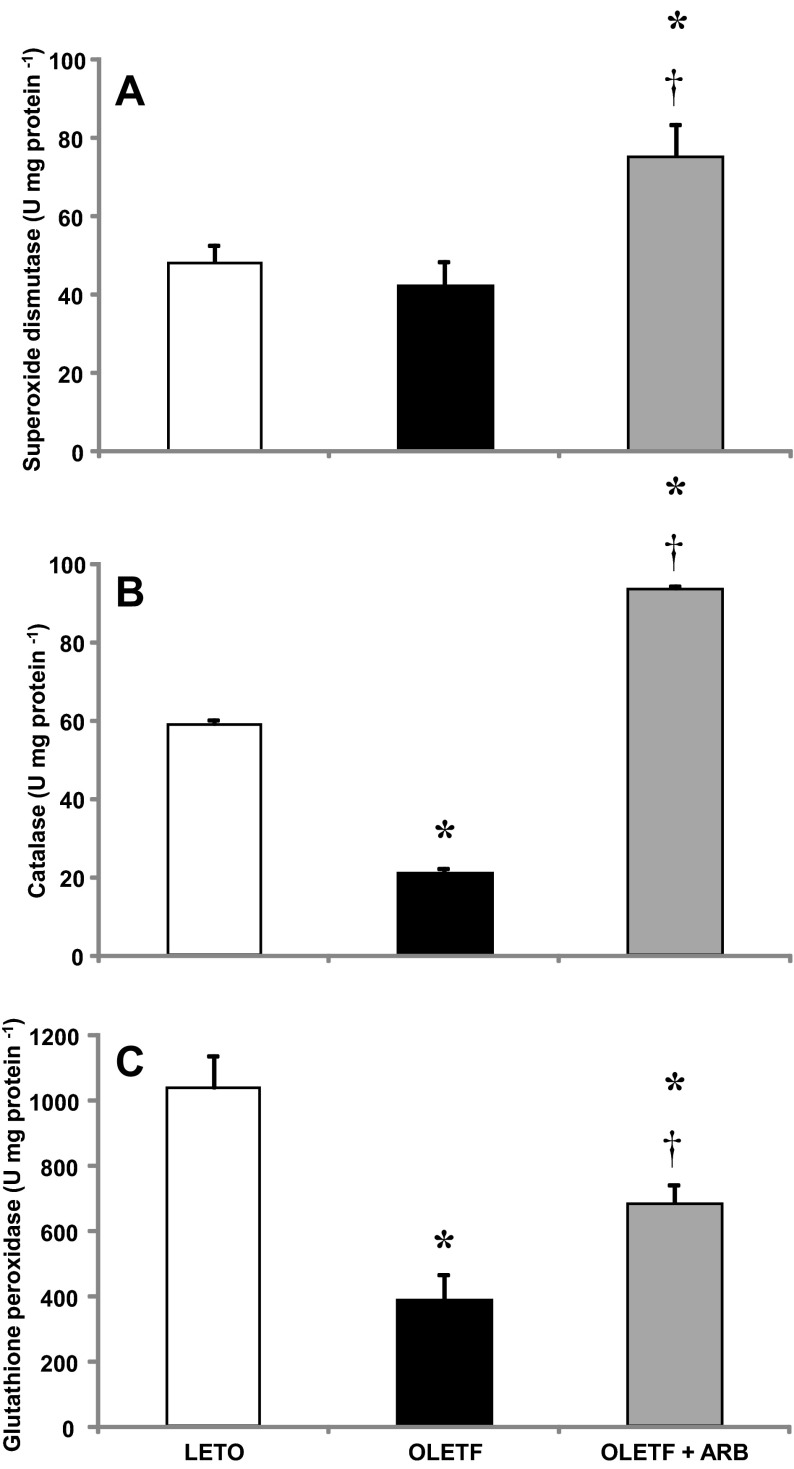
Activities of superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) were measured to assess the effects of the AT1-mediated oxidative stress on antioxidant capacity in the heart of obese, insulin-resistant rats. A strain effect on mean SOD activity was not detected, but ARB treatment increased activity levels 57% (Fig. 4A). Mean catalase activity decreased 66% in OLETF compared with LETO, and ARB treatment completely recovered activity levels with an additional 57% increase (Fig. 4B). Similarly, mean GPx activity decreased 63% in OLETF compared with LETO, and ARB treatment partially recovered activity levels to 35% of LETO (Fig. 4C). These changes in antioxidant activities suggest that AT1 activation diminishes the redox-mediated, antioxidant scavenging capacity in the heart of obese, insulin-resistant rats.
Fig. 4.
Mean ± SE values of cardiac catalase (A), superoxide dismutase catalase (B), and glutathione peroxidase activities (C) from LETO, OLETF, and OLETF + ARB rats. *Significant difference from LETO (P < 0.05). †Significant difference from OLETF (P < 0.05).
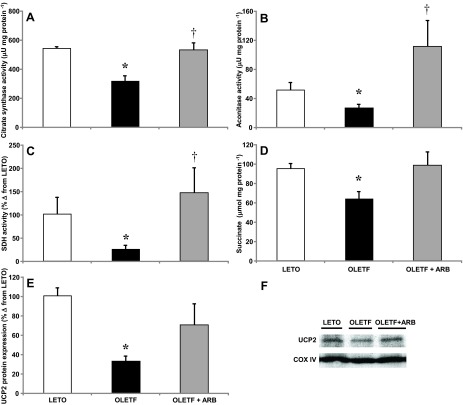
Mitochondrial enzyme activities, succinate content, and uncoupling protein 2 expression.
Mitochondrial citrate synthase (CS), aconitase and succinate dehydrogenase (SDH) activities, heart succinate levels, and uncoupling protein 2 (UCP2) protein expression were measured to assess the contributions of AT1 activation on mitochondrial function in the heart of obese, insulin-resistant rats. Mean activity of CS decreased 41% in OLETF compared with LETO, and was restored to LETO levels with ARB treatment (Fig. 5A). Mean aconitase activity decreased 50% in OLETF compared with LETO, and ARB treatment increased mean levels twofold compared with LETO (Fig. 5B). Mean SDH activity decreased 76% in OLETF compared with LETO, and ARB treatment completely restored activity (Fig. 5C). Mean heart succinate levels decreased 33% in OLETF compared with LETO, and again ARB treatment completely restored levels (Fig. 5D). Mean UCP2 protein expression decreased 68% in OLETF compared with LETO and were partially recovered by ARB treatment (Fig. 5, E and F). Overall, these results suggest that AT1 activation impairs mitochondrial function by targeting TCA cycle activity and uncoupling protein 2 in the heart of obese, insulin-resistant animals.
Fig. 5.
Mean ± SE values of cardiac citrate synthase (A), mitochondrial aconitase (B) and succinate dehydrogenase activities (C), succinate levels (D), and cardiac uncoupling protein 2 (UCP2) protein expression (E) from LETO, OLETF, and OLETF + ARB rats. F: representative Western blot for mitochondrial UCP2 expression in LETO, OLETF, and OLETF + ARB rats. *Significant difference from LETO (P < 0.05). †Significant difference from OLETF (P < 0.05).
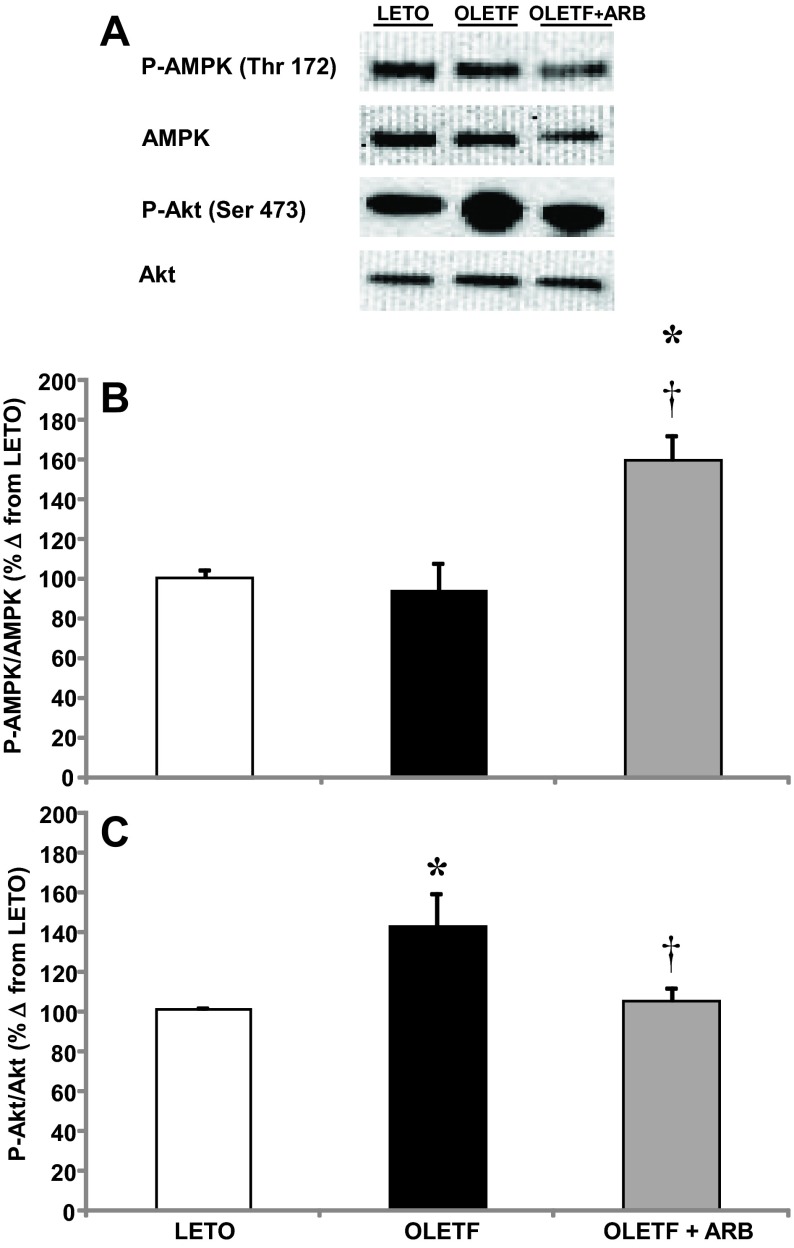
5′-Adenosine monophosphate protein kinase and Akt.
The phosphorylation levels of 5′-adenosine monophosphate protein kinase (AMPK) and protein kinase B (Akt) were measured as indicators of the contribution of AT1 activation on insulin signaling and cellular energy homeostasis in the heart of obese, insulin-resistant rats. The mean ratio of p-AMPK to AMPK was not different between LETO and OLETF, but phosphorylation levels increased 60% with ARB treatment (Fig. 6, A and B). The mean ratio of p-Akt to Akt increased 41% in OLETF compared with LETO, and ARB treatment normalized phosphorylation levels (Fig. 6, A and C). Collectively, these data suggest that AT1 activation impairs cellular energy balance, which is associated with the upregulation of insulin signaling in the heart of obese, insulin-resistant rats.
Fig. 6.
A: representative Western blots of phosphorylated AMPK and Akt. Mean ± SE phosphorylation levels of cardiac 5′-adenosine monophosphate protein kinase (AMPK; B) and protein kinase B (Akt; C) from LETO, OLETF, and OLETF + ARB rats. *Significant difference from LETO (P < 0.05). †Significant difference from OLETF (P < 0.05).
DISCUSSION
Oxidative stress derived from increased Nox2 is a common consequence of AT1 activation during cardiovascular and metabolic disease (25, 49, 50, 63). However, the effects of chronic AT1 activation on mitochondrial function, antioxidant capacity, and redox signaling in the heart of obese, insulin-resistant animals are not fully understood. In the present study, we demonstrated that, in addition to stimulating Nox2 and increasing oxidative damage, AT1 activation decreases cardiac redox signaling and antioxidant enzyme activities, and impairs mitochondrial function and overall cellular energy balance during insulin resistance.
Ang II signaling downstream of AT1 promotes the phosphorylation of the p47phox and p67phox cytosolic complexes required for Nox2 activation (12). Therefore, AT1 antagonism is an effective way to decrease Nox2-derived oxidative stress during glucose-induced cardiac dysfunction (41). The ability of ARB treatment to reduce Nox2 expression and recover the levels of oxidative damage in the heart suggests that the AT1-mediated oxidative damage in the heart is associated with upregulation of cardiac Nox2 in a model of metabolic syndrome. The results also demonstrated that the expression of cardiac Nox4 are not entirely associated with AT1 activation in the heart of insulin-resistant rats, despite the observed increases in the mRNA and protein expression (not statistically significant) of Nox4 in the OLEFT group. These increases are substantiated by previously reported increases in mice (1, 14).
In contrast to Nox2, Nox4 is localized to intracellular membranes (2, 7, 35, 61), does not require cytosolic activators (35), is constitutively active (39, 46, 56), and produces H2O2 instead of superoxide (45, 53). Therefore, increased Nox4 expression may be beneficial during cardiac remodeling, pressure overload, or myocardial infarction (42, 45, 62), since intracellular H2O2 can activate the Nrf2-mediated antioxidant response (9, 45). This Nrf2-mediated antioxidant response efficiently counteracts Nox2-derived oxidant generation and likely contributes to the prevention of oxidative damage. However, Nox4 overexpression may also result in overproduction of H2O2, which is associated with tissue hypertrophy and pro-inflammatory responses and may contribute to cardiac impairment in insulin resistance, metabolic dysfunction, and heart failure (8, 13, 64). Therefore, the fact that AT1 blockade further increased Nox4 expression may indicate incomplete cardiac protection in this condition.
The observed relationship between increased Nox4 expression, Nrf2, and antioxidant enzyme activities in ARB-treated animals suggests that cardiac AT1 activation reduces antioxidant enzyme activities and impairs the redox-mediated cellular antioxidant defense during metabolic syndrome. Another important finding of the present study is that AT1 activation also contributes to impaired mitochondrial function as indicated by the ability of the ARB treatment to normalize the reduced activities of CS, SDH, aconitase, and succinate levels and UCP2 expression in obese OLETF rats. This is important because it demonstrates that AT1-mediated oxidative stress via upregulation of Nox2 is associated with impaired mitochondrial function, antioxidant enzyme activities, and redox signaling in the heart of insulin-resistant animals. Mitochondrial dysfunction is well documented during diabetes (4, 47) and may contribute to increased oxidant generation during metabolic syndrome due to increases in glucose oxidation in the TCA cycle. This increase in glucose oxidation can eventually result in increased superoxide production (10) or NOS uncoupling (51). Moreover, AT1 activation induces mitochondrial dysfunction by activating Nox2 via a PKC pathway (18, 59), stimulating mitochondrial ROS formation and therefore contributing to mitochondrial dysfunction (16, 21, 48, 58). The improvement of mitochondrial function, the normalization of Akt phosphorylation, and the increase in AMPK activity with ARB treatment suggest that AT1 activation contributes to impaired insulin signaling and overall cellular energy balance in the heart during metabolic syndrome by targeting these two intracellular signaling pathways. Increased activation of AMPK with AT1 antagonism are consistent with results from in vitro models of cardiac injury (28), as well as with results from in vivo models of aging in diabetic mice (30), salt-sensitive hypertensive rats (60), and animals fed with a high-fructose (33) or high-fat diet (57). Collectively, this suite of data highlights the contribution of AT1 signaling in the heart to the manifestation of cardiovascular and metabolic disease (19, 29, 65). Thus this suite of conditions helps to define the pathological phenotype of the diabetic heart induced by AT1-mediated oxidative stress.
In summary, results of the present study suggest that AT1 activation increases Nox2-mediated oxidative damage and impairs redox signaling and mitochondrial function while altering insulin signaling and the overall energy balance in the heart during metabolic syndrome. Therefore, aside from decreasing blood pressure, AT1 antagonism may also improve cardiac function in the heart of obese, insulin-resistant animals by stimulating a redox-senstive protective mechanism that includes the upregulation of Nrf2 and an increase in antioxidant enzyme activities.
Perspectives
The present study reveals the impact of AT1 activation in the heart of insulin-resistant rats on Nox2-derived oxidative stress and the parallel impairment of mitochondrial function, redox signaling, and cellular energy balance. The ability of ARB treatment, for the most part, to ameliorate these detriments implicates AT1 activation as a potent contributing factor in the development of metabolic derangements in the heart during the manifestation of frank diabetes. In the scope of this study, ARB treatment provides the added benefits of improved cardiac cellular metabolism, which may translate into improved cardiac function during insulin resistance. Importantly, instead of reducing the expression of Nox4, which appears to play a role in the adaptive response to oxidative stress by activating Nrf2 (9, 45), AT1 antagonism increased Nox4, providing an alternative perspective of Nox4 function that deviates from its traditional role in promoting oxidative stress. Although a mechanistic explanation of the effects of AT1 signaling on Nox4 expression is still lacking and warrants further investigation, a compensatory mechanism derived from the downregulation of Nox2 during AT1 blockade in the insulin-resistant heart may explain the observed response. In the same way, and despite the observed associations of Nox2- and mitochondrial-derived oxidant production, insulin signaling, and cellular energetic balance, the specific mechanisms driving the impacts of cardiac AT1 activation on each of those variables during metabolic disease remain elusive. Nonetheless, this suite of measures can provide a first estimation of the representative heart phenotype of an obese, insulin-resistant animal.
GRANTS
J. P. Vázquez-Medina was supported by The University of California Institute for Mexico and The United States (UC MEXUS), and Mexico's National Council for Science and Technology (CONACYT). I.P. was supported by an American Physiological Society Undergraduate Summer Research Fellowship. J. A. Viscarra and R. Rodriguez were supported in part by T37 MD-001480 from the National Institute on Minority Health and Health Disparities. J. G. Sonanez-Organis was supported by a postdoctoral fellowship from UC MEXUS and CONACYT. R. M. Ortiz was partially supported by National Heart, Lung, and Blood Institute K02 HL-103787. Research was funded in parts by grants from American Diabetes Association Grant 1-11-BS-121 (J. Peti-Peterdi), National Institute on Minority Health and Health Disparities Grant T37 MD-001480, and National Heart, Lung, and Blood Institute Grant R01 HL-091767 (R. M. Ortiz). Olmesartan was kindly donated by Daiichi-Sankyo (Tokyo, Japan) to A. Nishiyama.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.P.V.-M., J.P.-P., A.N., and R.M.O. conception and design of research; J.P.V.-M., I.P., M.A.T., J.A.V., R.R., J.G.S.-O., and L.L. analyzed data; J.P.V.-M., J.A.V., J.G.S.-O., L.L., J.P.-P., D.N., A.N., and R.M.O. interpreted results of experiments; J.P.V.-M., J.A.V., and J.G.S.-O. prepared figures; J.P.V.-M., J.G.S.-O., and R.M.O. drafted manuscript; J.P.V.-M., M.A.T., J.A.V., R.R., J.G.S.-O., L.L., J.P.-P., D.N., A.N., and R.M.O. approved final version of manuscript; I.P., J.A.V., R.R., and D.N. performed experiments; J.P.-P., D.N., A.N., and R.M.O. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. N. Pelisch, A. Lee, J. Minas, and B. Martinez for laboratory assistance.
REFERENCES
- 1.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging (Milano) 2: 1012–1016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM. Nox4 and Nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol 28: 1347–1354, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38: 1278–1295, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105: 293–296, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Blendea MC, Jacobs D, Stump CS, McFarlane SI, Ogrin C, Bahtyiar G, Stas S, Kumar P, Sha Q, Ferrario CM, Sowers JR. Abrogation of oxidative stress improves insulin sensitivity in the Ren-2 rat model of tissue angiotensin II overexpression. Am J Physiol Endocrinol Metab 288: E353–E359, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci USA 106: 14385–14390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulden BM, Widder JD, Allen JC, Smith DA, Al-Baldawi RN, Harrison DG, Dikalov SI, Jo H, Dudley SC., Jr Early determinants of H2O2-induced endothelial dysfunction. Free Radic Biol Med 41: 810–817, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brewer AC, Murray TVA, Arno M, Zhang M, Anilkumar NP, Mann GE, Shah AM. Nox4 regulates Nrf2 and glutathione redox in cardiomyocytes in vivo. Free Radic Biol Med 51: 205–215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93: 802–805, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Choi H, Leto TL, Hunyady L, Catt KJ, Bae YS, Rhee SG. Mechanism of angiotensin II-induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. J Biol Chem 283: 255–267, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintrón M, Chen T, Marcinek DJ, Dorn GW, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Gαq overexpression-induced heart failure. Circ Res 108: 837–846, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 14: 173–194, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt H, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Campo L, Grande M, Diego J, Fuentes-Calvo I, Macias-Nunez J, Sánchez-Rodríguez A, Grande J, García-Ortiz L, Lopez-Novoa J, Martinez-Salgado C. Effect of different antihypertensive treatments on Ras, MAPK and Akt activation in hypertension and diabetes. Clin Sci 116: 165–173, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Ferrannini E, Buzzigoli G, Bonadonna R, Giorico MA, Oleggini M, Graziadei L, Pedrinelli R, Brandi L, Bevilacqua S. Insulin resistance in essential hypertension. N Engl J Med 317: 350–357, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Gao L, Mann GE. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 82: 9–20, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Helmcke I, Heumüller S, Tikkanen R, Schröder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal 11: 1279–1287, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Henriksen EJ, Jacob S, Kinnick TR, Teachey MK, Krekler M. Selective angiotensin II receptor antagonism reduces insulin resistance in obese zucker rats. Hypertension 38: 884–890, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 36: 1199–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Kakehi T, Yabe-Nishimura C. NOX enzymes and diabetic complications. Semin Immunopathol 30: 301–314, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract 24: S317–S320, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes 41: 1422, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Kim KS, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. β-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol 303: H1001–H1010, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong X, Zhang D, Wu H, Li F. Losartan and pioglitazone ameliorate nephropathy in experimental metabolic syndrome rats. Biol Pharm Bull 34: 693–699, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Kosugi R, Shioi T, Watanabe-Maeda K, Yoshida Y, Takahashi K, Machida Y, Izumi T. Angiotensin II receptor antagonist attenuates expression of aging markers in diabetic mouse heart. Circ J 70: 482, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47phox in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17: 3978–3988, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li P, Koike T, Jiang H, Wang Z, Kawata Y, Oshida Y. Acute treatment with candesartan cilexetil, an angiotensin II type 1 receptor blocker, improves insulin sensitivity in high-fructose-diet-fed rats. Horm Metab Res 44: 286–290, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 51: 319–325, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Montez P, Vázquez-Medina JP, Rodríguez R, Thorwald MA, Viscarra JA, Lam L, Peti-Peterdi J, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade recovers hepatic UCP2 expression and aconitase and SDH activities and ameliorates hepatic oxidative damage in insulin resistant rats. Endocrinology 153: 5746–5759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol 35: 851–859, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry 49: 2433–2442, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogihara T, Asano T, Ando K, Chiba Y, Sakoda H, Anai M, Shojima N, Ono H, Onishi Y, Fujishiro M, Katagiri H, Fukushima Y, Kikuchi M, Noguchi N, Aburatani H, Komuro I, Fujita T. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension 40: 872–879, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension 42: 206–212, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler Thromb Vasc Biol 31: 1368–1376, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Reaven GM. Role of insulin resistance in human disease. Diabetes 37: 1595–1607, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez R, Viscarra JA, Minas JN, Nakano D, Nishiyama A, Ortiz RM. Angiotensin receptor blockade increases pancreatic insulin secretion and decreases glucose intolerance during glucose supplementation in a model of metabolic syndrome. Endocrinology 53: 1684–1695, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 Is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406: 105–114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen GX. Mitochondrial dysfunction, oxidative stress and diabetic cardiovascular disorders. Cardiovasc Hematol Disord Drug Targets 12: 106–112, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Shen GX. Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can J Physiol Pharmacol 88: 241–248, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Sirker A, Zhang M, Murdoch C, Shah AM. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am J Nephrol 27: 649–660, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Sirker A, Zhang M, Shah A. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol 106: 735–747, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srinivasan S, Hatley ME, Bolick DT, Palmer LA, Edelstein D, Brownlee M, Hedrick CC. Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia 47: 1727–1734, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Sun QA, Hess DT, Nogueira L, Yong S, Bowles DE, Eu J, Laurita KR, Meissner G, Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc Natl Acad Sci USA 108: 16098–16103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest 118: 2526–2534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vázquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J Exp Biol 213: 2524–2530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Löhneysen K, Noack D, Hayes P, Friedman JS, Knaus UG. Constitutive NADPH oxidase 4 activity resides in the composition of the B-loop and the penultimate C terminus. J Biol Chem 287: 8737–8745, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Z, Koike T, Li P, Jiang H, Natsume Y, Mu L, Chen T, Oshida Y. Effects of angiotensin II AT1 receptor inhibition and exercise training on insulin action in rats on high-fat diet. Life Sci 90: 322–327, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JMD, Müller J, Schuhmacher S, Hortmann M, Baldus S, Gori T. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal 10: 1435–1448, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Wosniak J, Jr, Santos CXC, Kowaltowski AJ, Laurindo FRM. Cross-talk between mitochondria and NADPH oxidase: effects of mild mitochondrial dysfunction on angiotensin II-mediated increase in Nox isoform expression and activity in vascular smooth muscle cells. Antioxid Redox Signal 11: 1265–1278, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto E, Yamashita T, Tanaka T, Kataoka K, Tokutomi Y, Lai ZF, Dong YF, Matsuba S, Ogawa H, Kim-Mitsuyama S. Pravastatin enhances beneficial effects of olmesartan on vascular injury of salt-sensitive hypertensive rats, via pleiotropic effects. Arterioscler Thromb Vasc Biol 27: 556–563, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Nguyen MVC, Lardy B, Jesaitis AJ, Grichine A, Rousset F, Talbot M, Paclet MH, Qian G, Morel F. New insight into the Nox4 subcellular localization in HEK293 cells: first monoclonal antibodies against Nox4. Biochimie 93: 457–468, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Zhang M, Brewer AC, Schröder K, Santos CXC, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci USA 107: 18121–18126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang M, Perino A, Ghigo A, Hirsch E, Shah A. NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid Redox Signal 18: 1024–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Griendling KK, Dikalova A, Owens GK, Taylor WR. Vascular hypertrophy in angiotensin II-induced hypertension is mediated by vascular smooth muscle cell-derived H2O2. Hypertension 46: 732–737, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Zhou MS, Schulman IH, Raij L. Role of angiotensin II and oxidative stress in vascular insulin resistance linked to hypertension. Am J Physiol Heart Circ Physiol 296: H833–H839, 2009 [DOI] [PubMed] [Google Scholar]