Abstract
In endothelial cells (ECs), Ca2+-activated K+ channels KCa2.3 and KCa3.1 play a crucial role in the regulation of arterial tone via producing NO and endothelium-derived hyperpolarizing factors. Since a rise in intracellular Ca2+ levels and activation of p300 histone acetyltransferase are early EC responses to laminar shear stress (LS) for the transcriptional activation of genes, we examined the role of Ca2+/calmodulin-dependent kinase kinase (CaMKK), the most upstream element of a Ca2+/calmodulin-kinase cascade, and p300 in LS-dependent regulation of KCa2.3 and KCa3.1 in ECs. Exposure to LS (15 dyn/cm2) for 24 h markedly increased KCa2.3 and KCa3.1 mRNA expression in cultured human coronary artery ECs (3.2 ± 0.4 and 45 ± 10 fold increase, respectively; P < 0.05 vs. static condition; n = 8–30), whereas oscillatory shear (OS; ± 5 dyn/cm2 × 1 Hz) moderately increased KCa3.1 but did not affect KCa2.3. Expression of KCa2.1 and KCa2.2 was suppressed under both LS and OS conditions, whereas KCa1.1 was slightly elevated in LS and unchanged in OS. Inhibition of CaMKK attenuated LS-induced increases in the expression and channel activity of KCa2.3 and KCa3.1, and in phosphorylation of Akt (Ser473) and p300 (Ser1834). Inhibition of Akt abolished the upregulation of these channels by diminishing p300 phosphorylation. Consistently, disruption of the interaction of p300 with transcription factors eliminated the induction of these channels. Thus a CaMKK/Akt/p300 cascade plays an important role in LS-dependent induction of KCa2.3 and KCa3.1 expression, thereby regulating EC function and adaptation to hemodynamic changes.
Keywords: circulation, endothelial function, ion channels, Ca2+-dependent signaling
laminar shear stress (LS) is a physiological regulator for a number of important homeostatic functions in endothelial cells (ECs), such as vasodilator and anti-atherosclerotic functions, whereas oscillatory shear stress (OS), which occurs near arterial bifurcations, branch ostia, and curvatures, is associated with atheroma formation. LS quickly provokes an elevation of intracellular calcium concentration ([Ca2+]i) in ECs, originating from intracellular stores and from an influx (29). The rise in [Ca2+]i in turn activates multiple Ca2+-dependent cellular signaling and electrophysiological responses to maintain the athero-protective functions and regulate the adaptation to hemodynamic changes (28).
Endothelial Ca2+-activated K+ channels (KCa) play a crucial role in the regulation of vasomotor tone and blood pressure via controlling the production of major EC-derived relaxing factors such as NO, prostacyclin, and endothelium-derived hyperpolarizing factors (EDHF) by increasing Ca2+ influx through modulating membrane potential (21, 30–32). The defect or dysfunction of these channels is related to impaired EC-dependent vasodilator responses in diseases (21), whereas pharmacological activation of these channels lowers blood pressure by augmenting endothelial production of NO and EDHF (15, 39). There are five KCa subtypes: KCa1.1 (large conductance); KCa2.1, KCa2.2, and KCa2.3 (small conductance); and KCa3.1 (intermediate conductance). In general, ECs constitutively and predominantly express KCa2.3 and KCa3.1 in a variety of vasculatures of several species (21, 30). A recent study in mice deficient in either KCa2.3 or KCa3.1, or both, has demonstrated that NO-mediated vasodilations are associated with KCa2.3 activation, whereas EDHF-mediated responses are attributed to KCa3.1 activation (5). Thus determining the effect of shear patterns on the expression of KCa subtypes and the regulatory mechanisms in ECs is necessary for a better understanding of EC functions in physiological and pathophysiological conditions.
The Ca2+/calmodulin (CaM)-dependent protein kinase kinase (CaMKK), a serine/threonine-specific protein kinase, is the most upstream element of a CaM-kinase cascade and is thought to be directly activated by LS-induced elevation of [Ca2+]i in ECs (16). CaMKK has two isoforms: a 505-amino acid α-isoform and a slightly larger β-isoform composed of 587 amino acids. Each originates from separate genes (CAMKK1 and CAMKK2, respectively), and both are expressed in ECs as a monomer (37). Downstream targets of this kinase include Akt/PKB (50), Ca2+/CaM-dependent protein kinases (CaMKI and IV) (45), and AMP-activated protein kinase (AMPK) (14), which can also be activated by LS in ECs (13, 22) and subsequently phosphorylate and activate multiple transcription factors (1). Recently, LS has been shown to quickly increase the activity of the transcription coactivator histone acetyltransferases p300 (12), which binds and activates transcription factors, and to open chromatin in promoter regions via histone acetylation (4, 25). In addition, Huang and Chen have shown that Akt phosphorylation of p300 on serine 1834 is essential for its histone acetyltransferase activity (24). However, little is known about the role of a CaM-kinase cascade in the regulation of p300 activity and its involvement in shear-dependent regulation of KCa expression in ECs.
In the present study, we hypothesized that LS primarily increases KCa2.3 and KCa3.1 expression and that this upregulation is attributed to CaMKK activation followed by p300 stimulation via Akt.
MATERIALS AND METHODS
Materials.
All chemicals were obtained from Sigma-Aldrich, except for Compound C and Akt inhibitor IV (Calbiochem), apamin (Tocris Bioscience), and TRAM-34 (a gift from Dr. Heike Wulff, University of California Davis).
EC culture.
Human coronary artery ECs (HCAECs) were obtained from Cell Applications Cells and were maintained in a standard humidified incubator (37°C, 5% CO2) in MesoEndo Cell Growth Medium or Endothelial Cell Basal Medium (Cell Applications) for a 48-h starvation period before each experiment to eliminate KCa induction by growth factors (20) and minimize all background signaling. HCAECs from passages 5–6 were used in the following experimental protocols.
Shear stress studies.
HCAECs grown to confluent monolayers in 100-mm tissue culture dishes (Falcon) were exposed to static culture condition (ST) or arterial levels of shear stress via cone-and-plate shear apparatus for 0.25, 0.5, 1, or 24 h. LS at 5 or 15 dynes/cm2 was simulated by rotating a Teflon cone (0.5° cone angle) unidirectionally in the medium as previously described by our laboratory (35). To mimic unstable atherogenic OS, the cone was rotated bidirectionally in the medium using a stepping motor (Servo Motor) and computer program (DC Motor). ECs were exposed to OS at ± 5 dynes/cm2 with directional changes of flow at 1-Hz frequency (35). In some experiments, before exposure to ST or LS, cells were pretreated for 30 min with STO-609 (a specific inhibitor of CaMKK-α and -β; 10 μg/ml) (37, 46), Akt inhibitor IV (a specific Akt inhibitor; 1 μM) (11), KN-62 (an inhibitor of CaMKI, II, and IV; 10 μM) (40), compound C (an AMPK inhibitor; 10 μM) (23), or KG-501 [an inhibitor of p300 binding to transcription factors, such as cAMP response element binding protein (CREB), via KIX:KID domains interaction; 25 μM] (4).
RNA isolation and quantitative real-time PCR.
Transcripts for each endothelial KCa subtype were quantified as previously reported (47). Briefly, following experimental treatment, cells were harvested by scraping, and RNA was isolated and purified using TRIzol (Invitrogen). The remaining DNA was removed with the TURBO DNA-free kit (Ambion). RNA (1 μg) was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad). Real-time PCR (iCycler, Bio-Rad or 7900 HT Real-time PCR System, Applied Biosystem) was performed using the following FAM (6-carboxy-fluorescein)-labeled probes: KCa1.1 (assay ID: Hs00266938_m1), KCa2.1 (assay ID: Hs00158457_m1), KCa2.2 (assay ID: Hs01030641_m1), KCa2.3 (assay ID: Hs00158463_m1), KCa3.1 (assay ID: Hs00158470_m1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, assay ID: Hs99999905_m1) from Applied Biosystems. Each 20-μl PCR reaction consisted of 900 nM forward primer, 900 nM reverse primer, 250 nM probe, 25 ng of cDNA, and ×1 (final concentration) TaqMan Universal Master Mix (Applied Biosystems). PCR parameters were 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 1 min. To identify the amplification of specific transcripts, melting curve profiles were generated at the end of each PCR reaction. All reactions were carried out in triplicate and included controls without template. Threshold cycles (Ct) were calculated for the genes of interest and GAPDH. For each cDNA sample, the Ct for GAPDH was subtracted from the Ct for each gene of interest to give the parameter ΔCt, thus normalizing the initial amount of RNA used. Relative KCa expression (RQ) was calculated using the equation RQ = 2−ΔΔCt, where ΔΔCt is the difference between the ΔCt of the two cDNA samples to be compared.
Western blot analysis.
KCa2.3 and KCa3.1 protein expression, and phosphorylation levels of kinases and transcription factors were evaluated by Western blotting as previously reported (47). Briefly, cells were washed three times with ice-cold TBS and lysed with 200 μl of lysis buffer: 10 mM Tris·HCl, 1% CHAPS, 1 mM EDTA, 1% protease inhibitor cocktail (Sigma, P8340), 1 mM sodium orthovanadate, 10 mM sodium fluoride, 50 mM n-octyl-β-glucoside (pH 7.4). The lysates were further homogenized by a single round of freeze-thaw. The protein concentration of each sample was quantified using a RC DC Protein Assay (Bio-Rad). Ten micrograms of proteins were resolved on 10% SDS-polyacrylamide gels and subsequently transferred to a polyvinylidene difluoride membrane (Bio-Rad). The membrane was incubated with the primary antibody overnight at 4°C, followed by incubation with a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Protein expression was detected by a chemiluminescence method. The primary antibodies used were KCa2.3 (Santa Cruz), KCa3.1 (obtained from rabbit sera) (47); phospho-Akt (Thr308), phospho-Akt (Ser473), and total Akt (Cell Signaling); phospho-p300 (Ser1834) and total p300 (Thermo Scientific); phospho-CREB (Ser133) and total CREB (Cell Signaling); and β-actin (Santa Cruz). Representative immunoblots from three or more experiments are shown with results of densitometric analysis.
Electrophysiological studies.
The electrophysiological study was performed as our laboratory previously reported (47). HCAECs before and after exposure to LS were dissociated using Cellgro Cellstripper (Corning) and dropped on a bath mounted on the stage of an inverted microscope (T1-SM, Nikon, Japan). The bath was superfused at 5 ml/min, and whole-cell patch-clamp experiments were performed at room temperature (22–25°C). Patch pipettes with a free-tip resistance of 3–5 MΩ for whole-cell recording were connected to the head stage of a patch-clamp amplifier (Axopatch 200B, Molecular Devices, Sunnyvale, CA). pCLAMP software version 10.0 and Digidata 1440A (Molecular Devices) were used to acquire data and to apply command pulses. Whole-cell currents were recorded at 4 kHz and low-pass filtered at 2 kHz. Current traces were stored and analyzed using Clampfit version 10.0 and Origin version 5.0 (Microcal, Northampton, MA). For comparison of whole-cell currents between cells, the current amplitudes were normalized to the membrane area measured by electrical capacitance (pA/pF). The bath solution for the whole-cell patch-clamp contained (in mM) 135 NaCl, 5 KCl, 1.2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES adjusted to pH of 7.4 with Tris. The high Ca2+ (500 nm) pipette solution contained (in mM) 135 KCl, 7.74 CaCl2, 3 MgATP, 0.1 NaGTP, 2.5 creatine phosphate disodium, 10 EGTA, and 10 Hepes adjusted to pH 7.2 with Tris. A fast solution exchange system (AutoMate Scientific, Berkeley, CA) was used to test the effects of drugs on cells. Free Ca2+ concentrations were calculated by Maxchelator software (http://maxchelator.stanford.edu). HCAECs were dialyzed with 500 nM Ca2+ to activate KCa using whole-cell configurations. Whole-cell I-V relationships were measured by application of 400-ms-long voltage ramp from −80 to 80 mV. Apamin-sensitive (KCa2) and TRAM 34-sensitive (KCa3.1) currents were measured by subtraction of currents obtained at 40 mV before and after treatment with 300 nM apamin and 1 μM TRAM 34, respectively.
Statistical analysis.
All data are presented as means ± SE. Statistical analysis was assessed by Student's t-test or ANOVA for one-way and nonparametric tests for data, which are not distributed normally, where appropriate (47). All procedures were done using SigmaStat version 3 (SPSS). Statistical significance was defined as a value of P < 0.05.
RESULTS
LS increases KCa2.3 and KCa3.1 expression.
The effect of shear stress on the expression of each KCa channel subtype in HCAECs was determined by quantitative PCR (Tables 1 and 2). Under ST condition, the relative mRNA expression of KCa2.2 and KCa2.3 was significantly higher than the other subtypes, with KCa2.1 expression being the lowest among all subtypes. Exposure to LS (15 dynes/cm2) for 24 h markedly increased KCa3.1 mRNA expression, caused a moderate elevation in KCa1.1 and KCa2.3, and suppressed KCa2.1 and KCa2.2 expression. On the other hand, exposure to OS for 24 h had a relatively modest but significant effect on the expression of KCa3.1, exhibiting a twofold increase, without altering KCa1.1 and KCa2.3, whereas suppression of KCa2.1 and KCa2.2 expression, similar to LS, was observed (Table 2). The effect of LS on KCa3.1 expression was dose-dependent, since exposure to lower LS (5 dynes/cm2) for 24 h conferred a significantly lower induction of KCa3.1 (9.6 ± 2.0, P < 0.05 vs. LS of 15 dynes/cm2; n = 6), whereas the KCa2.3 expression was dose-independent (4.7 ± 1.2, P = not significant vs. LS of 15 dynes/cm2; n = 5). For the relative comparison of mRNA expression levels among KCa subtypes, each KCa mRNA level was normalized with the level of the housekeeping gene GAPDH and was expressed as the copy number of KCa mRNA out of the amount of template that contained 104 copies of GAPDH mRNA (Fig. 1). Under ST condition, the number of KCa2.2 and KCa2.3 copies per 104 GAPDH mRNA was significantly higher than the other three KCa subtypes. Under OS, KCa2.3 was the primary transcript among the subtypes, with KCa1.1, KCa2.2, and KCa3.1 being moderately and compatibly expressed. In contrast, exposure to LS produced a remarkably high number of transcripts for KCa2.3 and KCa3.1 compared with those for KCa1.1, KCa2.1, and KCa2.2. Taken together, these data indicate that LS increases KCa2.3 and KCa3.1 mRNA expression, whereas OS induces a modest increase in KCa3.1 transcripts, with KCa2.3 remaining as the primary transcript among the subtypes. These data suggest that KCa2.3 and KCa3.1 are the predominant KCa subtypes expressed in HCAECs under physiological conditions. To determine the signaling pathway responsible for the physiological induction of these channels, we performed the next studies at the arterial level of LS (15 dynes/cm2) (35).
Table 1.
mRNA expression levels of KCa subtypes in HCAECs under static condition
| KCa1.1 | KCa2.1 | KCa2.2 | KCa2.3 | KCa3.1 | |
|---|---|---|---|---|---|
| ΔCT values (CTx-CTGAPDH) | 13.6 ± 0.7 (n = 10) | 17.2 ± 0.4 (n = 10)* | 10.6 ± 0.3 (n = 10)† | 11.2 ± 0.1 (n = 23) | 13.4 ± 0.3 (n = 33) |
| Fold change vs. KCa3.1 | 0.9 | 0.1 | 6.7 | 4.5 | 1.0 |
Values are means ± SE. n Value indicates the number of experiments. HCAECs, human coronary artery endothelial cells.
Significant difference vs. the other four KCa subtypes (P < 0.05).
Significant difference vs. KCa1.1, KCa2.1, and KCa3.1 (P < 0.05). Fold change vs. KCa3.1 indicates 2ΔCT(KCa3.1) − ΔCT(X).
Table 2.
Effect of shear patterns on the mRNA expression of KCa subtypes in HCAECs
| Shear Pattern | KCa1.1 | KCa2.1 | KCa2.2 | KCa2.3 | KCa3.1 |
|---|---|---|---|---|---|
| LS | 9.5 ± 2.4 (n = 8)* | 0.3 ± 0.1 (n = 8)* | 0.3 ± 0.1 (n = 8)* | 3.2 ± 0.4 (n = 25)*,† | 44.7 ± 9.7 (n = 30)*,† |
| OS | 6.0 ± 2.7 (n = 5) | 0.2 ± 0.1 (n = 5)* | 0.3 ± 0.1 (n = 5)* | 1.0 ± 0.2 (n = 8) | 2.1 ± 0.4 (n = 9)* |
Values are means ± SE, and indicate the fold change of mRNA expression of each KCa subtype compared to the static (ST) condition [2ΔCT(X) − ΔCT(static)]. OS, oscillatory shear stress.
Significant difference vs. ST (P < 0.05).
Significant difference vs. OS (P < 0.05).
Fig. 1.
Laminar shear stress (LS) increases KCa3.1 and KCa2.3 expression in endothelial cells (ECs). Human coronary artery ECs (HCAECs) were exposed to static culture (ST), oscillatory shear stress (OS), or LS condition for 24 h, and the mRNA expression levels of each KCa subtype were compared based on the number of copies per 104 GAPDH mRNA. Under ST condition, transcripts for KCa2.2 and KCa2.3 were predominantly expressed compared with the other subtypes, whereas OS-exposed cells showed a moderate expression of KCa2.3. In contrast, KCa2.3 and KCa3.1 were the primary KCa subtypes after exposure to LS. aSignificant difference vs. KCa1.1, KCa2.1, and KCa3.1 in ST (P < 0.05). bSignificant difference vs. KCa1.1, KCa2.1, KCa2.2, and KCa3.1 in OS (P < 0.05). cSignficant difference vs. KCa1.1, KCa2.1, and KCa2.2 in LS (P < 0.05). *Signficant difference vs. ST (P < 0.05). #Signficant difference vs. OS (P < 0.05).
CaMKK activation mediates LS-induced increase in KCa2.3 and KCa3.1 expression through Akt phosphorylation.
We first tested the role of CaMKK in LS-induced upregulation of KCa2.3 and KCa3.1. HCAECs were exposed to LS for 24 h in the absence or presence of STO-609 (a specific inhibitor of CaMKK; 10 μg/ml) (37, 46). As shown in Fig. 2A, STO-609 had no effect on KCa3.1 expression under ST condition but strongly suppressed the LS-induced increase in KCa3.1 expression (LS + STO-609, 14 ± 2 fold change from ST, P < 0.05 vs. LS; 56 ± 8; n = 6). On the other hand, STO-609 potently attenuated KCa2.3 mRNA expression under both ST and LS conditions (LS + STO-609; 1.6 ± 0.3, P < 0.05 vs. LS, 3.4 ± 0.5; n = 8; Fig. 2B). Western blotting corroborated the effect of CaMKK inhibition on LS-dependent mRNA expression of KCa2.3 and KCa3.1, showing that an increase in KCa2.3 and KCa3.1 proteins in LS-exposed ECs was abolished by STO-609 (Fig. 2C). The electrophysiological study also confirmed these effects (Fig. 2D). LS induced the increase of TRAM-34-sensitive KCa3.1 and apamin-sensitive KCa2 currents (from 0.4 ± 0.1 to 2.7 ± 0.5 pA/pF and from 0.3 ± 0.1 to 2.7 ± 0.3 pA/pF, respectively). CaMKK inhibitor STO-609 significantly reduced LS-induced increase of KCa3.1 and KCa2 currents to 1.2 ± 0.1 and 0.6 ± 0.1 pA/pF, respectively. These data indicate that CaMKK plays an important role in mediating LS-induced upregulation of these channels in ECs.
Fig. 2.
CaMKK inhibition reduces LS-induced increase in the expression and activity of KCa3.1 and KCa2.3. HCAECs were exposed to LS for 24 h in the absence or presence of the CaMKK inhibitor STO-609 (10 μg/ml). LS-induced increase in KCa3.1 (A) and KCa2.3 (B) mRNA expression was significantly suppressed by CaMKK inhibition. C: Western blotting revealed that increased protein expression levels of KCa2.3 and KCa3.1 under LS condition were inhibited by CaMKK inhibition. Top: a representative image of immunoblots. Bottom: the summaries of densitometric analysis of KCa3.1 and KCa2.3 (n = 3). D: representative KCa currents in HCAECs after LS exposure (top). KCa3.1 and KCa2 currents were sensitive to 1 μM TRAM 34 and 300 nM apamin, respectively. Summary of KCa3.1 and KCa2 currents densities at 40 mV in HCAECs before and after LS exposure with or without STO-609 (n = 5; bottom). LS-induced increase in the channel activities of KCa3.1 and KCa2 under LS condition was diminished by CaMKK inhibition. *Signficant difference vs. ST (P < 0.05). #Signficant difference vs. LS (P < 0.05).
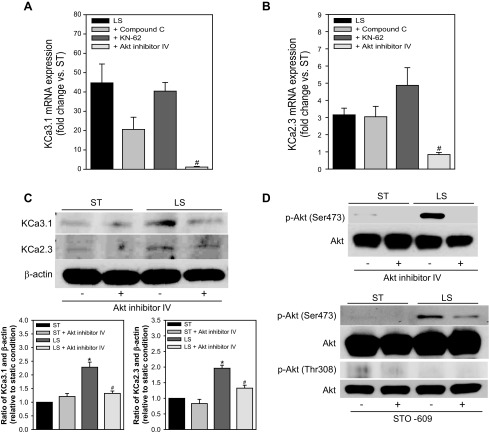
We next examined which downstream kinases were associated with the CaMKK-dependent increase in KCa2.3 and KCa3.1 expression in LS-sheared HCAECs. Akt inhibitor IV (1 μM) (11) completely blocked the induction of KCa3.1 (1.1 ± 0.3 fold of ST condition, n = 7, P < 0.05 vs. LS 45 ± 10, n = 30; Fig. 3A) and KCa2.3 mRNA expression (0.8 ± 0.1 fold of ST condition, n = 7, P < 0.05 vs. LS 3.2 ± 0.4, n = 25; Fig. 3B), whereas it modestly reduced the expression of KCa2.3 but not KCa3.1 under ST condition (data not shown). In contrast, KN-62 (an inhibitor of CaMKI, II, and IV; 10 μM) (40) did not affect LS-induced increase in the expression of KCa3.1 and KCa2.3. Compound C (an AMPK inhibitor; 10 μM) (23) tended to reduce LS-induced upregulation of KCa3.1 (21 ± 6 fold, n = 9, P = 0.06 vs. LS), but it had no effect on KCa2.3 upregulation. Neither compound C nor KN-62 had an effect on channel expression under ST condition (data not shown). Western blotting also confirmed the role of Akt activation, showing that Akt inhibition markedly reduces LS-dependent increased expression of KCa3.1 and KCa2.3 proteins (Fig. 3C). Akt activation was analyzed by Western blotting with antibodies specific for the phosphorylated (Thr308 or Ser473) and total forms of Akt. Exposure of ECs to LS for 60 min stimulated Ser473 phosphorylation, which was abolished in the presence of Akt inhibitor IV (Fig. 3D, top) (ratio of p-Akt and total Akt normalized to ST condition; LS + Akt inhibitor IV 0.7 ± 0.2, P < 0.05 vs. LS 1.6 ± 0.1, n = 3). CaMKK inhibition with STO-609 remarkably attenuated Ser473 phosphorylation of Akt in ECs exposed to 15 min of LS (Fig. 3D, bottom) (LS + STO-609 1.5 ± 0.1, P < 0.05 vs. LS 2.3 ± 0.1, n = 3). In contrast, Akt phosphorylation at Thr308 was not detected in the sheared or nonsheared ECs (data not shown; n = 3). Together, these data indicate that, in HCAECs, LS induction of KCa2.3 and KCa3.1 is dependent on Akt phosphorylation at Ser473 via CaMKK activation.
Fig. 3.
LS-induced upregulation of KCa3.1 and KCa2.3 expression is mediated by CaMKK-dependent Akt activation. A and B: HCAECs were exposed to LS for 24 h in the absence or presence of compound C (an AMPK inhibitor), KN-62 (a CaMK inhibitor), or Akt inhibitor IV. Compound C tended to reduce LS-induced upregulation of KCa3.1 mRNA expression (A) but did not change LS induction of KCa2.3 expression (B) (n = 9). KN-62 had no effect on the upregulation of KCa3.1 and KCa2.3 mRNA expression (n = 7–8). Akt inhibition completely blocked LS-induced upregulation of both KCa3.1 and KCa2.3 expression. C: LS-induced increase in KCa2.3 and KCa3.1 protein expression levels was suppressed by Akt inhibition. Top: a representative image of immunoblots. Bottom: the summaries of densitometric analysis of KCa3.1 and KCa2.3 signals (n = 3). D: Akt inhibitor IV abolished the phosphorylation of Akt at Ser473 in HCAECs exposed to 60-min LS (top). Levels of phospho-Akt (Ser473) were suppressed after inhibiting CaMKK with STO-609 in ECs exposed to 15 min of LS (bottom). Phosphorylation of Akt at Thr308 was not observed in ECs under ST or LS condition. #Signficant difference vs. LS (P < 0.05).
LS-induced CaMKK activation upregulates KCa2.3 and KCa3.1 expression via phosphorylating p300 through akt activation.
We next examined whether CaMKK/Akt regulation of LS-induced KCa2.3 and KCa3.1 induction is mediated by the activation of p300. To test this hypothesis, HCAECs were exposed to LS for 24 h in the absence or presence of KG-501, which inhibits p300 binding to transcription factors with KID domain (4). As shown in Fig. 4A, treatment with KG-501 (25 μM) decreased LS-induced mRNA levels of KCa3.1 without altering its expression under ST condition (16 ± 5 fold, P < 0.05 vs. LS, 33 ± 7 fold; n = 8). LS-induced increase in KCa2.3 expression was also abolished by KG-501 (0.9 ± 0.3 fold, P < 0.05 vs. LS; 2.9 ± 0.6 fold; n = 8; Fig. 4B), with lower basal expression levels under ST condition. Similarly, KG-501 strongly suppressed the KCa3.1 and KCa2.3 protein expression induced by LS (Fig. 4C). Next, the effect of LS on p300 activation and the role of CaMKK and Akt in this signaling were examined by Western blotting using antibodies for phosphorylated (Ser1834) and total p300. Increased phosphorylation of p300 at Ser1834 due to a 15- or 60-min exposure to LS was diminished by CaMKK blockade with STO-609 (Fig. 4D, top) and abolished by Akt inhibition (Fig. 4D, bottom) (ratio of p-p300 and total p300 normalized to ST condition; LS + STO-609, 0.8 ± 0.1, P < 0.05 vs. LS, 1.6 ± 0.2; n = 4). Although LS increased the levels of phosphorylated CREB (Ser133), a transcription factor interacting with p300 (4), these levels were augmented by STO-609 (ratio of p-CREB and total CREB normalized to ST condition; LS + STO-609, 2.8 ± 0.3, P < 0.05 vs. LS, 1.8 ± 0.1; n = 3) and slightly reduced by Akt inhibitor IV (LS + Akt inhibitor IV, 1.26 ± 0.01, P < 0.05 vs. LS, 1.34 ± 0.01; n = 3). KG-501 had no effect on CREB phosphorylation (data not shown). These data indicate that LS increases KCa2.3 and KCa3.1 expression via p300 phosphorylation and activation. Together, these experiments suggest that LS stimulates a CaMKK/Akt cascade leading to p300 phosphorylation and activation, and subsequent KCa3.1 and KCa2.3 gene expression in ECs.
Fig. 4.
CaMKK mediates LS-induced expression of KCa3.1 and KCa2.3 via activation of Akt and histone acetyltransferase p300. A and B: HCAECs were exposed to LS for 24 h in the absence or presence of KG-501 (an inhibitor of p300 binding to transcription factors, such as CREB, via KIX:KID domains interaction) (4). KG-501 inhibited LS-induced upregulation of KCa3.1 (A) and KCa2.3 (B) mRNA expression, whereas it lowered KCa2.3 but not KCa3.1 expression under ST condition. C: LS-induced increase in KCa2.3 and KCa3.1 protein expression levels was also suppressed by KG-501. Top: a representative image of immunoblots. Bottom: the summaries of densitometric analysis of KCa3.1 and KCa2.3 (n = 3). Electronically spliced and rearranged images are shown, as a white line indicates between the lanes, because responses to multiple inhibitors and a time course of shear stress were simultaneously examined in the original experiment. D: exposure to LS for 15 min induced phosphorylation of p300 (Ser1834) in HCAECs, which was abolished by CaMKK inhibition with STO-609 (top). In contrast, CREB phosphorylation was increased by STO-609 treatment under ST condition or after 30 min of LS. Electronically spliced and rearranged p-CREB and CREB images are shown due to a different arrangement in the original images. Akt inhibition with Akt inhibitor IV also dramatically abolished p300 phosphorylation induced by a 60 min of exposure to LS, whereas it had a modest inhibitory effect on phosphorylated CREB levels (bottom). *Signficant difference vs. ST (P < 0.05). #Signficant difference vs. LS (P < 0.05).
DISCUSSION
The major novel findings of this study are fivefold. First, LS induces a profound upregulation of both KCa2.3 and KCa3.1 mRNA and protein expression in HCAECs, whereas OS induces a relatively modest increase in KCa3.1 transcripts. Second, CaMKK activation is associated with LS-induced upregulation of these channels. Third, this upregulation is mediated by CaMKK-dependent Akt activation through Ser473 phosphorylation. Fourth, LS induces p300 activation in a CaMKK- and Akt-dependent manner. Finally, LS induction of KCa2.3 and KCa3.1 involves the binding of p300 with transcription factors via KIX:KID domain interaction. Taken together, these findings suggest that a CaMKK/Akt/p300 cascade mediates LS induction of endothelial KCa2.3 and KCa3.1 (Fig. 5).
Fig. 5.
Scheme for the proposed mechanism for LS-dependent regulation of KCa3.1 and KCa2.3 expression. LS increases intracellular Ca2+ concentration ([Ca2+]i) in ECs by stimulating Ca2+ release from endoplasmic reticulum (ER) and Ca2+ influx, which is maintained by KCa activation through membrane hyperpolarization (Em). Ca2+/CaM complex then activates CaMKK, leading to phosphorylation and activation of Akt, CaMK, and AMPK. Akt in turn phosphorylates p300 at Ser1834, which increases its histone acetyltransferase activity. Following its translocation to the nuclei, active p300 binds to transcription factors including CREB via KIX:KID domains complex and permits their access to promoter regions of KCa3.1 and KCa2.3 genes (e.g., CRE and AP-1 element) by opening chromatin in these regions via histone acetylation, in addition to a direct acetylation of the transcription factors.
The transcripts of all KCa subtypes were detected in HCAECs under ST, OS, or LS condition. LS significantly increased KCa1.1, KCa2.3, and KCa3.1 mRNA expression in HCAECs and decreased KCa2.1 and KCa2.2. Although KCa2.3 and KCa3.1 are considered to be constitutive and predominant KCa subtypes in primary ECs (21, 30), KCa1.1 activity is present in ECs from some vasculatures of several species such as human umbilical vein ECs (HUVEC) (27) and porcine renal arterial ECs (6). However, it is unlikely that endothelial KCa1.1 plays an important role in the regulation of vascular tone under physiological conditions for the following reasons. Three “small-conductance” KCa (KCa2.x) and the “intermediate-conductance” channel KCa3.1 are voltage-insensitive and are activated by [Ca2+]i below 1.0 μM, not far above resting [Ca2+]i levels (e.g., ∼120 nM for KCa3.1), in contrast to KCa1.1, which is activated by voltage and [Ca2+]i above 1.0 μM (48) (Fig. 5). Thus, when ECs are activated and hyperpolarized during NO and EDHF production in response to agonists (15, 39), KCa1.1 remains normally inactive, and other KCa subtypes can be opened by small fluctuations in [Ca2+]i. In addition, KCa3.1 inhibits KCa1.1 activity via a direct, membrane-delimited interaction between the channels in both native parotid acinar cells and in a heterologous expression system (44). Thus KCa1.1 could be electrophysiologically and physically inactivated when it coexists with KCa3.1 in activated ECs. Meanwhile, we also found that KCa2.1 and KCa2.2 were strongly suppressed in LS-exposed HCAECs. This finding is consistent with little or modest expression of KCa2.1 and KCa2.2 in ECs from human saphenous veins (42) and porcine coronary arteries (8). We therefore further focused on LS-dependent regulation of KCa2.3 and KCa3.1 expression in ECs.
The precise involvement of CaMKK in LS-dependent signaling remains elusive (16), although it can be activated during EC-dependent vasodilation to agonists (34, 41). To our knowledge, the present study provides the first evidence that LS induces CaMKK activation in ECs. In addition, we found that LS induction of both channels is abolished by inhibition of Akt but not of CaMK or AMPK, major substrates of CaMKK (14, 45, 50). This finding is consistent with the observed suppression of Akt phosphorylation at Ser473 by CaMKK inhibition. LS results in Akt phosphorylation at Ser473 and Thr308 in bovine aortic ECs (18, 22) but did not cause Thr308 phosphorylation in our experiments with HCAECs. Moreover, CaMKK phosphorylates Akt at Ser473 or Thr308, depending on the experimental conditions or stimuli (26, 50). Our study demonstrates that, in HCAECs, LS activates Akt through Ser473 phosphorylation via CaMKK. Inhibition of AMPK with compound C (23) tended to reduce LS-induced upregulation of KCa3.1 but not of KCa2.3. A complicated cross talk seems to exist between AMPK and Akt signaling (16). Thus further investigation is required to understand the detailed mechanisms of a possible involvement of AMPK in LS induction of KCa3.1. Furthermore, LS activates CaMII in an in vivo rat fistula vein model (13), whereas this kinase is inhibited by LS in bovine aortic ECs (9). Our study shows no discernible role for CaMK in LS-dependent upregulation of KCa2.3 and KCa3.1 expression. Together, these findings suggest that LS-dependent increase in KCa2.3 and KCa3.1 expression in HCAECs is mediated by Akt phosphorylation at Ser473 via CaMKK activation.
Since eukaryotic genes are assembled into units referred to as nucleosomes containing eight histone proteins clustered on 146 base pairs of DNA (25), transcription coactivator histone acetyltransferases such as p300 are required to permit the access of transcription factors to promoter regions, in addition to a direct acetylation of these factors. Our study shows that KG-501, which disrupts KIX:KID interaction, markedly inhibits LS-induced upregulation of both KCa2.3 and KCa3.1 in HCAECs, indicating that p300 activity is involved in this process. Importantly, Akt phosphorylation of p300 at Ser1834 is essential for its histone acetyltransferase activity (24). It is noted that inhibition of CaMKK or Akt abolished LS-induced phosphorylation of p300 at Ser1834 and hence its activation in HCAECs. These findings indicate that CaMKK and Akt-dependent activation of p300 play a crucial role in LS regulation of KCa2.3 and KCa3.1 in HCAECs.
Brakemeier et al. reported fourfold increases in KCa3.1 mRNA expression in HUVEC exposed to 24 h of LS via activation of the mitogen-activated protein kinase/extracellular-signal-regulated kinase kinase 1/2 (MEK/ERK) pathway (7). With the same duration and magnitude of LS, we observed over a 40-fold increase in KCa3.1 expression in HCAECs. Arterial ECs may therefore be more sensitive to LS-induced activation of KCa3.1 compared with venous ECs. In addition, MEK/ERK signaling is also associated with KCa3.1 induction in mitogen-activated fibroblasts (36). A more detailed signaling mechanism has been proposed in human T lymphocytes, where assembly of Fos/Jun heterodimers bind to activation protein-1 (AP-1) elements on an overlapping set of genes via activation of MEK/ERK and JNK (c-Jun NH2-terminal kinase) pathways (17). It should be noted that p300 stimulates transcription through an AP-1 site present in the promoter regions by interacting with c-jun and c-fos (2, 3), indicating a role for p300 in AP-1-mediated transcription. Thus our findings imply that a CaMKK/Akt/p300 cascade is activated in parallel to MEK/ERK and JNK pathways, leading to channel induction in a coordinated manner.
The cAMP response element (CRE) has also been identified in the promoter region of both KCa2.3 and KCa3.1 genes in addition to AP-1 elements (17, 43). LS-induced phosphorylation of CREB was insensitive to inhibition of CaMKK, Akt, and p300, whereas MEK/ERK pathway plays a crucial role in CREB activation (49). The present study did not intend to determine whether CREB directly contributes to LS-dependent induction of these channels, since more than 12 different transcription factors interact with p300 (19). Importantly, CREB bound to p300 increases the expression or activity of CRE-containing gene, c-fos, and AP-1 complexes (heterodimers of Fos and Jun family members) (33). Contrarily, AP-1 also transactivates CRE sequences, and Fos and Jun efficiently bind and cooperate in activating CRE promoter elements (38). Thus CREB may directly or indirectly be involved in LS-dependent transcriptional regulation of KCa2.3 and KCa3.1 via AP-1 or CRE promoter elements in a CaMKK/Akt/p300 cascade-dependent manner.
In contrast to LS, OS only induced a twofold increase in KCa3.1 transcripts with no change in KCa2.3. Chappell et al. observed that the mRNA expression of VCAM-1 peaked after 4 h of OS and then decreased after 6 h to eventually reach baseline levels after 24 h (10). In addition, LS-induced increases in mRNA expression and activity of KCa3.1 also peak then slightly decline after several hours of LS (7). It is therefore possible that OS-induced changes, at least in KCa2.3 and KCa3.1 mRNA expression levels, might be underestimated in our study. Further investigations should be performed to determine mechanisms of OS-dependent regulation of endothelial KCa subtype expression.
In conclusion, our study shows that LS upregulates KCa2.3 and KCa3.1 expression via activating CaMKK, leading to phosphorylation of Akt at Ser473 and subsequently of p300 at Ser1834. A CaMKK/Akt/p300 cascade may therefore play an important role in the regulation of NO and EDHF-mediated vasodilator responses via controlling LS-dependent induction of KCa2.3 and KCa3.1 expression, thereby maintaining EC homeostatic functions such as adaptation to hemodynamic changes.
GRANTS
This study was supported by grants from the National Heart, Lung, and Blood Institute (R01 HL-080173 and HL-080173-02S1), the National Center for Research Resources (P20 RR-018751), and the National Institute of General Medical Sciences (P20 GM-103513) from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.T., A.S., H.Z., M.O., L.M.F., R.T., and H.J. performed experiments; J.T., A.S., H.Z., M.O., L.M.F., V.L., and R.T. analyzed data; J.T., A.S., H.Z., M.O., R.T., and H.M. prepared figures; J.T., A.S., H.Z., and V.L. drafted manuscript; H.Z., S.D.K., L.M.F., V.L., R.T., and H.M. interpreted results of experiments; S.D.K., H.J., and H.M. conception and design of research; S.D.K., V.L., H.J., and H.M. edited and revised manuscript; H.M. approved final version of manuscript.
REFERENCES
- 1.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol 12: 141–151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature 370: 226–229, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Bannister AJ, Kouzarides T. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J 14: 4758–4762, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best JL, Amezcua CA, Mayr B, Flechner L, Murawsky CM, Emerson B, Zor T, Gardner KH, Montminy M. Identification of small-molecule antagonists that inhibit an activator:coactivator interaction. PNAS 101: 17622–17627, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de WC, Hoyer J, Kohler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119: 2323–2332, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Brakemeier S, Eichler I, Knorr A, Fassheber T, Kohler R, Hoyer J. Modulation of Ca2+-activated K+ channel in renal artery endothelium in situ by nitric oxide and reactive oxygen species. Kidney Int 64: 199–207, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brakemeier S, Kersten A, Eichler I, Grgic I, Zakrzewicz A, Hopp H, Kohler R, Hoyer J. Shear stress-induced up-regulation of the intermediate-conductance Ca2+-activated K+ channel in human endothelium. Cardiovasc Res 60: 488–496, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Burnham MP, Bychkov R, Feletou M, Richards GR, Vanhoutte PM, Weston AH, Edwards G. Characterization of an apamin-sensitive small-conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br J Pharmacol 135: 1133–1143, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai H, McNally JS, Weber M, Harrison DG. Oscillatory shear stress upregulation of endothelial nitric oxide synthase requires intracellular hydrogen peroxide and CaMKII. J Mol Cell Cardiol 37: 121–125, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res 82: 532–539, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Ganapathy S, Singh KP, Shankar S, Srivastava RK. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLos One 5: e15288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Bacanamwo M, Harrison DG. Activation of p300 histone acetyltransferase activity is an early endothelial response to laminar shear stress and is essential for stimulation of endothelial nitric-oxide synthase mRNA transcription. J Biol Chem 283: 16293–16298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YS, Lu MJ, Huang HS, Ma MC. Mechanosensitive transient receptor potential vanilloid type 1 channels contribute to vascular remodeling of rat fistula veins. J Vasc Surg 52: 1310–1320, 2010 [DOI] [PubMed] [Google Scholar]
- 14.da Silva CG, Jarzyna R, Specht A, Kaczmarek E. Extracellular nucleotides and adenosine independently activate AMP-activated protein kinase in endothelial cells: involvement of P2 receptors and adenosine transporters. Circ Res 98: 39–47, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de WC, Jensen BL, Simonsen U, Bie P, Wulff H, Kohler R. Pharmacological activation of KCa3.1/KCa23 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol 165: 223–234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105: 114–127, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Cahalan MD, Chandy KG. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem 275: 37137–37149, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Go YM, Boo YC, Park H, Maland MC, Patel R, Pritchard KA, Jr, Fujio Y, Walsh K, Darley-Usmar V, Jo H. Protein kinase B/Akt activates c-Jun NH2-terminal kinase by increasing NO production in response to shear stress. J Appl Physiol 91: 1574–1581, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Develop 14: 1553–1577, 2000 [PubMed] [Google Scholar]
- 20.Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Kohler R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. Arterioscler Thromb Vasc Biol 25: 704–709, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca2+-activated K+ channels in normal and impaired EDHF-dilator responses: relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol 157: 509–526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo D, Chien S, Shyy JY. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ Res 100: 564–571, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Hu Z, Chen J, Wei Q, Xia Y. Bidirectional actions of hydrogen peroxide on endothelial nitric-oxide synthase phosphorylation and function: co-commitment and interplay of Akt and AMPK. J Biol Chem 283: 25256–25263, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang WC, Chen CC. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol Cell Biol 25: 6592–6602, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khorasanizadeh S. The nucleosome: from genomic organization to genomic regulation. Cell 116: 259–272, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kimura T, Tomura H, Sato K, Ito M, Matsuoka I, Im DS, Kuwabara A, Mogi C, Itoh H, Kurose H, Murakami M, Okajima F. Mechanism and role of high density lipoprotein-induced activation of AMP-activated protein kinase in endothelial cells. J Biol Chem 285: 4387–4397, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler R, Schonfelder G, Hopp H, Distler A, Hoyer J. Stretch-activated cation channel in human umbilical vein endothelium in normal pregnancy and in preeclampsia. J Hypertens 16: 1149–1156, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol Cell Physiol 266: C628–C636, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Kwan HY, Leung PC, Huang Y, Yao X. Depletion of intracellular Ca2+ stores sensitizes the flow-induced Ca2+ influx in rat endothelial cells. Circ Res 92: 286–292, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology 21: 69–78, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization: contribution of nitric oxide and Ca2+-activated K+ channels. Circulation 99: 3132–3138, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca2+-activated K+ channels. Circulation 103: 1992–1998, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem 271: 14214–14220, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Mount PF, Lane N, Venkatesan S, Steinberg GR, Fraser SA, Kemp BE, Power DA. Bradykinin stimulates endothelial cell fatty acid oxidation by CaMKK-dependent activation of AMPK. Atherosclerosis 200: 28–36, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Mowbray AL, Kang DH, Rhee SG, Kang SW, Jo H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem 283: 1622–1627, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Pena TL, Chen SH, Konieczny SF, Rane SG. Ras/MEK/ERK up-regulation of the fibroblast KCa channel FIK is a common mechanism for basic fibroblast growth factor and transforming growth factor-beta suppression of myogenesis. J Biol Chem 275: 13677–13682, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Reihill JA, Ewart MA, Hardie DG, Salt IP. AMP-activated protein kinase mediates VEGF-stimulated endothelial NO production. Biochem Biophys Res Commun 354: 1084–1088, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sassone-Corsi P, Ransone LJ, Verma IM. Cross-talk in signal transduction: TPA-inducible factor jun/AP-1 activates cAMP-responsive enhancer elements. Oncogene 5: 427–431, 1990 [PubMed] [Google Scholar]
- 39.Sheng JZ, Ella S, Davis MJ, Hill MA, Braun AP. Openers of SKCa and IKCa channels enhance agonist-evoked endothelial nitric oxide synthesis and arteriolar vasodilation. FASEB J 23: 1138–1145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skelding KA, Rostas JA, Verrills NM. Controlling the cell cycle: the role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle 10: 631–639, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Stahmann N, Woods A, Carling D, Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol Cell Biol 26: 5933–5945, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sultan S, Gosling M, bu-Hayyeh S, Carey N, Powell JT. Flow-dependent increase of ICAM-1 on saphenous vein endothelium is sensitive to apamin. Am J Physiol Heart Circ Physiol 287: H22–H28, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Sun G, Tomita H, Shakkottai VG, Gargus JJ. Genomic organization and promoter analysis of human KCNN3 gene. J Hum Genet 46: 463–470, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Thompson J, Begenisich T. Membrane-delimited inhibition of maxi-K channel activity by the intermediate conductance Ca2+-activated K+ channel. J Gen Physiol 127: 159–169, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tokumitsu H, Enslen H, Soderling TR. Characterization of a Ca2+/calmodulin-dependent protein kinase cascade. Molecular cloning and expression of calcium/calmodulin-dependent protein kinase kinase. J Biol Chem 270: 19320–19324, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Tokumitsu H, Inuzuka H, Ishikawa Y, Ikeda M, Saji I, Kobayashi R. STO-609, a specific inhibitor of the Ca2+/calmodulin-dependent protein kinase kinase. J Biol Chem 277: 15813–15818, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin JE, Pratt PF, Hatoum OA, Gutterman DD, Harder DR, Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest 118: 3025–3037, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57: 463–472, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol 18: 1946–1955, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 396: 584–587, 1998 [DOI] [PubMed] [Google Scholar]