Abstract
Advanced age is associated with a disproportionate prevalence of cardiovascular disease (CVD). Intrinsic alterations in the heart and the vasculature occurring over the life course render the cardiovascular system more vulnerable to various stressors in late life, ultimately favoring the development of CVD. Several lines of evidence indicate mitochondrial dysfunction as a major contributor to cardiovascular senescence. Besides being less bioenergetically efficient, damaged mitochondria also produce increased amounts of reactive oxygen species, with detrimental structural and functional consequences for the cardiovascular system. The age-related accumulation of dysfunctional mitochondrial likely results from the combination of impaired clearance of damaged organelles by autophagy and inadequate replenishment of the cellular mitochondrial pool by mitochondriogenesis. In this review, we summarize the current knowledge about relevant mechanisms and consequences of age-related mitochondrial decay and alterations in mitochondrial quality control in the cardiovascular system. The involvement of mitochondrial dysfunction in the pathogenesis of cardiovascular conditions especially prevalent in late life and the emerging connections with neurodegeneration are also illustrated. Special emphasis is placed on recent discoveries on the role played by alterations in mitochondrial dynamics (fusion and fission), mitophagy, and their interconnections in the context of age-related CVD and endothelial dysfunction. Finally, we discuss pharmacological interventions targeting mitochondrial dysfunction to delay cardiovascular aging and manage CVD.
Keywords: oxidative stress, mitophagy, fusion and fission, neurodegeneration, resveratrol
this article is part of a collection on Mitochondria in Cardiovascular Physiology and Disease. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Advanced age is associated with excessive incidence and prevalence of cardiovascular disease (CVD). Over 80% of cases of coronary artery disease (CAD) and more than 75% of those of congestive heart failure (CHF) are observed in elderly patients (113). The incidence of CVD, including CAD, CHF, and stroke, increases from 4–10/1,000 person-years in adults aged 45–54 years to 65–75/1,000 person-years in those older than 85 (112). CVD is also a major cause of chronic disability in advanced age (140). Indeed, subclinical CVD is associated with a decline in physical and cognitive function equivalent to over 5 years of aging (136). Similarly, neurodegenerative disorders, such as vascular dementia and Alzheimer's disease (AD), pose a major problem to global public health (45, 90).
Although the long-term exposure to cardiovascular risk factors plays a major role in the etiopathogenesis of CVD and neurodegeneration, intrinsic aging of the heart and the vasculature greatly enhances the susceptibility to developing CVD and neurodegenerative conditions in late life (100). The cardiovascular system undergoes anatomical and functional changes over the course of aging, the interaction of which with CVD-specific mechanisms may eventually result in an excess risk for pathologies in late life.
The intimate mechanisms involved in cardiovascular senescence and neurodegeneration are not fully understood; yet, alterations in mitochondrial function are considered to be a major contributing factor (48). Dysfunctional mitochondria not only are less bioenergetically efficient, but also generate increased amounts of reactive oxygen species (ROS), interfere with cellular quality control (QC) mechanisms, and are more prone to triggering apoptosis (48). Due to the detrimental consequences of mitochondrial dysfunction, the removal of damaged mitochondria and their replacement with newly formed organelles are crucial for the maintenance of cellular homeostasis. However, the efficiency of these processes declines with advancing age, which may have important implications for cardiovascular senescence, CVD, and neurodegeneration (84, 117, 180).
In this review, we discuss relevant mechanisms linking mitochondrial dysfunction and abnormal ROS production to defective mitochondrial autophagy in the context of cardiovascular aging, CVD conditions common in advanced age, and neurodegeneration. The prospect of delaying cardiovascular senescence and treating CVD and neurodegenerative disorders by targeting mitochondrial dysfunction and autophagy is also illustrated.
Mechanisms of Mitochondrial Dysfunction and Altered Mitochondrial Quality Control in Cardiac Aging and Pathology
Role of mitochondrial oxidative stress in cardiac aging.
The heart is highly energy dependent, with about 90% of the energy consumed being supplied by mitochondrial oxidative phosphorylation. Hence, proper functioning of myocardial mitochondria is crucial for cardiac function. Damaged mitochondria not only produce less ATP but also release greater amounts of ROS and possess a higher propensity to induce apoptosis, all phenomena related to cardiac aging (48). In addition to being the major cellular source of ROS, mitochondria are highly susceptible to the oxidative stress they generate. This is mostly due to the close proximity of mitochondrial DNA (mtDNA) to the site of ROS generation, the lack of protective histones, and a less efficient DNA repair system as compared with nuclear DNA (222). Furthermore, the absence of introns in the mitochondrial genome makes each mutation highly likely to affect gene integrity.
To cope with the constant generation of ROS, the cell is equipped with antioxidant enzymes and nonenzymatic systems. The mitochondrial matrix contains manganese-dependent superoxide dismutase (MnSOD or Sod2) that converts superoxide anion (O2·−) into hydrogen peroxide (H2O2), the latter being further detoxified to harmless water by glutathione peroxidase (GPX) and thioredoxin enzymes (139). O2·− that diffuses in the cytoplasm is dismutated into H2O2 by copper-zinc-dependent SOD (CuZnSOD or Sod1).
Despite the cellular antioxidant defense systems, during aging or under pathological conditions (including CVD), ROS generation is amplified, resulting in an increased burden of oxidative stress, which may lead to detrimental functional and structural consequences (88). In many cases, such enhanced oxidative stress results from the accumulation of dysfunctional, ROS-leaking mitochondria, combined with the lack of proportional upregulation of antioxidant systems (125).
Damaged myocardial mitochondria generate up to 10-fold more H2O2 than healthy organelles (67). Enlarged mitochondria, characterized by matrix derangement, loss of cristae, and enhanced ROS generation, have been observed in several tissues from old rodents, including the myocardium (91, 161). In addition, high levels of oxidative damage to mitochondrial proteins, lipids, and nucleic acids have been detected in the aged myocardium (87, 103, 170). The frequency of the common 4,977-bp mtDNA deletion, a typical consequence of oxidative stress (159), increases with age in the human heart and is estimated to be 5- to 15-fold higher in people over 40 years of age relative to younger controls (111, 129). The 4977-bp deletion affects genes encoding 7 polypeptide components of the mitochondrial electron transport chain (ETC), and 5 of the 22 tRNAs required for mitochondrial protein synthesis. The bioenergetic consequences of the accumulation of 4977-bp deletions are thought to arise when the proportion of deleted mtDNA exceeds 50–55% of total mtDNA, and include declines in mitochondrial membrane potential (Δψm) and ATP synthesis rates and increases in ROS generation (82, 157).
The involvement of mtDNA mutations in cardiac aging is supported by findings in mice that express a proofreading-deficient version of mtDNA polymerase γ (PolG) (99, 186). A high load of mtDNA mutations and deletions accumulate in the heart of these mice, in conjunction with the early onset of several age-associated changes, including cardiac enlargement, fibrosis, and impairment of systolic and diastolic function (36). A reduced activity of ETC complexes and the accumulation of swollen and irregularly shaped mitochondria are also observed in the heart of PolG mice (186). In addition, protein carbonyls, a marker for protein oxidation, are increased in cardiac mitochondria of mtDNA-mutator rodents (36). PolG mice die prematurely from dilated cardiomyopathy, a phenomenon also observed in mice expressing a cardiac-specific proofreading-deficient PolG (227).
A further proof of principle for the role of mitochondria in cardiac aging has been provided by the characterization of mice with overexpression of the antioxidant enzyme catalase targeted to the mitochondrial matrix (mCAT) (36). In these rodents, cardiac enlargement, mtDNA mutation load, and mitochondrial levels of protein carbonyls are attenuated relative to age-matched wild-type controls (36). Furthermore, mCAT mice show increased mean and maximum lifespan and a delayed development of cardiac pathology (40, 166). At the cellular level, these animals are characterized by decreased rates of H2O2 production and reduced mitochondrial oxidative damage and mtDNA deletions (40, 166). Remarkably, the PolG heart phenotype, the cardiac mtDNA mutation load, and the extent of mitochondrial protein oxidation are partially rescued by mCAT overexpression (36).
The interpretation of age-related changes in cardiac mitochondrial function is complicated by the fact that two distinct populations of mitochondria exist in the myocardium: subsarcolemmal mitochondria (SSM), located beneath the plasma membrane, and interfibrillar mitochondria (IFM), arranged between the myofibrils (149). Seminal studies by Hoppel's group characterized relevant biochemical differences between IFM and SSM isolated from the rat heart (149). The activity of both citrate synthase and succinate dehydrogenase, state 3 respiration rates, abundance of respiratory cytochromes, and the activity of ETC complexes were found to be higher in IFM relative to SSM (148, 149).
These findings have instigated a great deal of research in an attempt to discern the specific contribution of IFM and SSM to myocardial aging and CVD. Fannin et al. (54) found that cardiac IFM isolated from old Fischer 344 rats exhibited reduced rates of oxidative phosphorylation and cytochrome c oxidase (COX) activity relative to young adult controls. No differences were observed for the SSM subpopulation between age groups. Data from our laboratory indicate that H2O2 production by SSM, but not IFM, increases with age (87). These results are in contrast with previous findings by Suh et al. (175) who reported an age-dependent increase in oxidant production by IFM but not SSM isolated from rat hearts. This seemingly opposite evidence could stem from methodological differences between the two studies with regard to the assessment of mitochondrial oxidant production: rate of oxidation of 2'7'-dihydrodichlorofluorescein that detects a variety of intramitochondrial oxidants [including H2O2 and nitric oxide (NO·)] (175) as opposed to the quantification of H2O2 released from intact mitochondria (87). It is noteworthy that in IFM from the same animals, Judge et al. (87) also observed increased activities of several antioxidant enzymes (SOD, GPX, and catalase), reduced glutathione concentrations, and elevated levels of oxidative damage. These findings may suggest that oxidant production within the matrix of old IFM is greater than in younger counterparts, which is in agreement with the results by Suh et al. (175).
The interpretation of the biochemical differences between IFM and SSM is further complicated by the fact that most isolation procedures yield either SSM alone or a mixed population of SSM and IFM. This may explain the lack of consistency regarding age-related changes in oxidative phosphorylation, protein yield, and enzymatic activities among studies (54, 87, 105, 148). In addition, cardiomyocytes with extremely dysfunctional mitochondria are likely eliminated via apoptosis and/or necrosis, so that only relatively healthy mitochondria are obtained upon isolation (88).
Despite some areas of uncertainty, studies in rodent models and observations in humans have made a strong case for damaged and dysfunctional mitochondria as a contributing factor to cardiac senescence. The available evidence also supports the therapeutic potential of improving mitochondrial redox homeostasis to prevent or delay cardiac aging. Although it stands reasonable to hypothesize that increasing antioxidant levels in an organism would provide overall beneficial effects and delay heart senescence, administration of antioxidant compounds have led to little or no cardioprotection in humans (13, 107, 185). A great deal of attention has therefore been diverted toward the optimization of mitochondrial QC to repair and/or remove damaged mitochondria, as discussed in the next sections. A synoptic overview of relevant findings on age-related changes in cardiac mitochondrial bioenergetics, oxidant generation, and QC is depicted in Table 1.
Table 1.
Synopsis of major findings on age-related changes in cardiac mitochondrial bioenergetics, oxidant generation, and quality control
| Experimental Model | Parameter(s) Examined | Findings | Reference |
|---|---|---|---|
| Tg-Sirt1 mice | Effects of cardiac-specific Sirt1 overexpression on cardiac aging | Attenuation of age-dependent cardiac hypertrophy, apoptosis/fibrosis, oxidative stress, and heart dysfunction by low to moderate (2.5- to 7.5-fold) Sirt1 overexpression; worsening of mitochondrial dysfunction, oxidative stress, and cardiomyopathy by high levels (12.5-fold) of Sirt1 overexpression | Alcendor et al. (4) |
| Polg/mCAT mice | Effects of mtDNA mutations on age-dependent heart structure and function; effects of mCAT overexpression on PolG phenotype | Marked cardiac hypertrophy and dilation, impairment of systolic and diastolic function, increased cardiac fibrosis; increased mtDNA deletions and protein oxidative damage; increased expression of apoptotic and senescence markers; decline in mitochondrial biogenesis signaling; partial rescue of PolG phenotype by mCAT | Dai et al. 2010 (36) |
| mCAT mice | Effects of mCAT overexpression on indexes of cardiac aging | Attenuated age-related cardiac dysfunction; reduced mitochondrial protein oxidation and mtDNA mutations; protection against cardiac hypertrophy and heart failure | Dai et al. 2009 (38) |
| Fischer 344 rats | Oxidative metabolism in SSM and IFM from 6-, 24-, and 28-month-old rats | Decreased rate of oxidative phosphorylation and cytochromic c oxidase activity in IFM from aged rats; no changes in SSM | Fannin et al. 1999 (54) |
| Fischer 344 rats | H2O2 and oxidative damage in SSM and IFM from 6- and 24-month-old rats | Age-dependent increase in H2O2 production by SSM, not by IFM; increased oxidative stress (4-HNE-modified proteins, protein carbonyls, and MDA) in IFM from old rats, only protein carbonyls in aged SSM; oxidative stress more severe in IFM than in SSM; age-related increases in MnSOD, GPX, and CAT activities in IFM; increased MnSOD and GPX activities and declined CAT activity in aged SSM | Judge et al. 2005 (87) |
| PolG mice | Oxidative stress and apoptosis in mice with homozygous mutation of PolG | Accumulation of mtDNA mutations with no increases in oxidative stress in isolated cardiac mitochondria; accelerated cardiac aging | Kujoth et al. 2005 (99) |
| Fischer 344 rats | Oxidative stress and mitochondrial apoptotic signaling in the heart from 6-, 16-, and 24-month-old 344 rats | Age-associated increase in MnSOD and GPX activity; elevated cytosolic cytochrome c content and decreased mitochondrial Bcl-2 with age | Phaneuf et al. 2001 (155) |
| Fischer 344 rats | Mitochondrial antioxidant capacity, oxidant generation, and oxidative damage to complex IV in IFM and SSM from 2- to 5- and 24- to 28-month-old rats | Higher rates of oxidant production and decline in mitochondrial ascorbate levels and GSH redox status only in IFM from old rats; fourfold increase in 4-HNE-modification and loss of complex IV activity limited to IFM from old rats | Suh et al. 2003 (175) |
| mCAT mice | Effects of mCAT overexpression on indexes of cardiac aging | Delayed cardiac aging, lower oxidative damage, H2O2 production and H2O2-induced aconitase inactivation, reduced mtDNA deletions; 18% lifespan extension | Schriner et al. 2005 (166) |
| PolG mice | Impact of homozygous mutation of PolG on mouse aging | Accumulation of cardiac mtDNA mutations associated with multiple signs of accelerated aging and reduced lifespan | Trifunovic et al. 2004 (186) |
| Cardiac-specific Atg5-deficient mice | Age-associated changes in autophagy in WT mouse heart; cardiac function and lifespan in Atg5-deficient mice | Reduction in the autophagy marker LC3-II with age; LV enlargement and decreased fractional shortening in Atg5-deficient mice; disorganized sarcomere structure and collapsed mitochondria with impaired respiration in Atg5-deficient mice | Taneike et al. 2010 (180) |
4-HNE, 4-hydroxynonenal; CAT, catalase; GPX, glutathione peroxidase; GSH, glutathione; IFM, intermyofibrillar mitochondria; LC3, light chain 3; mCAT, catalase targeted to the mitochondrial matrix; MDA, malondialdehyde; MnSOD, manganese-dependent superoxide dismutase; PolG, mtDNA polymerase-γ; SSM, subsarcolemmal mitochondria; Tg-Sirt1, Sirt1 transgenic; WT, wild-type.
Contribution of altered mitochondrial QC to cardiovascular aging and disease.
The maintenance of a healthy and functional mitochondrial pool within the cell relies on the efficiency of QC processes responsible for repairing or eliminating dysfunctional organelles (168). For instance, oxidatively modified and misfolded mitochondrial proteins are managed by a set of chaperones and proteases that function as a protein QC system (207). Mitochondrial fusion and fission ensure another level of QC by preventing the local accumulation of dysfunctional organelles and by segregating those that are irreversibly damaged or unnecessary from the vital mitochondrial pool (224). A specialized form autophagy, mitophagy, degrades mitochondria segregated by fission and is therefore placed at the end of the mitochondrial QC axis (189). Recent discoveries on the role played by altered mitochondrial dynamics and autophagy in the context of cardiovascular aging and CVD are discussed in the following subsections.
ROLE OF MITOCHONDRIAL DYNAMICS IN CARDIOVASCULAR PHYSIOLOGY, AGING, AND DISEASES.
Mitochondrial research has undergone a paradigm shift since groundbreaking data showed that mitochondria function in networks (5). Accordingly, mitochondria are no longer considered to be static, isolated organelles, the only function of which within the cell is to generate ATP. Rather, they appear to be highly mobile entities forming dynamic networks of long tubules. The functional and morphological behavior of mitochondrial networks largely influences the bioenergetic status of cells, tissues, and organs (11) and confers properties typical of complex systems, such as robustness, redundancy of function, and plasticity (6). These properties provide the system with the adaptive flexibility needed to adjust to changing stresses and metabolic demands (6).
The morphology and function of mitochondrial networks are regulated by continuous fusion and fission cycles that are crucial not only for determining organellar shape but also for transmitting redox-sensitive signals, redistributing metabolites and proteins, maintaining mtDNA integrity, performing metabolic processes, and regulating QC and cell death pathways (16, 165). For instance, the functionality of damaged mitochondria can be complemented and possibly restored by their fusion with neighboring intact mitochondria (189). Severely damaged mitochondria are segregated from the mitochondrial network through fission and eventually eliminated by mitophagy (189). Hence mitochondrial dynamics and autophagy form a QC axis, the dysfunction of which is invoked as a contributing factor to cardiovascular senescence and CVD (48) (Fig. 1).
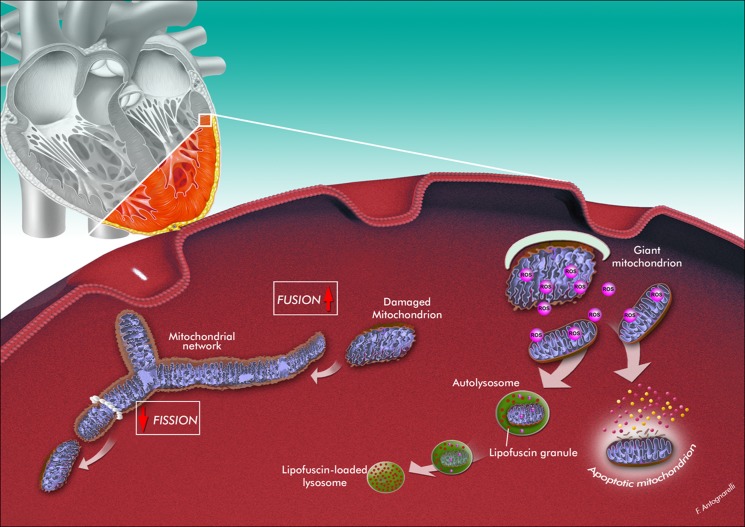
Fig. 1.
Hypothetical scenario of mitochondrial quality control failure, organellar dysfunction, and triggering of mitochondrion-mediated apoptosis during cardiac aging. The aged myocardium is characterized by the presence of enlarged mitochondria with aberrant morphology, reduced bioenergetic efficiency, and increased reactive oxygen species (ROS) production. The formation of giant mitochondria may be the consequence of a shift of mitochondrial dynamics toward fusion. This adaptation could allow diluting mitochondrial abnormalities along the network. Enlarged mitochondria are, however, less susceptible to mitophagic degradation because of their larger dimensions. The accumulation of lipofuscin within lysosomes may further impair the efficiency of mitochondrial quality control. Excessive oxidant production eventually triggers apoptosis. The artwork is by Francesco Antognarelli.
The balance between fusion and fission is dependent upon a complex mitochondrial dynamics machinery. Among the best characterized mitochondrial fusion factors in mammals are the dynamin-related GTPases mitofusin 1 and 2 (Mfn1 and Mfn2), that are responsible for tethering and fusion of outer mitochondrial membranes between two organelles (53), and optic atrophy protein 1 (OPA1), that controls inner membrane fusion (126). Mitochondrial fission is regulated by the dynamin-related protein 1 (Drp1) and fission protein 1 (Fis1) (131, 169).
The function of mitochondrial dynamics in the cardiovascular system has recently been investigated in primary vascular endothelial cells, vascular smooth muscle cells (VSMCs), cardiac cell lines, and neonatal cardiomyocytes, in which mitochondria are arranged in a filamentous network and constantly undergo fusion and fission processes (142). In adult cardiomyocytes, mitochondria, at least IFM, are organized into a more regular, crystal-like lattice arrangement (204), and it is unclear whether this spatial distribution may restrict fusion-fission processes. However, recent data suggest that mitochondrial dynamics are relevant to the physiology of adult heart despite the distinctive arrangement of mitochondria in this organ (73).
Alterations in mitochondrial dynamics may play a role in several cardiac and vascular events, including VSMC proliferation, cardiac development and differentiation, stem cell differentiation, cardiomyocyte hypertrophy, myocardial ischemia-reperfusion (I/R) injury, and CHF (reviewed in 142 and 143). For instance, excessive fission (and/or decreased fusion) may be detrimental in I/R injury (144), diabetes (115), hyperglycemia (225), and heart failure (23). In the setting of I/R, the inhibition of mitochondrial fission has been reported to be cardioprotective (144). Recent studies also suggest that the fusion proteins OPA1, Mfn1, and Mfn2 are required to maintain normal mitochondrial function and prevent the development of cardiac hypertrophy and heart failure (24, 150, 151, 156).
It is noteworthy that some of the mitochondrial-shaping proteins possess pleiotropic effects independent of their ability to modulate mitochondrial morphology. Paradigmatic in this context is Mfn2 that not only mediates fusion but is also involved in apoptosis (by interacting with Bak and Bax) (80), mitophagy (being a substrate of the mitophagy-related protein Parkin) (61), and in the tethering between mitochondria and the endoplasmic reticulum (41). Interestingly, Mfn2 inhibits VSMC proliferation in a variety of vasculo-proliferative conditions (22, 70) and triggers oxidative stress-mediated VSMC apoptosis (69).
In old and postmitotic human umbilical vein endothelial cells (HUVECs), a well-established cell culture model to follow mitochondrial activity and dysfunction during vascular aging, mitochondria show distinct morphological alterations, loss of Δψm, and mtDNA depletion (85). Both fusion and fission activities are decreased in old HUVECs, indicating that these processes are altered during aging, which could contribute to the accumulation of damaged mitochondria (85).
Although very preliminary, the available evidence suggests that optimization of mitochondrial dynamics may be harnessed as therapeutic means against cardiovascular senescence and CVD. Further studies are, however, necessary to fully comprehend the role mitochondrial fission and fusion play in the cardiovascular system in both health and disease.
MECHANISMS OF AUTOPHAGY.
Autophagy is a self-eating process through which cells degrade their own components, recycling amino acids and other building blocks that eventually can be reused (98). Macroautophagy is the best characterized autophagic pathway, and consists in a catabolic process that degrades damaged or unnecessary cellular proteins and organelles through sequestration into a double-membrane structure known as the autophagosome (98). Autophagosomes then fuse with lysosomes to form autolysosomes, wherein the enveloped content is degraded. Although initially considered a bulk degradation pathway, it is now widely accepted that macroautophagy can proceed either nonselectively or selectively (vide infra) (98).
Two other autophagic pathways include microautophagy and chaperone-mediated autophagy (CMA). During micropautophagy, cellular components are directly sequestered through arm-like projections or invaginations of the lysosomal membrane (124). Both nonselective and selective microautophagy-dependent lysosomal degradative pathways have been described. Nonselective microautophagy involves the degradation of randomly sequestered soluble intracellular substrates and is regularly observed in mammalian cells (124). Selective microautophagy sequesters and degrades specific organelles (e.g., micropexophagy, piecemeal microautophagy of the nucleus, micromitophagy), but its occurrence and significance in mammals are still controversial (108). CMA recognizes only a particular pool of cytosolic proteins containing a motif that is biochemically related to the pentapeptide KFERQ (7). Such proteins are recognized by the 70-kDa heat shock cognate protein (hsc70), which delivers them to lysosomes for degradation (25). CMA is maximally activated during times of stress such as exposure to toxic compounds, long-term starvation, or oxidative stress (92).
MOLECULAR MACHINERY AND REGULATION OF MACROAUTOPHAGY.
The process of macroautophagy, hereby referred to as autophagy, can be divided into discrete steps, known as induction and nucleation, expansion, fusion, and degradation. Each of these phases is regulated by autophagy-related (Atg) proteins, of which over 35 have been identified. The ULK1-Atg13-FIP200 kinase complex is required for the induction phase (220). The vacuolar protein sorting-34 (Vps34), a class III phosphatidylinositol-3-kinase (PI3K), is necessary for the nucleation stage, during which Vps34 associates with Beclin1 and subsequently recruits Atg14 and Vps15 (p150) to the pre-autophagosomal structure (177). Elongation and expansion of the phagophore membrane involve two ubiquitin-like conjugation systems: Atg12 (conjugated to Atg5) and Atg8/light chain 3 (LC3, conjugated to phosphatidyl ethanolamine), along with other Atg proteins such as Atg9 and Atg16 (220). For a detailed description of the function of these proteins, the reader is referred to specialized reviews (for instance, 96).
In response to starvation, nonselective autophagy is activated, which serves to provide cells with nutrients necessary for survival. In contrast, selective autophagy occurs to specifically remove protein aggregates or damaged or superfluous organelles and can be operative also under nutrient-rich conditions. So far, several cargo-specific autophagic processes have been identified in yeasts and mammals, and each of them has been named based on the nature of the target. Examples include pexophagy for the disposal of peroxisomes, ERphagy for the degradation of endoplasmic reticulum, ribophagy for the elimination of ribosomes, and aggrephagy for the removal of aggregates (96). In addition, autophagy can be specifically targeted toward the degradation of mitochondria (mitophagy) (39). This process regulates mitochondrial number to match metabolic demands and also serves as a QC mechanism to remove damaged mitochondria (16). Mitophagy is preceded by mitochondrial fission, which divides mitochondria into pieces of manageable size for engulfment and mediates the segregation of damaged material for subsequent disposal (188). The loss of Δψm acts as a major trigger for mitophagy (188). The opening of the mitochondrial permeability transition pore (mPTP) may also be required for the selective removal of damaged mitochondria by inducing Δψm dissipation (21). Apart from the degradation of damaged mitochondria under stress conditions, mitophagy is essential for mitochondrial turnover in the basal state and during cell differentiation, such as the maturation of reticulocytes into mature red blood cells (224).
Parkin and PTEN-induced putative kinase (PINK1) are believed to play important roles in the selective degradation of damaged mitochondria (43). Parkin is a cytosolic E3-ubiquitin ligase that is selectively recruited to dysfunctional mitochondria and assists in their removal by mitophagy (135). In the steady state, PINK1 is imported into healthy mitochondria through a Δψm-dependent process and is rapidly degraded by the presenilin-associated rhomboid-like (PARL) protease (119). When Δψm becomes dissipated, PINK1 accumulates on the mitochondrial surface, leading to the recruitment of Parkin that ubiquitinates outer membrane proteins, including Mfn1, Mfn2, and the voltage-dependent anion channel (VDAC) (61, 62). Ubiquitin-tagged mitochondrial proteins, in turn, serve as binding partners for p62/sequestosome 1 (p62/SQSTM1), a ubiquitin-binding scaffold protein assisting in the recruitment of autophagosomal membranes to mitochondria (62). Parkin can also interact with Ambra1 (activating molecule in Beclin1-regulated autophagy), which in turn stimulates the activity of PI3K that is essential for the formation of new phagophores (203). Interestingly, PINK1-null fruit flies display defects in flight and mitochondrial bioenergetics, which are both rescued by vitamin K2 supplementation (208). This effect appears to be mediated by the transport of electrons by vitamin K2 from complex I and II to complex III of the ETC, similar to its function in prokaryotic membranes.
Priming of mitochondria to autophagy can also occur via PINK1/Parkin-independent mechanisms. For instance, damaged mitochondria can increase the expression of FUN14 domain-containing 1 (FUNDC1), which recruits autophagosomes to mitochondria by direct interaction with LC3/Atg8 (110). In addition, upon mitochondrial depolarization, SMAD-specific E3 ubiquitin protein ligase 1 (SMURF1) targets mitochondria to mitophagy, most likely via the ubiquitination of mitochondrial proteins (146). Finally, the apoptotic proteins BNIP3 (Bcl-2 and adenovirus E1B 19-kDa-interacting protein 3) and NIX (Nip3-like protein X) are thought to trigger selective mitophagy via several mechanisms involving mitochondrial depolarization, competitive disruption of the inhibitory interaction between Bcl-2 and Beclin1, and direct interaction with LC3/Atg8 (228).
Although the molecular regulation of mitophagy has not yet been elucidated completely, the mammalian target of rapamycin (mTOR)/AMP-activated protein kinase (AMPK) axis is proposed to be a major checkpoint (78). Importantly, AMPK, in addition to stimulating mitochondrial removal through autophagy, enhances the activity of sirtuin 1 (Sirt1) and its downstream target peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), resulting in stimulation of mitochondrial biogenesis (19).
CONTRIBUTION OF ALTERED MITOCHONDRIAL AUTOPHAGY TO HEART SENESCENCE.
During aging, a decreased efficiency of autophagy has been reported, especially in the liver (44) and postmitotic tissues such as the nervous system (42, 122) and the heart (84, 180). Because the generation of ROS and the accumulation of oxidatively damaged components are enhanced in old age, a concomitant increase in the autophagic flux could be expected to be beneficial (48). Indeed, experimental inhibition of autophagy results in the accumulation of dysfunctional and bioenergetically inefficient mitochondria, which can result in further ROS generation (91, 184). Cardiac-specific inhibition of autophagy by Atg5 knockdown results in left ventricular hypertrophy (LVH), reduced fractional shortening, disorganization in sarcomere structure, and accumulation of dysfunctional mitochondria with respiratory defects (180). It is important to note that enlarged mitochondria are hypothesized to be less autophagocytosed because of their larger dimensions (184) (Fig. 1).
In addition to the accumulation of damaged mitochondria, the myocardium of aged rodents is also characterized by the buildup of a nondegradable, yellow-brown pigment called lipofuscin (182). Lipofuscin is formed within lysosomes by ROS-inflicted modifications of proteins and lipids. ROS are usually generated by peroxide-induced Fenton reactions within lysosomes containing damaged mitochondria, or can diffuse into lysosomes from the cytosol (89, 183). Lipofuscin-loaded lysosomes consume a major part of newly produced lysosomal hydrolases that, however, cannot digest lipofuscin. At the same time, a smaller amount of lysosomal enzymes remains available for autophagic degradation, including mitophagy. The progressive accumulation of lipofuscin eventual overburdens the autophagy-lysosomal system and results in a decreased degradative capacity (89, 183) (Fig. 1). This series of events forms the basis for the garbage catastrophe theory of aging, also known as the mitochondrial-lysosomal axis theory of aging (181, 184).
A recent study using mice with cardiac-specific deletion of lysosomal DNase II exposed to pressure overload has demonstrated that mtDNA escaping from autophagy activates inflammatory processes in myocytes via a Toll-like receptor 9-mediated pathway (141). Cell-autonomous activation of inflammatory processes is a hallmark of cardiovascular senescence, and the aforementioned finding raises the possibility that age-related alterations of mitochondrial autophagy may also be causally linked to the exacerbation of cardiac inflammation in advanced age.
As discussed further on, abnormalities in mitochondrial structure and function have been associated with a number of pathological conditions affecting the cardiovascular system (48). Autophagy induction under such circumstances can be viewed as a rescue process through the removal of aberrant mitochondria.
ROLE OF MITOCHONDRIAL DYSFUNCTION AND ALTERED AUTOPHAGY IN CARDIAC PATHOLOGY.
Mitochondrial dysfunction has been proposed to contribute to cardiomyocyte injury during I/R, where energy depletion may result in mPTP opening and subsequent cardiomyocyte injury (106). Autophagy is enhanced during I/R and this is usually associated with improved cell survival, most probably by maintaining adequate levels of substrates during times of energy depletion (120).
The inhibition of autophagy by pharmacological (3-methyladenine, wortmannin) or genetic interventions (overexpression of dominant negative Atg5 or RNAi-mediated knockdown of Beclin1) increases apoptosis in cardiomyocytes subjected to simulated I/R (74). Upregulation of autophagy via rapamycin treatment or Beclin1 overexpression, on the other hand, improves cardiomyocyte survival in such experimental paradigm (74). In another model, transgenic animals with cardiac-specific deletion of the autophagy gene Atg5 showed mitochondrial aggregation and misalignment in response to pressure overload induced by thoracic transverse aortic constriction (133). These animals develop LVH, ventricular dilatation, and contractile dysfunction. Furthermore, Atg5-deficient mice show enhanced susceptibility to β-adrenergic stimulation and develop left ventricular dilatation and cardiac dysfunction when exposed to isoproterenol, suggesting that autophagy may serve a beneficial role under such circumstances (133). Finally, transgenic rats expressing human renin and angiotensin genes accumulate enlarged mitochondrial clusters and develop angiotensin-II-mediated LVH (56). Four weeks of 40% calorie restriction (CR) in these animals induced autophagy, improved cardiac ultrastructural remodeling, and reduced mortality (56).
The accumulation of dysfunctional mitochondria due to inefficient cardiac autophagy is proposed to be involved in the pathogenesis of diabetic cardiomyopathy (221). Downregulation of cardiomyocyte autophagy, secondary to reduced AMPK activity, has been documented in diabetic mice (221). Ultrastructurally, hearts from these rodents display several morphological aberrations, including aggregation of chaotically distributed mitochondria. The inhibition of AMPK by overexpression of a cardiac-specific dominant negative AMPK gene further reduces autophagy, exacerbates ultrastructural aberrations, worsens cardiac dysfunction, and increases mortality in diabetic mice (221). In contrast, treatment with metformin significantly enhances autophagy and ameliorates cardiomyocyte ultrastructural abnormalities, while preserving cardiac function. Such benefits are not observed in rodents expressing a dominant negative AMPK, indicating that cardioprotection by metformin is achieved through an AMPK-mediated upregulation of autophagy (221).
In contrast to the above studies showing a beneficial role of autophagy, in a heart failure model induced by intramuscular injections of diphtheria toxin in transgenic mice overexpressing cardiac-specific diphtheria toxin receptor, cardiomyocytes showed a degenerated heart phenotype, with morphological features of autophagic cell death (2). Furthermore, Matsui et al. (120) have shown that upregulation of autophagy during reperfusion is maladaptive and increases infarct size. These findings indicate that, depending on the context under which it is induced and the extent and duration of activation, autophagy can serve either beneficial or detrimental functions in the setting of CVD. Hence, interventions that fine tune the autophagic process, without inducing too low or excessive activation of this self-digestion process, may provide substantial therapeutic gain in the context of several cardiac diseases.
Mechanisms of Mitochondrial Dysfunction and Altered Mitochondrial Quality Control in Vascular Aging and Pathology
Role of mitochondrial oxidative stress and altered autophagy in vascular aging.
With advancing age, mitochondrial ROS production increases significantly in the vasculature, in both endothelial cells and VSMCs (192, 200, 201). The molecular mechanisms responsible for the increased mitochondrial oxidative stress in the aged vascular system are multifaceted and likely involve pathways similar to those contributing to enhanced mitochondrial ROS production in the old heart. These mechanisms encompass ETC dysfunction (196), activation of NOX4 (NADPH oxidase 4, located in the mitochondrial membrane) (1), declines in glutathione content (28), and impairments in antioxidant defenses (34, 193, 201). In addition, an age-related dysregulation of NF-E2-related factor 2 (Nrf2)-mediated antioxidant response is likely to contribute to mitochondrial oxidative stress in the vascular system (191, 192, 195).
In the vasculature of young animals, in response to increased mitochondrial ROS generation, an adaptive Nrf2-driven antioxidant defense mechanism takes place, which upregulates antioxidant response element (ARE)-mediated expression of a wide range of antioxidant enzymes as well as glutathione biosynthesis (192, 193). This homeostatic response attenuates oxidative damage to mitochondria and cytosolic components and limits cellular dysfunction induced by oxidative stress. Recent studies have demonstrated that the increased production of ROS by mitochondria and other cellular sources fails to activate Nrf2 in aged arteries, resulting in an amplified cellular sensitivity to the deleterious effects of oxidative stressors (191, 192, 199). Recent investigations have also shown that the mitochondrial enzyme p66Shc is involved in the regulation of mitochondrial redox homeostasis (135). It is significant that the genetic depletion of p66Shc in mice reduces mitochondrial ROS production, which is associated with a 30% increase in lifespan and improved endothelial function (18, 57, 223).
Because mitochondrial macromolecules, including mtDNA, are particularly susceptible to oxidative damage, an effective control of mitochondrial turnover is critical for the maintenance of a healthy mitochondrial phenotype in vascular cells (39, 200). One can hypothesize that the age-related dysregulation of mitochondrial turnover may contribute to mitochondrial dysfunction, oxidative stress, and altered signal transduction during the aging process. In support to this hypothesis, previous studies demonstrated that aging is associated with impaired mitochondrial biogenesis and reduced mitochondrial mass in vascular endothelial cells and VMSCs (14, 15, 198). Our current understanding is that in vascular cells of aged animals, due to an increased production of O2·− and downregulation and uncoupling of endothelial nitric oxide synthase (eNOS), NO· bioavailability is significantly decreased (196). This results in PGC-1α downregulation and consequential ETC dysfunction (198). It is likely that an inadequate NO· bioavailability also underlies the impaired mitochondrial biogenesis observed in other organ systems during aging (114, 137). It is tempting to speculate that an age-dependent decline in autophagy may also contribute to the accumulation of damaged, nonfunctional mitochondria in the vasculature, promoting the development of vascular pathologies (164). Indeed, it has recently been reported that markers of autophagy in blood vessels are reduced in aged mice and that treatment with the autophagy-enhancing agent trehalose improves endothelial function (101). On the other hand, the exposure of cultured murine hippocampal slices to amyloid β40 induces endothelial autophagy, which associates with impaired neurovascular regeneration, indicating that dysregulation of autophagy plays a complex role in age-related vascular pathophysiology (77).
In addition to the aforementioned cell-autonomous effects, aging also alters endocrine/paracrine regulatory mechanisms, which have a significant impact on vascular mitochondrial integrity. There is increasing evidence that a causal relationship exists between age-related decreases in insulin-like growth factor 1 (IGF-1) levels and cardiovascular pathologies in aged laboratory animals and elderly humans (reviewed in 171 and 194). Previous studies suggest that the cardiovascular protective effects of IGF-1 may be, at least in part, related to mitochondria-protective mechanisms. It is significant that in vitro treatment of endothelial cells and cardiomyocytes with recombinant IGF-1 decreases mitochondrial H2O2 production (29). Treatment of cultured endothelial cells with IGF-1 also preserves Δψm, maintaining the mitochondrial retention of cytochrome c and reducing caspase-3 activation upon exposure to H2O2 (76). Furthermore, overexpression of IGF-1 protects mice against high-fat diet feeding-induced increases in ROS generation, thus preventing mitochondrial damage (227). In contrast, low circulating levels of IGF-1 in Ames dwarf mice are associated with increased mitochondrial production of ROS both in the vasculature and the myocardium (29), mimicking the aging phenotype.
There are studies extant demonstrating that the administration of IGF-1 to aged rodents confers mitochondrial protection, including attenuation of ROS production in the liver (159). There is also evidence that treatments that increase circulating IGF-1 levels exert cardiovascular protective effects during aging (194). Future studies are required to elucidate the role of mitochondrial mechanisms (including mitochondrial disposal through autophagy and inhibition of mitochondrial pathways of apoptosis) in the beneficial effects of IGF-1 treatment in the aged heart and vasculature.
Previous investigations have revealed an association between upregulation of tissue renin-angiotensin system (RAS) and structural remodeling and functional impairments in large arteries of aged animals and humans (86, 128, 173, 212–214). Importantly, recent studies demonstrate that mitochondrial ROS play a critical role in mediating the cellular effects of angiotensin II in the cardiovascular system (37, 153). It has been proposed that angiotensin II binds to angiotensin receptor 1 (ATR1), thereby activating NADPH oxidases (NOX2 and NOX4), which then leads to increased mitochondrial ROS production in both vascular endothelial cells and VSMCs as well as in cardiac myocytes (39, 46, 95). Although it is possible that upregulation of local RAS contributes to increased mitochondrial oxidative stress in the aged vasculature, further studies are needed to provide definitive experimental evidence in support to this hypothesis. A synoptic overview of relevant findings on age-related changes in vascular mitochondrial bioenergetics, oxidant generation, and QC is depicted in Table 2.
Table 2.
Synopsis of major findings on age-related changes in vascular mitochondrial bioenergetics, oxidant generation, and quality control
| Experimental Model | Parameter(s) Examined | Findings | Reference |
|---|---|---|---|
| Macaca nemestrina | Mitochondrial mass, ATP synthesis, and ATPase activity in capillary endothelium from the frontal and occipital cortex of 4-, 10-, and 20-year-old primates | Age-dependent decrease in mitochondrial number, ATP synthesis, and ATPase activity | Burns et al. 1979 (14) |
| Macaque monkeys Sprague-Dawley rats | Mitochondrial mass in cerebral capillary from 4-, 10-, and 20-year-old primates and from 1-, 14-, 35-, 180-, and 800-day-old rats | Age-associated decline in cerebral capillary endothelial mitochondrial content in monkeys and rats | Burns et al. 1981 (15) |
| p66Shc−/− mice | Oxidant generation and expression of antioxidant enzymes (CuZnSOD and MnSOD) in aortic ring endothelium from 6- and 18-month-old WT and p66Shc−/− mice | Age-related increase in endothelial oxidant production in WT, but not in p66Shc−/− mice; no changes with age in antioxidant enzyme expression in either group | Francia et al. 2004 (57) |
| HUVECs | Effect of aging on mitochondrial morphology and dynamics | Alterations in overall morphology and fine structure, loss of mitochondrial membrane potential in old HUVECs; decreased intact mtDNA and impaired fusion/fission in old HUVECs | Jendrach et al. 2005 (85) |
| Macaca mulatta | Nrf2-driven free radical detoxification, oxidative stress (8-iso-PGF2α and 4-HNE), and vascular inflammation in VMSCs from 10- and 20-year-old primates | Age-dependent Nrf2 dysfunction associated with mitochondrial oxidant generation, oxidative stress, and vascular inflammation | Ungvari et al. 2011 (192) |
| Fischer 344 × Brown Norway rats | Nrf2-driven free radical detoxification and vascular inflammation in the aorta from 3-, 12-, 18-, 24-, and 28-month-old rats | Age-dependent Nrf2 dysfunction associated with increased oxidant generation and vascular inflammation | Ungvari et al. 2011 (193) |
| Fischer 344 rats | Mitochondrial oxidant generation, mitochondrial mass, expression of mitochondrial biogenesis factors (Tfam and PGC-1α), expression of ETC components, and COX activity in carotid arteries, and aorta of 3-, 18-, and 24-month-old rats | Age-related increase in mitochondrial ROS production, decline in mitochondrial mass, reduced expression of mitochondrial biogenesis factors, altered expression of ETC components, and decrease in COX expression and activity | Ungvari et al. 2008 (196) |
| Fischer 344 rats | Mitochondrial oxidant generation and vascular inflammation (NF-κB activation) in carotid arteries and aorta of 3-, 18-, and 24-month-old rats | Age-related increase in mitochondrial oxidant production and NF-κB activation | Ungvari et al. 2007 (198) |
COX, cytochrome c oxidase; CuZnSOD, copper-zinc-dependent superoxide dismutase; ETC, electron transport chain; HUVECs, human umbilical vein endothelial cells; Nrf2, NF-E2-related factor 2; p66Shc, 66-kDa isoform of the growth factor adapter Shc; PCG-1α, peroxisome proliferator-activated receptor γ coactivator 1-α; ROS, reactive oxygen species; Tfam, transcription factor A, mitochondrial; VSMCs: vascular smooth muscle cells.
Role of mitochondrial decay and altered autophagy in endothelial dysfunction and neurodegeneration.
Mitochondria-derived ROS likely contribute to a variety of macromolecular oxidative modifications in the vasculature, which in turn may play a role in the pathogenesis of several disease conditions. CR, an intervention known to attenuate mitochondrial oxidative stress in vascular cells (31), has been shown to decrease oxidative macromolecular damage to the aorta of aged rats (27, 71). Mitochondrial ROS are thought to impact endothelium-dependent vasodilation (60, 216), in part by increasing cytoplasmic levels of ROS and decreasing NO· bioavailability. It is well established that an increased production of ROS leads to endothelial dysfunction during aging both in laboratory animals and humans (196), but the specific role mitochondria-derived ROS play in this process has not been completely understood.
Recent studies demonstrate that the administration of coenzyme Q10 to late middle-aged persons (∼60 years) significantly improves flow-induced arterial vasodilation (59), suggesting that mitochondrial oxidative stress may play a direct role in endothelial dysfunction. Yet, oxidative stress-induced endothelial dysfunction in cerebral vessels is not worsened by MnSOD deficiency in mice (128). Mitochondria-derived H2O2 is also likely to contribute to the development of chronic, low-grade vascular inflammation at old age by activating redox-sensitive signaling pathways, including NF-κB (195, 198). Furthermore, as previously mentioned, mCAT overexpression confers cardioprotection to aged mice (166). Future studies are warranted to determine whether mCAT overexpression also promotes vasoprotection by attenuating vascular inflammation.
The age-related oxidative stress in VSMCs also leads to the development of a secretory phenotype (32), which induces proliferation, invasion, matrix fragmentation, and collagenization (211). SOD2+/− mice exhibit a greater age-related mitochondrial O2·− production in the aortic wall, which is associated with increased aorta stiffness (231). Recent studies also suggest that mitochondria-derived ROS play a role in the accelerated development of a senescent phenotype in endothelial cells, for instance by activating protein kinase B (PKB/Akt) (51).
Endothelial cell senescence has significant pathophysiological implications since it may impair the regenerative and angiogenic capacity of the vascular endothelium. Another potentially important link between mitochondrial oxidative stress and vascular aging is the induction of endothelial cell apoptosis (155). Indeed, an increased rate of apoptosis has been documented in the endothelium of aged rodents (33, 152) and old nonhuman primates (8). The finding that endothelial cell apoptosis in coronary microvessels of aged rats is associated with increased activation of caspase-9 suggests that mitochondrial cell death pathways may play a key role in this process. It is logical to speculate that an age-related increase in the rate of endothelial apoptosis contributes to microvascular rarefaction, thus impairing blood supply to the heart and brain (reviewed in 195).
The role of autophagy in disease conditions affecting the vasculature, such as diabetes, is still unclear (111). There are studies extant suggesting that hypoxia activates autophagy, promoting survival of endothelial progenitor cells (210), and that the autophagic machinery regulates angiogenesis during tumor development (102). In addition, as previously mentioned, amyloid β40-dependent induction of endothelial autophagy has been linked to impaired neurovascular regeneration, which may contribute to the initial progression of AD (77).
Cerebrovascular endothelial cells are rich in mitochondria, and normal mitochondrial function is essential to maintain the integrity of the blood-brain barrier (BBB). Accordingly, alterations of Δψm in endothelial cells have been shown to directly affect BBB permeability (104). On the basis of the available data with mitochondrial inhibitors (94), it can be hypothesized that the age-related mitochondrial dysfunction contributes to BBB breakdown, thereby promoting neuroinflammation and neurodegeneration (55).
In recent years, several important observations have been published linking mitochondrial dysfunction and age-related microvascular pathologies to the development of AD. Amyloid deposition in the cerebrovasculature, known as cerebral amyloid angiopathy, occurs in 80–90% of AD patients (63, 66). There is increasing clinical and experimental evidence suggesting that cerebral amyloid angiopathy is causally related to BBB breakdown and, importantly, contributes to the pathogenesis of neurodegeneration and plaque formation in AD (66, 232). The mitochondrial antioxidant enzyme MnSOD has an important role in preserving the structural and functional integrity of the cerebral microcirculation, thereby conferring protection against the development of cerebral amyloid angiopathy in AD (52). Accordingly, inactivation of one MnSOD allele (SOD2+/−) exacerbates cerebrovascular amyloidosis and accelerates the development of behavioral alterations in a mouse model of AD [human amyloid precursor protein (hAPP) transgenic mice] (52). Electronmicroscopic evaluation of cerebral microvessels in hAPP/SOD2+/− mice revealed the presence of prominent vascular amyloid deposits in the smooth muscle layer and alterations of the basal membrane, which were often associated with gliosis and dystrophic neurites (52).
An enhanced generation of free radicals appears to contribute to increasing cerebrovascular permeability also in humans (58). Adults with trisomy 21 (Down syndrome) develop AD progressively with age (217), and this has been associated with increased mitochondrial oxidative stress (147), microvascular degeneration (127), impaired BBB permeability (50), and generalized endothelial dysfunction (20).
Despite significant advances, the mechanisms by which mitochondrial oxidative stress and dysfunction contribute to the disruption of the BBB and the accumulation of amyloid deposits are not completely understood. Recent studies demonstrate that overexpression of SOD2 in a mouse model of AD [Tg19959 mice expressing human APP695 with 2 familial AD mutations (KM670/671NL and V717F)] mitigates neurodegeneration and improves clinical outcomes (47). Furthermore, the polyphenolic compound resveratrol, which attenuates mitochondrial oxidative stress in endothelial cells (197) and exerts multifaceted cerebromicrovascular protective effects (145), has shown to confer protection both in vitro and in vivo in models of AD (reviewed in 3). Similarly, the mitochondria-targeted antioxidant 10-(6'-ubiquinonyl)-decyltriphenylphosphonium (MitoQ) has recently been demonstrated to improve cognitive function and prevent early neuropathology in a transgenic mouse model of AD (123).
Further studies are evidently needed to completely understand the effects of SOD2 overexpression, resveratrol, and mitochondria-targeted antioxidants on cerebromicrovascular pathologies in experimental models of AD, before translation to humans is explored.
Mitochondrial Dysfunction as a Pharmacological Target Against Cardiovascular Aging and CVD.
Although the exact mechanisms responsible for cardiovascular aging are not fully understood, pathways leading to the progressive accrual of oxidative damage, accumulation of dysfunctional mitochondria, and impairments in mitochondrial QC are assumed to play a crucial role in this process and have consequently emerged as candidate therapeutic targets to manage age-related CVD (118).
The critical role postulated for mitochondria-driven oxidative damage in CVD (and age-related pathology in general) would suggest that the administration of antioxidants might mitigate the burden of CVD. However, most clinical trials that have tested the effects of systemic, nontargeted supplementation with antioxidants to attenuate the progression of CVD failed to show any positive cardiovascular outcome (13). Unexpectedly, chronic administration of β-carotene, vitamin A, or vitamin E could even induce pro-oxidant responses and increase cardiovascular mortality (13).
Nonetheless, recent studies suggest that targeting molecules with antioxidant properties to mitochondria could be a much more effective strategy to slow cardiovascular aging and manage CVD (160, 206). Indeed, the development of antioxidant molecules that achieve concentrations in mitochondria 100- to 1,000-fold higher than in the cytosol, such as Szeto-Schiller (SS) synthetic antioxidant peptides (26, 178, 179, 230), MitoQ (176, 205), and Euk-8 (a SOD/catalase mimetic and antioxidant) (94, 202), have demonstrated some efficacy in models of cardiovascular stress. A recent study in spontaneous hypertensive rats showed that MitoQ treatment for 8 weeks significantly reduced systolic blood pressure, improved endothelial function, and attenuated cardiac hypertrophy (64). SS-31 has been shown to mitigate I/R injury and reperfusion arrhythmia and preserve myocardial function in various infarct models (reviewed in 179). Moreover, SS-31 ameliorates cardiomyopathy resulting from prolonged angiotensin-II stimulation and Gαq overexpression in mice, in the absence of any blood pressure lowering effect (35). Euk-8 treatment protects both oxidative stress-prone Harlequin mice and wild-type controls from pressure overload-induced left ventricular remodeling and cardiac decompensation, and extended the overall survival of these animals (202). Several clinical trials are ongoing to test the efficacy of mitochondria-targeted antioxidants in various CVD conditions. It will also be important to establish whether these agents can delay human cardiovascular aging.
The regulation of mitochondrial turnover and function appears to be impaired as a function of age in both the heart and the vasculature (101), which may contribute to cardiovascular degeneration. Therefore, it is proposed that interventions that fine tune mitochondrial turnover and/or reverse mitochondrial dysfunction may serve as novel therapeutic approaches to counter cardiovascular aging (reviewed in 39, 48, and 132). In this context, CR, resveratrol administration, and sirtuin(s) pathway(s) activation could be especially relevant to delay cardiac aging and manage age-related CVD (65, 199).
An increasing amount of data suggests that CR, defined as a reduction in food intake without malnutrition, confers cardiovascular protection during aging and in pathological conditions associated with accelerated cardiovascular aging (reviewed in 199 and 215). In the vasculature, CR appears to protect against endothelial dysfunction and arterial stiffness and to attenuate atherogenesis by improving several cardiometabolic risk factors (215). In the heart, CR mitigates age-related changes in the myocardium (e.g., reduction in fibrosis and apoptosis, prevention of myosin isoform switch) (215).
CR conveys beneficial effects on the cardiovascular system through different mechanisms, including attenuation of mitochondrial ROS production (114, 138) and consequent inhibition of signaling pathways regulated by ROS (e.g., NF-κB) (28), and optimization of mitochondrial autophagy (48, 168, 172, 218). Studies also suggest that CR improves mitochondrial biogenesis (113, 116, 138), although this point remains controversial (75). The cellular pathways involved in mitochondrial protection induced by CR appear to converge on the expression/activity of the dyad AMPK/Sirt1 (27, 116, 172) and activation of downstream effectors, including PGC-1α (113, 114). Sirt1 confers vasoprotection by reducing endothelial ROS production, inhibiting NF-κB signaling, and attenuating vascular inflammation (174). The effects mediated by Sirt1 are likely potentiated by an increased bioavailability of NO· (121). Interestingly, Sirt1 appears to exert a hormetic action on cardiomyocyte physiology (4). Low (2.5-fold) to moderate (7.5-fold) transgenic overexpression of Sirt1 in the mouse heart attenuates age-dependent hypertrophy and reduces the severity of apoptosis/fibrosis and cardiac dysfunction (4). In contrast, high levels (12.5-fold) of Sirt1 expression increase the extent of cardiomyocyte apoptosis and the degree of hypertrophy, while decreasing cardiac function, thereby promoting the development of cardiomyopathy (4).
AMPK has recently emerged as the master regulator of several mitochondrial functions, including biogenesis, mitophagy, mitochondrial metabolism, and mitochondrial antioxidant defense (reviewed in 162). Accumulating data suggest that AMPK upregulates Sirt1 activity (19) and that AMPK activation may contribute to the cardiovascular protection elicited by CR (49, 172). CR can also activate the transcription factor Nrf2, thus modulating the expression of numerous ROS-detoxifying and antioxidant genes that regulate mitochondrial redox homeostasis (199).
Despite the host of health benefits brought about by CR, it is likely that most people will not be able to sustain drastic food restrictions for the long term because of several possible adverse events (118). Thus considerable effort has been directed toward the discovery of drugs that could mimic the effects of CR without requiring dietary restriction (83). Promising CR mimetics with mitochondrial protective properties are those that intersect with the critical signaling pathways previously discussed and include resveratrol, affecting sirtuin activity (130), and biguanides such as metformin, activating the AMPK pathway (219).
The polyphenol resveratrol (3,5,4'-trihydroxystilbene) is believed to be the active compound responsible for the cardioprotective effects of red wine (97). Studies in rodents have shown that resveratrol inhibits cardiomyocyte apoptosis, protects the myocardium against I/R injury, prevents LVH, improves endothelial function, inhibits platelet aggregation, and reduces inflammation (reviewed in 154). Resveratrol has also been shown to mimic the cardiovascular protective adaptations induced by CR (9, 10, 152), including the induction of mitochondrial biogenesis (30) and the attenuation of mitochondrial oxidative stress in both vascular endothelial cells and cardiomyocytes (190, 197). The beneficial effects of resveratrol on CVD are partly due to its ability to activate Sirt1 (79), enhance endothelial NO· signaling (209), and activate Nrf2 in endothelial cells (190). Interestingly, just like its putative target Sirt1, resveratrol seems to exert cardiovascular effects in a hormetic fashion. Indeed, in H9c2 heart myoblasts, low doses of resveratrol promote cell survival by eliciting a protective preconditioning-like effect through the induction of autophagy (72). Conversely, higher doses of this compound inhibit protective autophagy and slightly reduce cell survival (72).
Resveratrol and other polyphenols may also activate AMPK (226), and this can represent another CR-mimetic effect whereby these compounds protect against CVD. In this context, the AMPK activator metformin has been shown to ameliorate cardiac ischemia (12), myocardial infarction severity (17), diabetic cardiomyopathy (221), and heart failure (68, 163). Furthermore, metformin improves age-related cardiomyocyte dysfunction (187) and endothelial vasodilation in rodent models of accelerated vascular aging (81).
In conclusion, preclinical studies indicate that interventions aimed at reducing mitochondrial-specific oxidative burden, improving mitochondrial function, and optimizing mitochondrial turnover may represent a novel and promising strategy to manage cardiovascular deterioration with age. Further studies are warranted to determine whether these interventions confer mitochondrial protective effects and promote cardiovascular health in old humans.
Conclusions and Future Perspectives
Advancing age per se is a major risk factor for CVD and neurodegeneration. Several lines of evidence from both experimental models and human subjects indicate that mitochondria play a critical role in cardiovascular aging. The inherent complexity of mitochondrial processes related to energy provision, redox homeostasis, cellular and intra-inter-organellar QC, regulation of cell death/survival pathways, and their hierarchical network organization pose significant obstacles to understanding the precise role these organelles play in the pathogenesis of CVD. The same complexity hampers the identification of potential therapeutic targets for the prevention of age-related CVD. For instance, although a crucial role for mitochondrial dysfunction is postulated in CVD, a consensus has not been reached as to whether cardiovascular damage is primarily mediated by oxidative stress or by other mechanisms for which mitochondria-derived ROS function as signaling molecules (e.g., alterations in autophagy, mitochondrial dynamics, apoptotic cell death, etc.).
Furthermore, some key cellular processes, such as mitophagy, exhibit a Janus-like behavior. Indeed, basal levels of mitophagy are fundamental for maintaining cellular homeostasis and protecting cells against the accumulation of dysfunctional mitochondria. On the other hand, upregulation of autophagy can lead to excessive removal of mitochondria, loss of cardiac myocytes, and development of CVD. It should also be considered that most autophagy/mitophagy mediators have multiple functions, implying that their genetic or pharmacologic manipulation may produce unrelated and possibly undesirable effects. Moreover, both cardiac and vascular aging involve neurohormonal signaling (e.g., alterations in renin-angiotensin and IGF-1 signaling) and cell-autonomous mechanisms that can impact age-related mitochondrial changes, thus adding a further level of complexity that needs to be fully elucidated.
The quest for effective treatments that improve mitochondrial function, attenuate oxidative stress, and optimize mitochondrial QC both in cardiac and vascular tissues has been an intriguing yet unresolved issue for cardiologists and geriatricians. Nonetheless, promising therapeutic strategies to manage age-related CVD have recently emerged. In particular, mitochondria-targeted antioxidants have proven effective in various animal models, and preliminary clinical data show positive results in humans. In addition, pharmacological or nutritional interventions (e.g., CR and CR mimetics like resveratrol) acting on evolutionarily conserved, Nrf2/ARE-driven, or sirtuin-dependent pro-survival pathways that upregulate intrinsic antioxidant systems in mitochondria, can be exploited for therapeutic advantage. Interventions that modulate processes involved in the regulation of mitochondrial turnover are also of particular interest.
In summary, an extraordinary research effort is required to untangle the complexity of the network of multileveled, interrelated pathways that regulate mitochondrial homeostasis. This knowledge will likely provide clinicians with novel and highly effective therapeutics to delay cardiovascular aging and manage CVD and neurodegeneration.
GRANTS
This work was partly supported by funds from the Centro Studi Achille e Linda Lorenzon (to E. Marzetti), the American Federation for Aging Research (to A. Csiszar), the Oklahoma Center for the Advancement of Science and Technology (to A. Csiszar), the University of Oklahoma College of Medicine Alumni Association (to A. Csiszar), the American Heart Association (to A. Csiszar), NIH-AG031085 (to A. Csiszar), the University of Arkansas Medical Center Claude Pepper Older Americans Independence Center (to A. Csiszar), the Italian Ministry of Economy and Finance to the CNR for the Project FaReBio di Qualità (to R. Calvani), the University of Florida's Institute on Aging and Claude D. Pepper Older Americans Independence Center (NIA 1P30AG028740 to C. Leeuwenburgh), NIA RO1-AG21042 and NIDDK RO1-DK090115-01A1 (to C. Leeuwenburgh).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.M., A.C., and C.L. provided the conception and design of research; E.M., A.C., D.D., G.B., R.C., and C.L. drafted manuscript; E.M., A.C., D.D., G.B., R.C., and C.L. edited and revised manuscript; E.M., A.C., D.D., G.B., R.C., and C.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We recognize that not all of the excellent scientific work in this area could be included or cited due to the vast literature on the subject and space limitations. We thank Francesco Antognarelli for invaluable assistance with illustrations.
REFERENCES
- 1.Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging (Albany NY) 2: 1012–1016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akazawa H, Komazaki S, Shimomura H, Terasaki F, Zou Y, Takano H, Nagai T, Komuro I. Diphtheria toxin-induced autophagic cardiomyocyte death plays a pathogenic role in mouse model of heart failure. J Biol Chem 279: 41095–41103, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Albani D, Polito L, Signorini A, Forloni G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors 36: 370–376, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Aon MA. From isolated to networked: a paradigmatic shift in mitochondrial physiology. Front Physiol 1: 20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aon MA, Cortassa S. Mitochondrial network energetics in the heart. Wiley Interdiscip Rev Syst Biol Med 4: 599–613, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol 23: 184–189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asai K, Kudej RK, Shen YT, Yang GP, Takagi G, Kudej AB, Geng YJ, Sato N, Nazareno JB, Vatner DE, Natividad F, Bishop SP, Vatner SF. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arterioscler Thromb Vasc Biol 20: 1493–1499, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp Gerontol 43: 859–866, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 3: e2264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benard G, Rossignol R. Ultrastructure of the mitochondrion and its bearing on function and bioenergetics. Antioxid Redox Signal 10: 1313–1342, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol 103: 274–284, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297: 842–857, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Burns EM, Kruckeberg TW, Comerford LE, Buschmann MT. Thinning of capillary walls and declining numbers of endothelial mitochondria in the cerebral cortex of the aging primate, Macaca nemestrina. J Gerontol 34: 642–650, 1979 [DOI] [PubMed] [Google Scholar]
- 15.Burns EM, Kruckeberg TW, Gaetano PK. Changes with age in cerebral capillary morphology. Neurobiol Aging 2: 283–291, 1981 [DOI] [PubMed] [Google Scholar]
- 16.Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem 394: 393–414, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 57: 696–705, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Camici GG, Cosentino F, Tanner FC, Luscher TF. The role of p66Shc deletion in age-associated arterial dysfunction and disease states. J Appl Physiol 105: 1628–1631, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cappelli-Bigazzi M, Santoro G, Battaglia C, Palladino MT, Carrozza M, Russo MG, Pacileo G, Calabro R. Endothelial cell function in patients with Down's syndrome. Am J Cardiol 94: 392–395, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy 6: 462–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP, Tang J. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 6: 872–883, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res 84: 91–99, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y, Liu Y, Dorn GW., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109: 1327–1331, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246: 382–385, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis 18: 215–220, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev 130: 518–527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csiszar A, Labinskyy N, Perez V, Recchia FA, Podlutsky A, Mukhopadhyay P, Losonczy G, Pacher P, Austad SN, Bartke A, Ungvari Z. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am J Physiol Heart Circ Physiol 295: H1882–H1894, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297: H13–H20, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csiszar A, Sosnowska D, Tucsek Z, Gautam T, Toth P, Losonczy G, Colman RJ, Weindruch R, Anderson RM, Sonntag WE, Ungvari Z. Circulating factors induced by caloric restriction in the non-human primate Macaca mulatta activate angiogenic processes in endothelial cells. J Gerontol A Biol Sci Med Sci 68: 235–249, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci 67: 811–820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004 [DOI] [PubMed] [Google Scholar]
- 34.D'Armiento FP, Bianchi A, de Nigris F, Capuzzi DM, D'Armiento MR, Crimi G, Abete P, Palinski W, Condorelli M, Napoli C. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke 32: 2472–2479, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 58: 73–82, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai DF, Chen T, Wanagat J, Laflamme M, Marcinek DJ, Emond MJ, Ngo CP, Prolla TA, Rabinovitch PS. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9: 536–544, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 108: 837–846, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med 19: 213–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119: 2789–2797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Del Roso A, Vittorini S, Cavallini G, Donati A, Gori Z, Masini M, Pollera M, Bergamini E. Ageing-related changes in the in vivo function of rat liver macroautophagy and proteolysis. Exp Gerontol 38: 519–527, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Ding WX, Yin XM. Mitophagy, mechanisms, pathophysiological roles, and analysis. Biol Chem 393: 547–564, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donati A, Cavallini G, Paradiso C, Vittorini S, Pollera M, Gori Z, Bergamini E. Age-related changes in the autophagic proteolysis of rat isolated liver cells: effects of antiaging dietary restrictions. J Gerontol A Biol Sci Med Sci 56: B375–B383, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci 33: 494–501, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J 23: 2459–2466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res 110: 1125–1138, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech Ageing Dev 131: 739–742, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emerson JF, Kesslak JP, Chen PC, Lott IT. Magnetic resonance imaging of the aging brain in Down syndrome. Prog Clin Biol Res 393: 123–138, 1995 [PubMed] [Google Scholar]
- 51.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol 106: 326–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puolivali J, Scearce-Levie K, Masliah E, Mucke L. Reduction in mitochondrial superoxide dismutase modulates Alzheimer's disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci 26: 5167–5179, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eura Y, Ishihara N, Yokota S, Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem 134: 333–344, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys 372: 399–407, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease—systematic review and meta-analysis. Neurobiol Aging 30: 337–352, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Finckenberg P, Eriksson O, Baumann M, Merasto S, Lalowski MM, Levijoki J, Haasio K, Kyto V, Muller DN, Luft FC, Oresic M, Mervaala E. Caloric restriction ameliorates angiotensin II-induced mitochondrial remodeling and cardiac hypertrophy. Hypertension 59: 76–84, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, Pelicci PG, Schiavoni M, Luscher TF, Volpe M, Cosentino F. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 110: 2889–2895, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Fraser PA. The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med 51: 967–977, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis 221: 311–316, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Gao Q, Zhao X, Ahmad M, Wolin MS. Mitochondrial-derived hydrogen peroxide inhibits relaxation of bovine coronary arterial smooth muscle to hypoxia through stimulation of ERK MAP kinase. Am J Physiol Heart Circ Physiol 297: H2262–H2269, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19: 4861–4870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension 54: 322–328, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 333: 1109–1112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke 35: 2616–2619, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Grivennikova VG, Kareyeva AV, Vinogradov AD. What are the sources of hydrogen peroxide production by heart mitochondria? Biochim Biophys Acta 1797: 939–944, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 104: 403–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo X, Chen KH, Guo Y, Liao H, Tang J, Xiao RP. Mitofusin 2 triggers vascular smooth muscle cell apoptosis via mitochondrial death pathway. Circ Res 101: 1113–1122, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Guo YH, Chen K, Gao W, Li Q, Chen L, Wang GS, Tang J. Overexpression of Mitofusin 2 inhibited oxidized low-density lipoprotein induced vascular smooth muscle cell proliferation and reduced atherosclerotic lesion formation in rabbit. Biochem Biophys Res Commun 363: 411–417, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Guo ZM, Yang H, Hamilton ML, VanRemmen H, Richardson A. Effects of age and food restriction on oxidative DNA damage and antioxidant enzyme activities in the mouse aorta. Mech Ageing Dev 122: 1771–1786, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Gurusamy N, Lekli I, Mukherjee S, Ray D, Ahsan MK, Gherghiceanu M, Popescu LM, Das DK. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res 86: 103–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hall AR, Hausenloy DJ. The shape of things to come: mitochondrial fusion and fission in the adult heart. Cardiovasc Res 94: 391–392, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281: 29776–29787, 2006 [DOI] [PubMed] [Google Scholar]
- 75.Hancock CR, Han DH, Higashida K, Kim SH, Holloszy JO. Does calorie restriction induce mitochondrial biogenesis? A reevaluation. FASEB J 25: 785–791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hao CN, Geng YJ, Li F, Yang T, Su DF, Duan JL, Li Y. Insulin-like growth factor-1 receptor activation prevents hydrogen peroxide-induced oxidative stress, mitochondrial dysfunction and apoptosis. Apoptosis 16: 1118–1127, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Hayashi S, Sato N, Yamamoto A, Ikegame Y, Nakashima S, Ogihara T, Morishita R. Alzheimer disease-associated peptide, amyloid beta40, inhibits vascular regeneration with induction of endothelial autophagy. Arterioscler Thromb Vasc Biol 29: 1909–1915, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Hirota Y, Kang D, Kanki T. The physiological role of mitophagy: new insights into phosphorylation events. Int J Cell Biol 2012: 354914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Ihara S, Maeda-Takekoshi F, Takekoshi M, Yokoyama M, Sakuma S, Watanabe Y. Evidence that the tyrosine kinase domain of a small fraction of epidermal growth factor receptor molecules is exposed on the outer surface of A431 cells. Cell Struct Funct 16: 217–223, 1991 [DOI] [PubMed] [Google Scholar]
- 81.Iida KT, Kawakami Y, Suzuki M, Shimano H, Toyoshima H, Sone H, Shimada K, Iwama Y, Watanabe Y, Mokuno H, Kamata K, Yamada N. Effect of thiazolidinediones and metformin on LDL oxidation and aortic endothelium relaxation in diabetic GK rats. Am J Physiol Endocrinol Metab 284: E1125–E1130, 2003 [DOI] [PubMed] [Google Scholar]
- 82.Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion 7: 106–118, 2007 [DOI] [PubMed] [Google Scholar]
- 83.Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell 5: 97–108, 2006 [DOI] [PubMed] [Google Scholar]
- 84.Inuzuka Y, Okuda J, Kawashima T, Kato T, Niizuma S, Tamaki Y, Iwanaga Y, Yoshida Y, Kosugi R, Watanabe-Maeda K, Machida Y, Tsuji S, Aburatani H, Izumi T, Kita T, Shioi T. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation 120: 1695–1703, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Jendrach M, Pohl S, Voth M, Kowald A, Hammerstein P, Bereiter-Hahn J. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev 126: 813–821, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, Lakatta EG. Increased aortic calpain-1 activity mediates age-associated angiotensin II signaling of vascular smooth muscle cells. PLoS ONE 3: e2231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 19: 419–421, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol 292: C1983–C1992, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Jung T, Bader N, Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann N Y Acad Sci 1119: 97–111, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke 43: 2526–2534, 2012 [DOI] [PubMed] [Google Scholar]
- 91.Karbowski M, Kurono C, Wozniak M, Ostrowski M, Teranishi M, Nishizawa Y, Usukura J, Soji T, Wakabayashi T. Free radical-induced megamitochondria formation and apoptosis. Free Radic Biol Med 26: 396–409, 1999 [DOI] [PubMed] [Google Scholar]
- 92.Kaushik S, Cuervo AM. Chaperone-mediated autophagy. Methods Mol Biol 445: 227–244, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawakami S, Matsuda A, Sunagawa T, Noda Y, Kaneko T, Tahara S, Hiraumi Y, Adachi S, Matsui H, Ando K, Fujita T, Maruyama N, Shirasawa T, Shimizu T. Antioxidant, EUK-8, prevents murine dilated cardiomyopathy. Circ J 73: 2125–2134, 2009 [DOI] [PubMed] [Google Scholar]
- 94.Kim GW, Gasche Y, Grzeschik S, Copin JC, Maier CM, Chan PH. Neurodegeneration in striatum induced by the mitochondrial toxin 3-nitropropionic acid: role of matrix metalloproteinase-9 in early blood-brain barrier disruption? J Neurosci 23: 8733–8742, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension 45: 438–444, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Klionsky DJ, Baehrecke EH, Brumell JH, Chu CT, Codogno P, Cuervo AM, Debnath J, Deretic V, Elazar Z, Eskelinen EL, Finkbeiner S, Fueyo-Margareto J, Gewirtz D, Jaattela M, Kroemer G, Levine B, Melia TJ, Mizushima N, Rubinsztein DC, Simonsen A, Thorburn A, Thumm M, Tooze SA. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 7: 1273–1294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur J Endocrinol 138: 619–620, 1998 [DOI] [PubMed] [Google Scholar]
- 98.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell 40: 280–293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309: 481–484, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Lakatta EG. Heart aging: a fly in the ointment? Circ Res 88: 984–986, 2001 [DOI] [PubMed] [Google Scholar]
- 101.LaRocca TJ, Henson GD, Thorburn A, Sindler AL, Pierce GL, Seals DR. Translational evidence that impaired autophagy contributes to arterial ageing. J Physiol 590: 3305–3316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee SJ, Kim HP, Jin Y, Choi AM, Ryter SW. Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy 7: 829–839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leeuwenburgh C, Wagner P, Holloszy JO, Sohal RS, Heinecke JW. Caloric restriction attenuates dityrosine cross-linking of cardiac and skeletal muscle proteins in aging mice. Arch Biochem Biophys 346: 74–80, 1997 [DOI] [PubMed] [Google Scholar]
- 104.Lenzser G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res 1051: 72–80, 2005 [DOI] [PubMed] [Google Scholar]
- 105.Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Turkaly PJ, Hoppel CL. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol 33: 37–47, 2001 [DOI] [PubMed] [Google Scholar]
- 106.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol 33: 1065–1089, 2001 [DOI] [PubMed] [Google Scholar]
- 107.Levonen AL, Vahakangas E, Koponen JK, Yla-Herttuala S. Antioxidant gene therapy for cardiovascular disease: current status and future perspectives. Circulation 117: 2142–2150, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Li WW, Li J, Bao JK. Microautophagy: lesser-known self-eating. Cell Mol Life Sci 69: 1125–1136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu H, Yu S, Xu W, Xu J. Enhancement of 26S proteasome functionality connects oxidative stress and vascular endothelial inflammatory response in diabetes mellitus. Arterioscler Thromb Vasc Biol 32: 2131–2140, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, Huang L, Xue P, Li B, Wang X, Jin H, Wang J, Yang F, Liu P, Zhu Y, Sui S, Chen Q. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol 14: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 111.Liu VW, Zhang C, Nagley P. Mutations in mitochondrial DNA accumulate differentially in three different human tissues during ageing. Nucleic Acids Res 26: 1268–1275, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119: 480–486, 2009 [DOI] [PubMed] [Google Scholar]
- 113.Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A 103: 1768–1773, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol 43: 813–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia 53: 1783–1794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martin-Montalvo A, Cabo RD. Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid Redox Signal. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Martin LJ. Biology of mitochondria in neurodegenerative diseases. Prog Mol Biol Transl Sci 107: 355–415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marzetti E, Wohlgemuth SE, Anton SD, Bernabei R, Carter CS, Leeuwenburgh C. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin Geriatr Med 25: 715–732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189: 211–221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100: 914–922, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neurosignals 16: 75–84, 2008 [DOI] [PubMed] [Google Scholar]
- 123.McManus MJ, Murphy MP, Franklin JL. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer's disease. J Neurosci 31: 15703–15715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mijaljica D, Prescott M, Devenish RJ. Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy 7: 673–682, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Miro O, Casademont J, Casals E, Perea M, Urbano-Marquez A, Rustin P, Cardellach F. Aging is associated with increased lipid peroxidation in human hearts, but not with mitochondrial respiratory chain enzyme defects. Cardiovasc Res 47: 624–631, 2000 [DOI] [PubMed] [Google Scholar]
- 126.Misaka T, Miyashita T, Kubo Y. Primary structure of a dynamin-related mouse mitochondrial GTPase and its distribution in brain, subcellular localization, and effect on mitochondrial morphology. J Biol Chem 277: 15834–15842, 2002 [DOI] [PubMed] [Google Scholar]
- 127.Miyakawa T, Kuramoto R. Ultrastructural study of senile plaques and microvessels in the brain with Alzheimer's disease and Down's syndrome. Ann Med 21: 99–102, 1989 [DOI] [PubMed] [Google Scholar]
- 128.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol 296: H1914–H1919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mohamed SA, Hanke T, Erasmi AW, Bechtel MJ, Scharfschwerdt M, Meissner C, Sievers HH, Gosslau A. Mitochondrial DNA deletions and the aging heart. Exp Gerontol 41: 508–517, 2006 [DOI] [PubMed] [Google Scholar]
- 130.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis 1: e10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol 151: 367–380, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nair S, Ren J. Autophagy and cardiovascular aging: lesson learned from rapamycin. Cell Cycle 11: 2092–2099, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13: 619–624, 2007 [DOI] [PubMed] [Google Scholar]
- 134.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA 100: 2112–2116, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183: 795–803, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Newman AB, Arnold AM, Naydeck BL, Fried LP, Burke GL, Enright P, Gottdiener J, Hirsch C, O'Leary D, Tracy R. “Successful aging”: effect of subclinical cardiovascular disease. Arch Intern Med 163: 2315–2322, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003 [DOI] [PubMed] [Google Scholar]
- 138.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314–317, 2005 [DOI] [PubMed] [Google Scholar]
- 139.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31: 1287–1312, 2001 [DOI] [PubMed] [Google Scholar]
- 140.Odding E, Valkenburg HA, Stam HJ, Hofman A. Determinants of locomotor disability in people aged 55 years and over: the Rotterdam Study. Eur J Epidemiol 17: 1033–1041, 2001 [DOI] [PubMed] [Google Scholar]
- 141.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485: 251–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ong SB, Hausenloy DJ. Mitochondrial morphology and cardiovascular disease. Cardiovasc Res 88: 16–29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121: 2012–2022, 2010 [DOI] [PubMed] [Google Scholar]
- 145.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci 1: 4, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Orvedahl A, Sumpter R, Jr, Xiao G, Ng A, Zou Z, Tang Y, Narimatsu M, Gilpin C, Sun Q, Roth M, Forst CV, Wrana JL, Zhang YE, Luby-Phelps K, Xavier RJ, Xie Y, Levine B. Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature 480: 113–117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pagano G, Castello G. Oxidative stress and mitochondrial dysfunction in Down syndrome. Adv Exp Med Biol 724: 291–299, 2012 [DOI] [PubMed] [Google Scholar]
- 148.Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys 236: 691–702, 1985 [DOI] [PubMed] [Google Scholar]
- 149.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem 252: 8731–8739, 1977 [PubMed] [Google Scholar]
- 150.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O'Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31: 1309–1328, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Papanicolaou KN, Ngoh GA, Dabkowski ER, O'Connell KA, Ribeiro RF, Jr, Stanley WC, Walsh K. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol 302: H167–H179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pepe M, Mamdani M, Zentilin L, Csiszar A, Qanud K, Zacchigna S, Ungvari Z, Puligadda U, Moimas S, Xu X, Edwards JG, Hintze TH, Giacca M, Recchia FA. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res 106: 1893–1903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci 1215: 22–33, 2011 [DOI] [PubMed] [Google Scholar]
- 155.Phaneuf S, Leeuwenburgh C. Cytochrome c release from mitochondria in the aging heart: a possible mechanism for apoptosis with age. Am J Physiol Regul Integr Comp Physiol 282: R423–R430, 2002 [DOI] [PubMed] [Google Scholar]
- 156.Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, Mateo P, Fortin D, Huynh le H, Nicolas V, Alavi MV, Brenner C, Ventura-Clapier R, Veksler V, Joubert F. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res 94: 408–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Porteous WK, James AM, Sheard PW, Porteous CM, Packer MA, Hyslop SJ, Melton JV, Pang CY, Wei YH, Murphy MP. Bioenergetic consequences of accumulating the common 4977-bp mitochondrial DNA deletion. Eur J Biochem 257: 192–201, 1998 [DOI] [PubMed] [Google Scholar]
- 158.Prithivirajsingh S, Story MD, Bergh SA, Geara FB, Ang KK, Ismail SM, Stevens CW, Buchholz TA, Brock WA. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett 571: 227–232, 2004 [DOI] [PubMed] [Google Scholar]
- 159.Puche JE, Garcia-Fernandez M, Muntane J, Rioja J, Gonzalez-Baron S, Castilla Cortazar I. Low doses of insulin-like growth factor-I induce mitochondrial protection in aging rats. Endocrinology 149: 2620–2627, 2008 [DOI] [PubMed] [Google Scholar]
- 160.Rocha M, Apostolova N, Hernandez-Mijares A, Herance R, Victor VM. Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Curr Med Chem 17: 3827–3841, 2010 [DOI] [PubMed] [Google Scholar]
- 161.Sachs HG, Colgan JA, Lazarus ML. Ultrastructure of the aging myocardium: a morphometric approach. Am J Anat 150: 63–71, 1977 [DOI] [PubMed] [Google Scholar]
- 162.Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 11: 230–241, 2012 [DOI] [PubMed] [Google Scholar]
- 163.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation 119: 2568–2577, 2009 [DOI] [PubMed] [Google Scholar]
- 164.Sazonova M, Budnikov E, Khasanova Z, Sobenin I, Postnov A, Orekhov A. Studies of the human aortic intima by a direct quantitative assay of mutant alleles in the mitochondrial genome. Atherosclerosis 204: 184–190, 2009 [DOI] [PubMed] [Google Scholar]
- 165.Schafer A, Reichert AS. Emerging roles of mitochondrial membrane dynamics in health and disease. Biol Chem 390: 707–715, 2009 [DOI] [PubMed] [Google Scholar]
- 166.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909–1911, 2005 [DOI] [PubMed] [Google Scholar]
- 167.Shinmura K, Tamaki K, Sano M, Murata M, Yamakawa H, Ishida H, Fukuda K. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol 50: 117–127, 2011 [DOI] [PubMed] [Google Scholar]
- 168.Shutt TE, McBride HM. Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim Biophys Acta 1833: 417–424, 2013 [DOI] [PubMed] [Google Scholar]
- 169.Smirnova E, Griparic L, Shurland DL, van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech Ageing Dev 74: 121–133, 1994 [DOI] [PubMed] [Google Scholar]
- 171.Sonntag WE, Csiszar A, deCabo R, Ferrucci L, Ungvari Z. Diverse roles of growth hormone and insulin-like growth factor-1 in mammalian aging: progress and controversies. J Gerontol A Biol Sci Med Sci 67: 587–598, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med 32: 159–221, 2011 [DOI] [PubMed] [Google Scholar]
- 173.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol 24: 1397–1402, 2004 [DOI] [PubMed] [Google Scholar]
- 174.Stein S, Schafer N, Breitenstein A, Besler C, Winnik S, Lohmann C, Heinrich K, Brokopp CE, Handschin C, Landmesser U, Tanner FC, Luscher TF, Matter CM. SIRT1 reduces endothelial activation without affecting vascular function in ApoE−/− mice. Aging (Albany NY) 2: 353–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Suh JH, Heath SH, Hagen TM. Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic Biol Med 35: 1064–1072, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Supinski GS, Murphy MP, Callahan LA. MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol 297: R1095–R1102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J 20: 5971–5981, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Szeto HH. Development of mitochondria-targeted aromatic-cationic peptides for neurodegenerative diseases. Ann N Y Acad Sci 1147: 112–121, 2008 [DOI] [PubMed] [Google Scholar]
- 179.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10: 601–619, 2008 [DOI] [PubMed] [Google Scholar]
- 180.Taneike M, Yamaguchi O, Nakai A, Hikoso S, Takeda T, Mizote I, Oka T, Tamai T, Oyabu J, Murakawa T, Nishida K, Shimizu T, Hori M, Komuro I, Takuji Shirasawa TS, Mizushima N, Otsu K. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6: 600–606, 2010 [DOI] [PubMed] [Google Scholar]
- 181.Terman A. Garbage catastrophe theory of aging: imperfect removal of oxidative damage? Redox Rep 6: 15–26, 2001 [DOI] [PubMed] [Google Scholar]
- 182.Terman A, Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res 68: 355–365, 2005 [DOI] [PubMed] [Google Scholar]
- 183.Terman A, Gustafsson B, Brunk UT. Mitochondrial damage and intralysosomal degradation in cellular aging. Mol Aspects Med 27: 471–482, 2006 [DOI] [PubMed] [Google Scholar]
- 184.Terman A, Kurz T, Navratil M, Arriaga EA, Brunk UT. Mitochondrial turnover and aging of long-lived postmitotic cells: the mitochondrial-lysosomal axis theory of aging. Antioxid Redox Signal 12: 503–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tinkel J, Hassanain H, Khouri SJ. Cardiovascular antioxidant therapy: a review of supplements, pharmacotherapies, and mechanisms. Cardiol Rev 20: 77–83, 2012 [DOI] [PubMed] [Google Scholar]
- 186.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423, 2004 [DOI] [PubMed] [Google Scholar]
- 187.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell 9: 592–606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777: 1092–1097, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ungvari Z, Bailey-Downs L, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363–H372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-κB activation in the non-human primate Macaca mulatta. J Gerontol Biol Med Sci 66: 866–875, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363–H372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci 67: 599–610, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci 65: 1028–1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol 294: H2121–H2128, 2008 [DOI] [PubMed] [Google Scholar]
- 197.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 199.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res 102: 519–528, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ungvari Z, Sonntag WE, Csiszar A. Mitochondria and aging in the vascular system. J Mol Med (Berl) 88: 1021–1027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Luscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.van Empel VP, Bertrand AT, van Oort RJ, van der Nagel R, Engelen M, van Rijen HV, Doevendans PA, Crijns HJ, Ackerman SL, Sluiter W, De Windt LJ. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J Am Coll Cardiol 48: 824–832, 2006 [DOI] [PubMed] [Google Scholar]
- 203.Van Humbeeck C, Cornelissen T, Hofkens H, Mandemakers W, Gevaert K, De Strooper B, Vandenberghe W. Parkin interacts with Ambra1 to induce mitophagy. J Neurosci 31: 10249–10261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. Am J Physiol Cell Physiol 288: C757–C767, 2005 [DOI] [PubMed] [Google Scholar]
- 205.Vergeade A, Mulder P, Vendeville-Dehaudt C, Estour F, Fortin D, Ventura-Clapier R, Thuillez C, Monteil C. Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: prevention by the targeted antioxidant MitoQ. Free Radic Biol Med 49: 748–756, 2010 [DOI] [PubMed] [Google Scholar]
- 206.Victor VM, Rocha M. Targeting antioxidants to mitochondria: a potential new therapeutic strategy for cardiovascular diseases. Curr Pharm Des 13: 845–863, 2007 [DOI] [PubMed] [Google Scholar]
- 207.Voos W. Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases. Res Microbiol 160: 718–725, 2009 [DOI] [PubMed] [Google Scholar]
- 208.Vos M, Esposito G, Edirisinghe JN, Vilain S, Haddad DM, Slabbaert JR, Van Meensel S, Schaap O, De Strooper B, Meganathan R, Morais VA, Verstreken P. Vitamin K2 is a mitochondrial electron carrier that rescues pink1 deficiency. Science 336: 1306–1310, 2012 [DOI] [PubMed] [Google Scholar]
- 209.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106: 1652–1658, 2002 [DOI] [PubMed] [Google Scholar]
- 210.Wang HJ, Zhang D, Tan YZ, Li T. Autophagy in endothelial progenitor cells is cytoprotective in hypoxic conditions. Am J Physiol Cell Physiol 304: C617–C626, 2013 [DOI] [PubMed] [Google Scholar]
- 211.Wang M, Monticone RE, Lakatta EG. Arterial aging: a journey into subclinical arterial disease. Curr Opin Nephrol Hypertens 19: 201–207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 41: 1308–1316, 2003 [DOI] [PubMed] [Google Scholar]
- 213.Wang M, Zhang J, Jiang LQ, Spinetti G, Pintus G, Monticone R, Kolodgie FD, Virmani R, Lakatta EG. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 50: 219–227, 2007 [DOI] [PubMed] [Google Scholar]
- 214.Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats. Am J Pathol 167: 1429–1442, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol 301: H1205–H1219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wenzel P, Schuhmacher S, Kienhofer J, Muller J, Hortmann M, Oelze M, Schulz E, Treiber N, Kawamoto T, Scharffetter-Kochanek K, Munzel T, Burkle A, Bachschmid MM, Daiber A. Manganese superoxide dismutase and aldehyde dehydrogenase deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc Res 80: 280–289, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wisniewski KE, Dalton AJ, McLachlan C, Wen GY, Wisniewski HM. Alzheimer's disease in Down's syndrome: clinicopathologic studies. Neurology 35: 957–961, 1985 [DOI] [PubMed] [Google Scholar]
- 218.Wohlgemuth SE, Julian D, Akin DE, Fried J, Toscano K, Leeuwenburgh C, Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res 10: 281–292, 2007 [DOI] [PubMed] [Google Scholar]
- 219.Xie Z, He C, Zou MH. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy 7: 1254–1255, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol 9: 1102–1109, 2007 [DOI] [PubMed] [Google Scholar]
- 221.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60: 1770–1778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA 94: 514–519, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yamamori T, White AR, Mattagajasingh I, Khanday FA, Haile A, Qi B, Jeon BH, Bugayenko A, Kasuno K, Berkowitz DE, Irani K. P66shc regulates endothelial NO production and endothelium-dependent vasorelaxation: implications for age-associated vascular dysfunction. J Mol Cell Cardiol 39: 992–995, 2005 [DOI] [PubMed] [Google Scholar]
- 224.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science 337: 1062–1065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res 79: 341–351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006 [DOI] [PubMed] [Google Scholar]
- 227.Zhang D, Mott JL, Farrar P, Ryerse JS, Chang SW, Stevens M, Denniger G, Zassenhaus HP. Mitochondrial DNA mutations activate the mitochondrial apoptotic pathway and cause dilated cardiomyopathy. Cardiovasc Res 57: 147–157, 2003 [DOI] [PubMed] [Google Scholar]
- 228.Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ 16: 939–946, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang Y, Yuan M, Bradley KM, Dong F, Anversa P, Ren J. Insulin-like growth factor 1 alleviates high-fat diet-induced myocardial contractile dysfunction: role of insulin signaling and mitochondrial function. Hypertension 59: 680–693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 230.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]
- 231.Zhou RH, Vendrov AE, Tchivilev I, Niu XL, Molnar KC, Rojas M, Carter JD, Tong H, Stouffer GA, Madamanchi NR, Runge MS. Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function. Arterioscler Thromb Vasc Biol 32: 745–755, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12: 723–738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]