Abstract
We have previously shown that myocardial infarct size in nonreperfused hearts of mice with a functional deletion of the circadian rhythm gene mPer2 (mPer2-M) was reduced by 43%. We hypothesized that acute ischemia-reperfusion injury (I/R = 30 min I/2 h R) would also be reduced in these mice and that ischemic preconditioning (IPC) (3 × 5 min cycles) before I/R, which enhances protection in wild-type (WT) hearts, would provide further protection in mPer2-M hearts. We observed a 69 and 75% decrease in infarct size in mPer2-M mouse hearts compared with WT following I/R and IPC, respectively. This was coincident with 67% less neutrophil infiltration and 57% less apoptotic cardiomyocytes. IPC in mPer2-M mice before I/R had 48% less neutrophil density and 46% less apoptosis than their WT counterparts. Macrophage density was not different between WT and mPer2-M I/R, but it was 45% higher in mPer2-M IPC mouse hearts compared with WT IPC. There were no baseline differences in cardiac mitochondrial function between WT and mPer2-M mice, but, following I/R, WT exhibited a marked decrease in maximal O2 consumption supported by complex I-mediated substrates, whereas mPer2-M did not, despite no difference in complex I content. Moreover, cardiac mitochondria from WT mice exhibited a very robust increase in ADP-stimulated O2 consumption in response to exogenously added cytochrome c, along with a high rate of reactive oxygen species production, none of which was exhibited by cardiac mitochondria from mPer2-M following I/R. Taken together, these findings suggest that mPer2 deletion preserves mitochondrial membrane structure and functional integrity in heart following I/R injury, the consequence of which is preservation of myocardial viability. Understanding the mechanisms connecting cardiac events, mitochondrial function, and mPer2 could lead to preventative and therapeutic strategies for at risk populations.
Keywords: ischemia-reperfusion, heart, circadian, mitochondrial function
in mice with functional deletion of the mPer2 gene (mPer2-M), we have previously shown that infarct size and inflammatory cell infiltration in nonreperfused myocardium are greatly reduced at 4 days postmyocardial infarction (35). Acute ischemia-reperfusion (I/R) injury is a complex process involving vascular and endothelial dysfunction, metabolic and mitochondrial dysfunction, necrosis, apoptosis, and functional deficits, even in tissue without permanent cellular injury (8, 19, 22, 32). A common feature of I/R injury is an inflammatory reaction, with infiltration of polymorphonuclear leukocytes, predominantly neutrophils that have been causally linked to myocardial damage due to increased oxidative stress during reperfusion injury (22). Specifically, Per2 has been shown to modulate cytokine release in some inflammatory cells (25). Furthermore, oxidant stress, a finely modulated event in normal physiology, may be directly influenced by clock genes. The most common sources of routine oxidant burdens result from normal metabolic activity and mitochondrial electron transfer to molecular oxygen in the mitochondria at complexes I and III of the electron transport chain, producing superoxide and other reactive oxygen species (ROS). Mice deficient in mPer2 have been reported to be cancer-prone, and this may be related to oxidative stress (20, 24). Clearly, there is strong confluence between cardiac metabolic processes and those regulated by circadian genes (4–6, 11, 14, 39). However, specific studies examining the effects of circadian genes in myocardial I/R injury and its modulation by ischemic preconditioning (IPC) have not been investigated. We hypothesized that mPer2-M mice would have less infarct damage than wild-type (WT) mice undergoing I/R surgery, and, moreover, IPC would further attenuate ischemic injury. We predicted that this would be due to more robust activation of cardioprotective pathways, reduced oxidative stress due to preserved mitochondrial integrity and function, and reduced inflammation.
EXPERIMENTAL PROCEDURES
Animals.
Female WT C57BL/6J mice (aged 8–10 wk) were obtained from Jackson Laboratories (Bar Harbor, ME). Mutant mPer2 mice (mPer2-M), obtained from Jackson Laboratories (mPer2 Brdm1, stock no. 003819), were bred on a C57BL/6J background. All animals were individually housed in a light-proof chamber and entrained in a 12:12-h light-dark cycle for at least 10 days before surgery and had access to food and water ad libitum throughout the study. All procedures were approved by the East Carolina University Institutional Animal Care and Use Committee and are in compliance with National Institutes of Health (NIH) guidelines.
Surgical procedure.
Female mice were anesthetized with 2,2,2-tribromoethanol (20 μl/g Avertin ip), intubated, and ventilated using a Kent Scientific TOPO ventilator. A depilatory (Nair) was used to remove the fur on the chest. The surgical area was cleaned with Betadine and alcohol, and the thoracic cavity was opened to expose the heart. An 8–0 suture was passed under the left anterior descending (LAD) coronary artery, and a 5-mm piece of PE-90 tubing was placed on the heart to permit temporary ligation of the coronary artery (9, 36). After 30 min ischemia, the hearts were reperfused for 2 h. For IPC, three cycles of 5 min ischemia and 5 min reperfusion preceeded the 30 min ischemia and 2 h reperfusion. All surgeries were performed from 9:00 A.M. to 3:00 P.M., and subsequent measures are derived from tissue collected at the end of the 2-h reperfusion period.
Morphometry and histology.
At the end of the 2-h reperfusion period, all mice were anesthetized with an intraperitoneal injection of pentobarbital (dose) or ketamine/xylazine (dose). The LAD was religated at the original point of occlusion. Evan's blue and 2,3,5-triphenyltetrazolium chloride (TTC) staining (WT n = 6, mPer2 n = 6) were used to determine the infarct size as a percentage of the left ventricle (LV) at risk [area at risk (AAR)]. Briefly, a solution containing 1% Evan's blue was injected in the aorta. After processing, sections were incubated in a 1% solution of TTC to assess infarct size. Both sides of all sections were photographed and analyzed using NIH Image J software to determine LV tissue area and area of infarct. The infarct was expressed as a percentage of the AAR, and the values for all sections from each heart were averaged (9).
Immunohistochemistry.
Tissue sections were deparaffinized and rehydrated, and endogenous peroxidases were quenched. After being rinsed in PBS, slides were incubated with anti-Ly6G (no. 550291; 1:100; Pharmingen) for neutrophils, anti-CD45 (rat anti-mouse monoclonal; no. 550539; 1:2,000; Pharmingen) for macrophages, or were TUNEL stained with an In Situ Cell Death Detection Kit, POD (11684817910; Roche), to label apoptotic cells followed by monoclonal anti-α-sarcomeric actin (A2172; 1:4,000; Sigma) to stain cardiomyocytes. The first primary reaction product was visualized with DAB (SK-4100; Vector), which is a brown chromogen, and the second primary reaction product by Vector Red (SK-5100; Vector), which is red. Neutrophil and macrophage density was measured in six fields per specimen at ×20 and expressed per 0.1 mm2 as measured by NIH Image J. TUNEL-positive cardiomyocytes (DAB+/Vector Red+) were counted by inspecting random fields throughout the infarct until a total of 500 cardiomyocyte nuclei (centrally located in cross sections of myocytes) had been counted in two sections containing infarct regions (apical), and the data were expressed as a percentage of the total cardiomyocyte nuclei.
Whole heart protein extraction and mitochondrial protein fractionation.
Whole LV were snap-frozen in liquid nitrogen. For protein extraction, samples (n = 3/group) were homogenized using HEPES/protease inhibitor. Briefly, homogenates were incubated for 60 min and centrifuged at 15,000 g for 25 min at 4°C, and the supernatant was removed. Protein quantification was performed using the EZ Q kit (Invitrogen), and aliquots were stored at −80°C until use.
Using the method adapted from Boehm et al. (3), mitochondria from the LV of WT and mPer2-M hearts subjected to either I/R or IPC (n = 5/group) were isolated. Briefly, buffers containing sucrose, Na-HEPES, and EDTA with and without BSA in combination with trypsin were used to homogenize the tissue. The initial suspension was centrifuged at 600 g for 10 min at 4°C, and subsequently the supernatant was centrifuged and resuspended twice more at 8,000 g for 15 min at 4°C to obtain the purified mitochondrial protein fraction.
Western blotting for Bag-1, bcl-2, bax, NF-kB, glycogen synthase kinase (GSK)-3β and phospho (p)-GSK-3β, protein kinase B (Akt) and p-Akt, poly ADP ribose polymerase (PARP) and cleaved PARP, and caspase-3 (Santa Cruz and Cell Signaling) was run on whole heart (n = 3) and/or mitochondrial (n = 5) extracts. Total mitochondrial OXPHOS immunoblots were obtained using whole LV homogenates probed with an antibody cocktail that recognizes subunits of respiratory complexes I-IV and the mitochondrial ATPase (MitoSciences, Eugene, OR). ImageQuant TL was used for densitometric analysis of the blots. Protein expression in whole LV homogenates was normalized to GAPDH (Millipore) and to voltage-dependent anion channel (Abcam) in mitochondrial extracts.
Preparation of permeabilized cardiac myofibers.
Small portions (<20 mg) of LV tissue immediately distal to the site of LAD occlusion were dissected and placed in ice-cold buffer X containing (in mM) 50 2-(N-morpholino)ethanesulfonic acid (MES), 7.23 K2EGTA, 2.77 CaK2EGTA, 20 imidazole, 0.5 DTT, 20 taurine, 5.7 ATP, 14.3 phosphocreatine, and 6.56 MgCl2-6 H2O (pH 7.1, 290 mosmol/kgH2O). The muscle was trimmed of all noticeable connective and vascular tissue. Five or six small muscle bundles (∼4–6 mm in length, 1.0–2.5 mg wet wt) were prepared from each animal. These bundles were gently separated along their longitudinal axis with a pair of needle-tipped forceps under magnification (MX6 Stereoscope; Leica Microsystems, Wetzlar, Germany). Cardiac myofiber bundles were then permeabilized with 50 μg/ml saponin in ice-cold buffer X, and incubated on a rotor for 30 min at 4°C. Following permeabilization, the fibers used for mitochondrial O2 (mO2) consumption experiments were placed in buffer Z containing (in mM) 105 K-MES, 30 KCl, 1 EGTA, 10 K2HPO4, and 5 MgCl2-6H2O, 0.005 glutamate, and 0.002 malate with 5.0 mg/ml BSA (pH 7.4, 290 mosmol/kgH2O).
Measurement of mO2 and ROS production.
All mitochondrial measurements were performed at 37°C in buffer Z + 5 mg/ml BSA containing 20 μM blebbistatin to prevent contraction during the course of the experiments. The respiration medium was hyperoxygenated to ∼400 μM O2 before the start of each respiration experiment. The Oroboros O2K Oxygraph system (Oroboros Instruments, Innsbruck, Austria) was used for O2 consumption (mO2) measurements. The H2O2 measurements were performed in a spectrofluorometer (Photon Technology Instruments, Birmingham, NJ) equipped with a thermojacketed cuvette chamber. During the course of the mO2 experiments, all substrates, nucleotides, and inhibitors were used as indicated in Figs. 1–7 and the figure legends. Mitochondrial H2O2 measurements were performed in the presence of 75 μM ADP, 5 mM glucose, and 1 U/ml hexokinase to keep the mitochondria in a permanent, submaximal phosphorylating state, to best mimic the in vivo condition. The formation of H2O2 was detected by inclusion of 10 μM Amplex Red, 1 U/ml horseradish peroxidase, and 25 U/ml superoxide dismutase in the reaction buffer. During the course of the H2O2 experiments, all substrates, nucleotides, and respiratory inhibitors were provided as indicated in the legends for Figs. 1–7. Rates of mitochondrial H2O2 were calculated according to the rate of increase in resorufin formation (i.e., oxidized Amplex Red) as outlined previously (2). At the conclusion of all experiments, fibers were rinsed in ddH2O, lyophilized in a freeze-dryer (Labconco, Kansas City, MO) for >2 h, and weighed on a microscale. Data are expressed as picomole per minute per milligram dry weight.
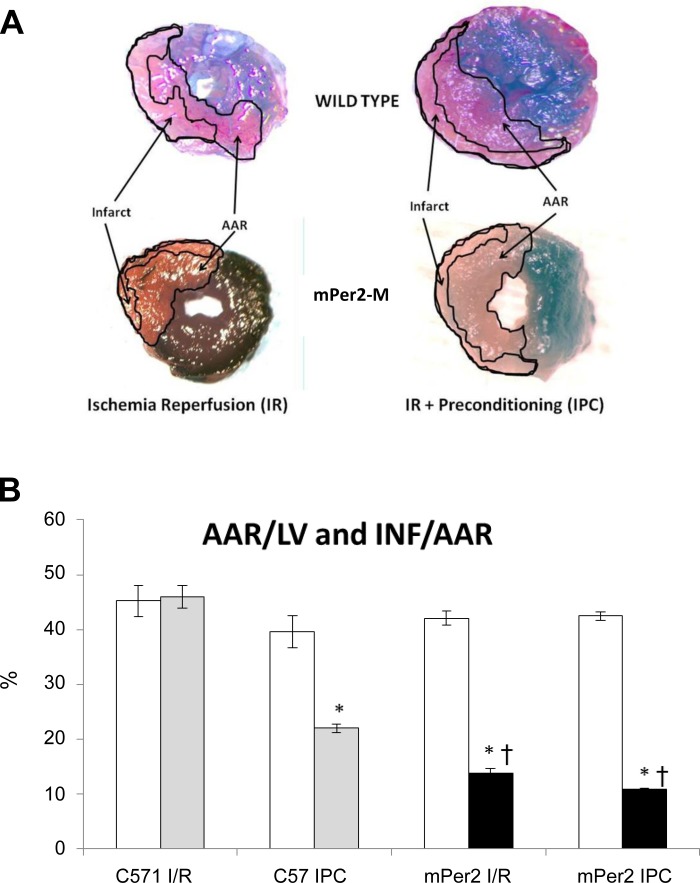
Fig. 1.
Evan's blue- and 2,3,5-triphenyltetrazolium chloride (TTC)-stained hearts and morphometric measures of area at risk (AAR)/left ventricle (LV) and infarct area (INF)/AAR. The images in A are representative images of Evan's blue- and TTC-stained hearts from each of the four experimental groups (×20). The graph in B shows the AAR as a function of the LV (white bars) and the INF as a function of the AAR (gray and black bars). There were no differences in AAR/LV between the 4 experimental groups. As expected, ischemic preconditioning (IPC) in WT C57 mouse hearts reduced the INF/AAR by 52% (*P < 0.001). The INF/AAR in mPer2-M hearts in both the ischemia-reperfusion (I/R) and IPC groups was reduced by 67 and 74%, respectively (*P < 0.001), and both groups were significantly less than the C57 IPC group (†P < 0.001). There was no difference in INF/AAR between the mPer I/R and IPC groups.
Fig. 7.
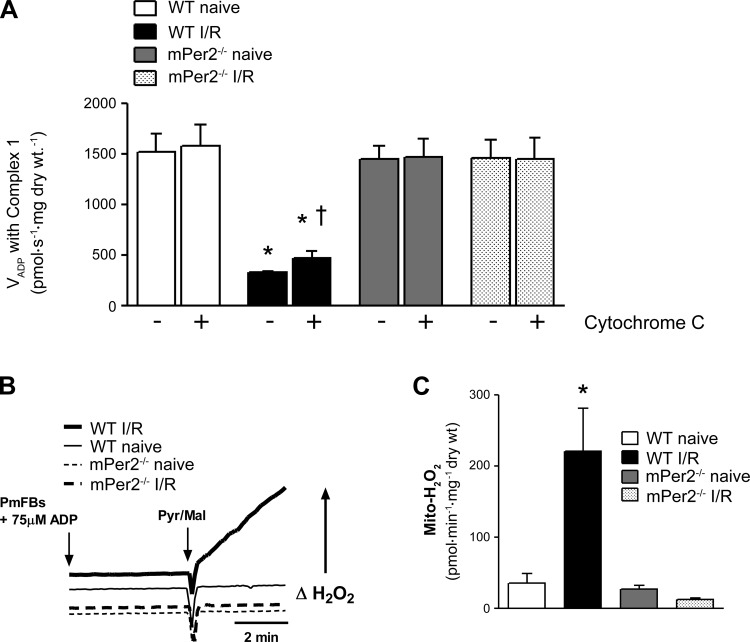
Mitochondrial outer membrane integrity and ROS production. Intactness of the outer mitochondrial membrane was used as an index of the structural integrity of mitochondria by measuring the rates of maximal ADP-stimulated O2 consumption before (−) and after (+) exogenous cytochrome c supported by pyruvate/glutamate/malate, as shown in A. Representative experimental traces of mitochondrial ROS production (H2O2) supported by pyruvate/glutamate/malate + 75 μM ADP are shown in B from all 4 experimental groups. Quantified rates of mitochondrial H2O2 production are presented in C. Values are means ± SE (n = 5). *P < 0.01 vs. all other groups for that condition. †P < 0.05, main effect of cytochrome c within group.
Determination of mitochondrial outer membrane integrity.
Following permeabilization and wash in buffer Z, fibers were placed in an O2K chamber in buffer Z. A maximal rate of mO2 was established in the permeabilized fibers with 5 mM ADP in the presence of 5 mM pyruvate, 2 mM malate, and 5 mM glutamate. After establishment of a steady O2 flux, a bolus of 20 μM cytochrome c (Sigma-Aldrich) was added to the respiration medium. Rates of maximal mO2 before and after addition of cytochrome c were compared to determine the intactness of the outer mitochondrial membrane.
Statistics.
Data are expressed as means ± SE. Statistical significance between groups was determined by ANOVA, and significance levels were P < 0.05.
RESULTS
Infarct size.
Representative Evan's blue and TTC stains are shown in Fig. 1A. There was no difference in the AAR as a ratio of the LV across the four groups, but INF/AAR was 69% reduced in mPer2-M I/R mouse hearts (Fig. 1B). Although the expected reduction in INF/AAR in the C57 IPC group occurred compared with C57 I/R, there was no further reduction in infarct size in the mPer2-M IPC group compared with the mPer2-M I/R group.
Inflammatory infiltrate.
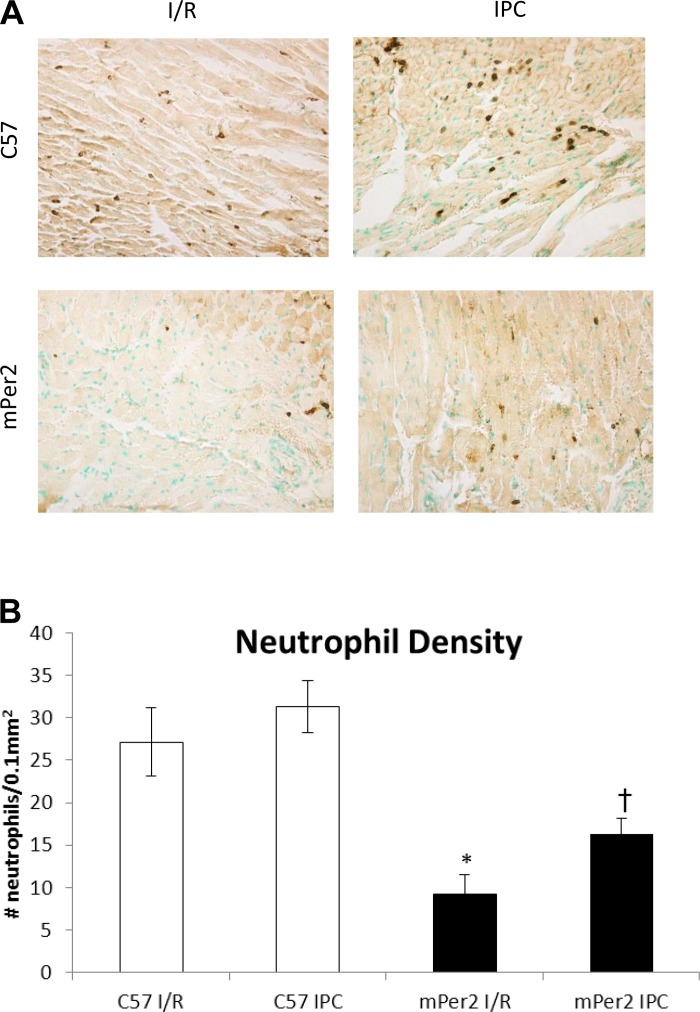
The density of neutrophils (Ly6G+-labeled cells) per 0.1 mm2 of the infarcted area (Fig. 2) was significantly decreased (P < 0.001) in mPer2-M I/R (27 ± 4) compared with WT I/R (9 ± 2) and in mPer2-M IPC (16 ± 2) vs. WT IPC (31 ± 3; P < 0.01), but there were no differences between I/R and IPC in the WT hearts or the mPer2-M hearts.
Fig. 2.
Neutrophil density in the infarct zone. The images in A illustrate the density of neutrophils in the infarct zone of mouse hearts from C57 and mPer2-M mice exposed to either I/R or IPC (×400). The graph in B shows that the relative density of neutrophils (Ly6G+-labeled cells) per 0.1 mm2 of the infarcted area was decreased 67% in mPer2-M I/R compared with WT I/R (*P < 0.001) and 48% in mPer2-M IPC vs. WT IPC (†P < 0.01), but there were no differences between I/R and IPC in the WT hearts or the mPer2-M hearts.
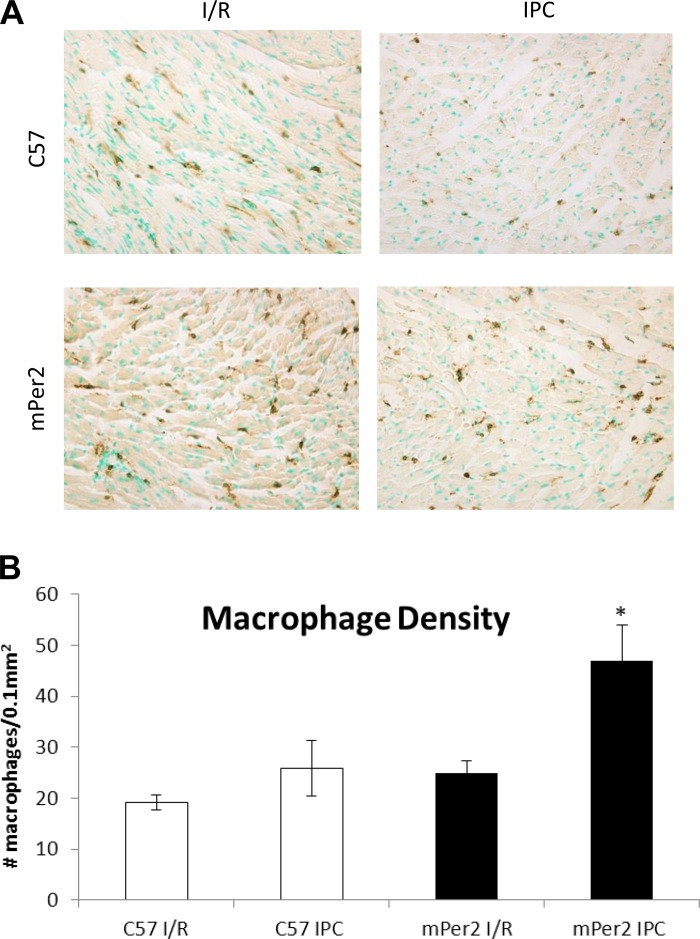
The images and graph in Fig. 3 illustrate the density of macrophages (CD45+-labeled cells) per 0.1 mm2 of the infarcted area. There were 47 ± 7 macrophages in mPer2-M IPC hearts, which was significantly more than compared with only 25 ± 2 mPer2-M I/R hearts, 19 ± 3 in WT I/R hearts, and 26 ± 5 in the WT IPC group.
Fig. 3.
Leukocyte density in the infarct zone. The images in A show the density of leukocytes in the infarct zone of mouse hearts from C57 and mPer2-M mice exposed to either I/R or IPC (×400). The graph in B shows the density of macrophages (CD45+-labeled cells) per 0.1 mm2 of the infarcted area. There was a 47% increase in macrophage density in mPer2-M IPC hearts compared with mPer2-M I/R hearts, and this represents a 45% increase compared with the WT IPC group. *P < 0.01 compared with both mPer2-M I/R C57 and I/R + IPC.
Cardiomyocyte apoptosis.
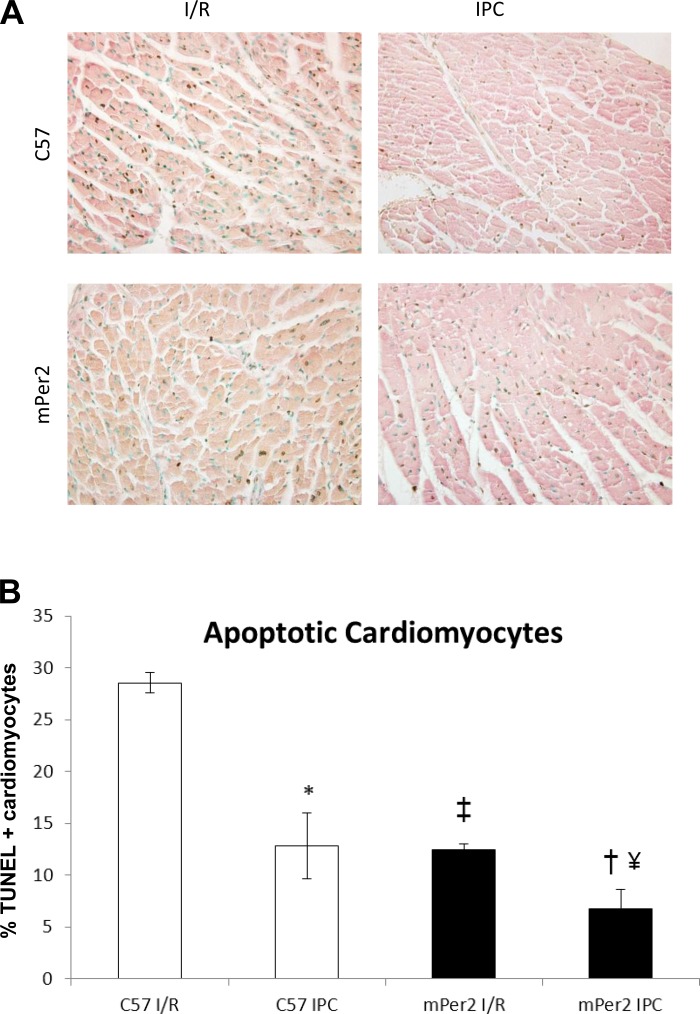
Figure 4 shows representative images of the infarct zone of C57 and mPer2-M I/R and IPC hearts. The number of TUNEL+ cardiomyocytes (out of 500; Fig. 4B) in C57 I/R hearts was significantly higher (29 ± 1) than all other groups. TUNEL+ cardiomyocytes in the mPer2-M I/R group were 12 ± 1, which was not different from the C57 IPC group (13 ± 3). Similarly, the TUNEL+ cardiomyocytes (out of 500) in the infarct zone of the mPer2-M IPC group (7 ± 2) were significantly lower compared with both the mPer2 I/R group and the C57 IPC group.
Fig. 4.
Apoptotic cardiomyocytes in the infarct zone. Immunohistochemical images of TUNEL+ cardiomyocyte nuclei in wild-type (WT) and mPer-M I/R and IPC hearts (×400). The percentage of TUNEL+ cardiomyocyte nuclei decreased 54% in C57 IPC hearts compared with C57 I/R hearts (P < 0.001). mPer2-M IPC hearts had 46% less apoptotic cardiomyocytes than C57 IPC hearts (‡P < 0.05). The no. of apoptotic cardiomyocyte nuclei in mPer2-M I/R hearts was 57% less than C57 I/R hearts (†P < 0.001) and 42% less in mPer2-M IPC hearts compared with mPer2-M I/R hearts (#P < 0.05), but there was no difference in the no. of TUNEL+ cardiomyocytes in mPer2-M I/R hearts compared with C57 IPC hearts.
Western blotting in LV homogenates and mitochondrial extracts.
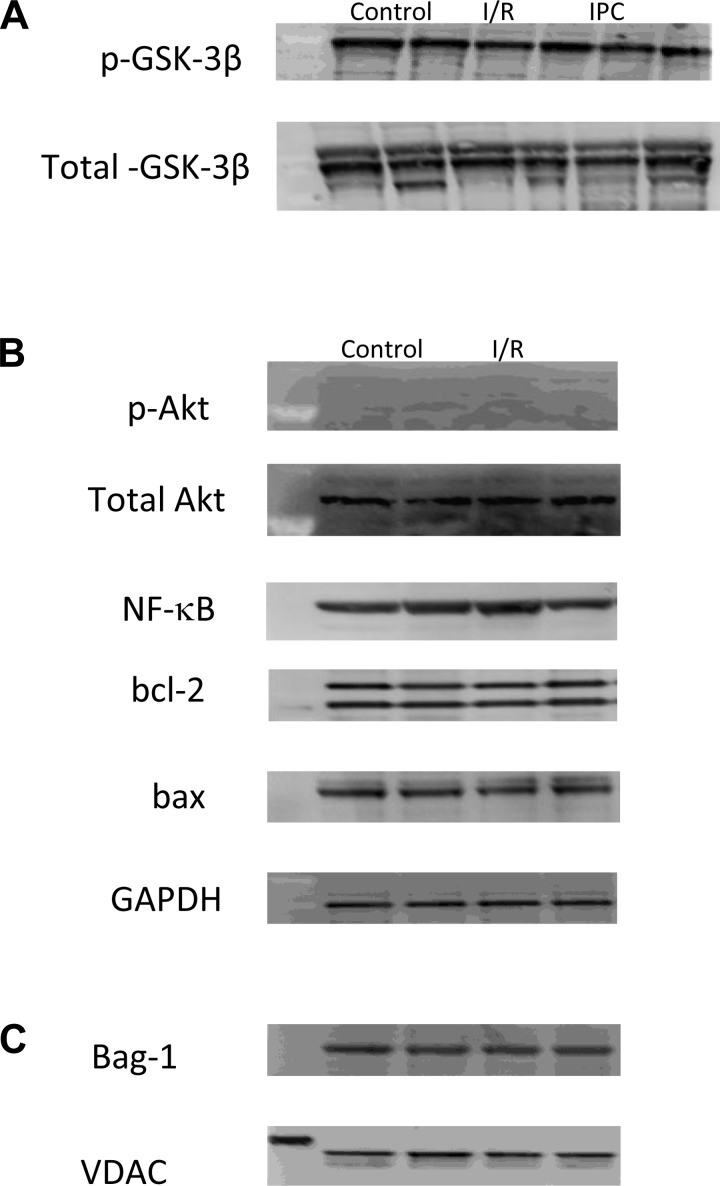
Representative Western blots for phospho (p)-GSK-3β/total GSK-3β (A), and Akt, p-Akt, bcl-2, bax, and bag-1 (B) in LV homogenates, and bag-1 in mitochondrial extracts (C) are shown in Fig. 5. The results for caspase-3, cleaved caspase-3, PARP, and cleaved PARP were the same as those observed with Akt and p-Akt (i.e., no signal for cleaved caspase-3 or cleaved PARP) so these data are not shown. No differences in expression level were observed in any of the proteins examined.
Fig. 5.
Representative Western blotting of LV and mitochondrial extracts. The protein expression level of phospho (p)-glycogen synthase kinase (GSK)-3β/total GSK-3β (A) and p-protein kinase B (Akt)/Akt, NF-κB, bcl-2, and bax/GAPDH (B) in whole LV homogenates and Bag-1/voltage-dependent anion channel (VDAC) in mitochondrial extracts (C) did not change significantly between the groups.
Mitochondrial function and structural integrity.
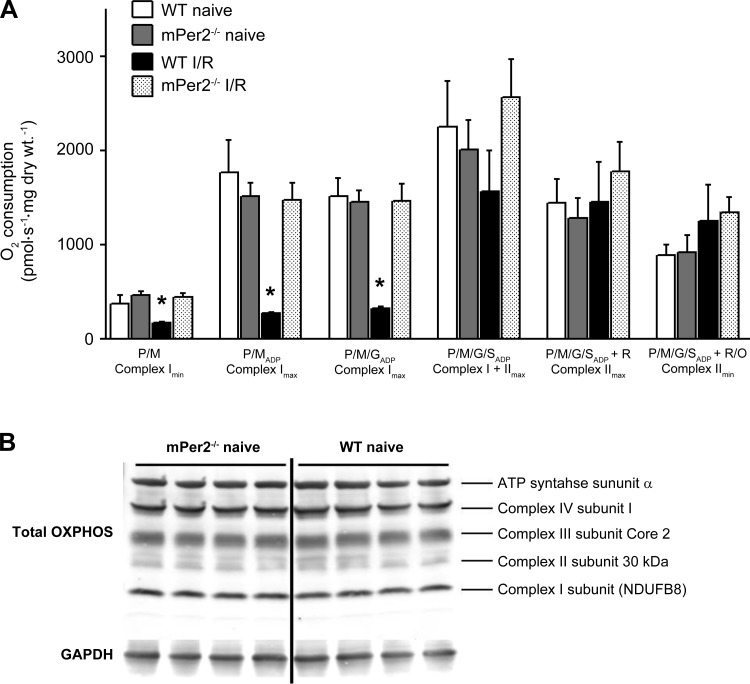
Mitochondria in WT and mPer2-M mice were identical in uninjured, normal hearts, as indicated by similar rates of basal and maximal ADP-stimulated mO2 supported by pyruvate/malate, glutamate, and succinate in permeabilized cardiac myofibers (Fig. 6). Following I/R injury, mitochondrial dysfunction was markedly induced in WT hearts, as indicated by lack of ADP-stimulated mO2 in the presence of pyruvate/malate and glutamate. This decrease in ADP-stimulated respiration was not evident under succinate-supported conditions (i.e., complex II), suggesting a complex I-specific defect. Interestingly, mitochondria in mPer-M mice had absolutely no dysfunction following I/R injury, under any oxidative substrate condition tested. Moreover, the intactness of the outer mitochondrial membrane, as determined by the mO2 response to exogenous cytochrome c, was preserved in permeabilized myofibers prepared from mPer2-M hearts subjected to I/R injury while clearly becoming ruptured in mitochondria from WT hearts (Fig. 7).
Fig. 6.
Mitochondrial O2 consumption and respiratory protein content. Rates of mitochondrial O2 consumption in the presence of 5 mM pyruvate/2 mM malate (P/M, Complex Imin); P/M + 5 mM ADP (P/MADP, Complex Imax); P/MADP + 5 mM glutamate (P/M/GADP, Complex Imax); P/M/GADP + 5 mM succinate (P/M/G/SADP, Complex I + IImax); and P/M/G/SADP + 1 μM rotenone (Complex IImax) are shown in A. Shown in B are representative immunoblots (n = 4) of mitochondrial respiratory enzyme subunits of complexes I-IV + complex V (H+-ATPase) in naïve mPer2−/− and WT myocardium; Values are means ± SE (n = 5). *P < 0.01 vs. all other groups for that condition.
DISCUSSION
Irreversible ischemic injury begins within 40 min of severe ischemia in vivo and, in the absence of reperfusion, is complete within 1 h (21, 31). In normal animals, the inflammatory response begins within hours of the onset of ischemia. Neutrophil transmigration into the ischemic myocardium, which begins within 1 h of injury, peaks after 6 h of reperfusion, but continues for days and is accompanied by evolution of ROS, proteolytic enzymes, and cytokines, which exacerbate the injury (13, 15, 17, 18, 38). Subsequent infiltration by macrophages results in release of a variety of factors, including chemokines such as monocyte chemoattractant protein-1, cytokines such as IL-1β and granulocyte colony-stimulating factor, and reactive oxygen and nitrogen species (7, 15, 16, 23, 28, 30). These molecules can also damage surrounding viable cells and result in further spreading of the infarct. Reperfusion therapy salvages myocardium but is accompanied by further oxidative burden. To date, however, reperfusion has been proven to be the most effective therapy to reduce remodeling, improve ventricular function, and reduce mortality. It is possible that the increased macrophage density in mPer2-M hearts promotes more rapid clearing of damaged tissue, thereby enhancing infarct healing (34). In our hands, the reduction of AAR in mPer2-M hearts in the acute studies represents an increased resistance to ischemic injury.
Mitochondrial function measurements indicate that there are no differences between uninjured WT, mPer2-M, or mPer2-M I/R, whereas the WT I/R respiratory capacity was significantly compromised. Although there were significant differences in the density of the inflammatory infiltrates, there were no differences between mPer2-M I/R and IPC. Combined with the fact that there was no difference in mitochondrial function parameters between controls and mPer2-M I/R, this indicated that it would be unnecessary to repeat these mitochondrial functional assays in mPer2-M IPC tissue. In addition to differences in baseline metabolism between strains, it is possible that the capacity to endure severe stress is performing maximally in these young mPer2-M mice and, therefore, may not be enhanced any further. Interestingly, IPC is not effective in aging hearts (1), and it has also been shown that mPer2-null mice tend to age prematurely (37). It is conceivable that the accelerated senescence may reflect exhaustion of the physiological mechanisms responsible for the observed tolerance in young mice. Further studies in aged mice are necessary to evaluate this.
Use of the germline mPer2-M mouse does not permit us to draw conclusions regarding the contributions of different tissues to the observed phenotype, and thus additional studies using tissue-specific Per2 deletion are warranted. Additionally, other groups have reported opposing findings regarding mPer2, which indicate that the role of mPer2 in modulating myocardial metabolism may be a time-dependent phenomenon. Because the timing of I/R injury in our experiments was likely not aligned with Eckle and colleagues (12), it is difficult to state with certainty why our data contradicts theirs. It is possible that the increased damage associated with the longer ischemic period (60 min vs. our 30 min), time of day of the surgery, fed or fasted state of the animals, and/or differences in the type of anesthesia used caused the divergent results. In any case, the mere fact that our groups have opposing results regarding the role of mPer2 in cardiac I/R injury reflects the complex nature of circadian gene expression and the difficulties in studying their role in health and disease (12, 26, 33).
In summary, our data show a significant relationship between the circadian rhythm gene mPer2 and acute cardiac injury. We observed a 69% decrease in the ratio of infarct size to AAR in mPer2-M mice vs. WT. Reduced infiltration of neutrophils indicates an attenuated early inflammatory response in the mPer2-M mice. The density of apoptotic cardiomyocytes was reduced in mPer2-M vs. WT in both I/R and IPC, and this difference in the IPC cohort correlated with the trends observed in INF/AAR, although the infarct size in mPer2-M IPC was not significantly smaller. From these results, it is not possible to definitively determine whether the reduced injury is an inherent property or an inducible phenomenon. However, the fact that the protection from I/R and IPC in mPer2-M hearts is greater than that observed in WT IPC but IPC in mPer2-M does not confer additional protection over that observed in I/R alone would seem to suggest that it is inherent. Interestingly, there were no differences in protein expression levels of canonical IPC pathway mediators such as Bag-1, bcl-2, bax, NF-kB, GSK-3β and p-GSK-3β, Akt and p-Akt, PARP and cleaved PARP, and caspase-3 in whole LV homogenates and/or mitochondrial fractions in the mPer2-M I/R or IPC compared with WT IPC (10, 27, 29). Taken together, these observations suggest that traditional cardioprotective mechanisms are likely not responsible for the tolerance of mPer2-M myocardium to ischemic injury. Other cell death pathways (necrosis, autophagy) may be involved in ischemic tolerance. Further investigation of the type and quantity of cytokines and ROS elaborated by injured myocardium and infiltrating inflammatory cells in mPer2-M mice as well as shifts in metabolic substrate utilization may help uncover the mechanisms behind the protection afforded by functional deletion of the mPer2 circadian rhythm gene in ischemic injury response. This may ultimately lead to new treatment options.
GRANTS
A portion of this project was funded by National Heart, Lung, and Blood Institute Grant HL-098780 to E. J. Anderson, and all authors would like to acknowledge the Departments of Physiology, Pharmacology and Toxicology, and Comparative Medicine in the Brody School of Medicine at East Carolina University for their support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.A.I.V., E.J.A., J. Ding, and R.M.L. conception and design of research; J.A.I.V., E.J.A., S.D.K., H.D.B., T.J., F.M., J. DeAntonio, K.T., and R.M.L. performed experiments; J.A.I.V., E.J.A., S.D.K., H.D.B., T.J., F.M., J. DeAntonio, K.T., and R.M.L. analyzed data; J.A.I.V., E.J.A., S.D.K., H.D.B., T.J., F.M., J. DeAntonio, K.T., J. Ding, and R.M.L. interpreted results of experiments; J.A.I.V., E.J.A., S.D.K., H.D.B., and K.T. prepared figures; J.A.I.V. drafted manuscript; J.A.I.V., E.J.A., S.D.K., K.T., J. Ding, and R.M.L. edited and revised manuscript; J.A.I.V., E.J.A., S.D.K., H.D.B., T.J., F.M., J. DeAntonio, K.T., J. Ding, and R.M.L. approved final version of manuscript.
REFERENCES
- 1.Abete P, Testa G, Cacciatore F, Della-Morte D, Galizia G, Langellotto A, Rengo F. Ischemic preconditioning in the younger and aged heart. Aging Dis 2: 138–148, 2011 [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem 282: 31257–31266, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Boehm EA, Jones BE, Radda GK, Veech RL, Clarke K. Increased uncoupling proteins and decreased efficiency in palmitate-perfused hyperthyroid rat heart. Am J Physiol Heart Circ Physiol 280: H977–H983, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 294: H1036–H1047, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Bray MS, Young ME. Diurnal variations in myocardial metabolism. Cardiovasc Res 79: 228–237, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bray MS, Young ME. The role of cell-specific circadian clocks in metabolism and disease. Obesity Rev 10, Suppl 2: 6–13, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Byrne JA, Grieve DJ, Cave AC, Shah AM. Oxidative stress and heart failure. Arch Mal Coeur Vaisseaux 96: 214–221, 2003 [PubMed] [Google Scholar]
- 8.Cohen MV, Yang XM, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 70: 231–239, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Cozzi E, Hazarika S, Stallings HW, 3rd, Cascio WE, Devlin RB, Lust RM, Wingard CJ, Van Scott MR. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 291: H894–H903, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev 12: 181–188, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Durgan DJ, Young ME. Linking the cardiomyocyte circadian clock to myocardial metabolism. Cardiovasc Drugs Ther 22: 115–124, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 18: 774–782, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Entman ML, Michael L, Rossen RD, Dreyer WJ, Anderson DC, Taylor AA, Smith CW. Inflammation in the course of early myocardial ischemia. FASEB J 5: 2529–2537, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Esser KA, Young ME. The role of clock genes in cardiometabolic disease. J Appl Physiol 107: 1316–1317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal 8: 1907–1939, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Frangogiannis NG, Entman ML. Chemokines in myocardial ischemia. Trends Cardiovasc Med 15: 163–169, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 53: 31–47, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Frangogiannis NG, Youker KA, Entman ML. The role of the neutrophil in myocardial ischemia and reperfusion. Exs 76: 263–284, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest 115: 500–508, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardeland R, Coto-Montes A, Poeggeler B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int 20: 921–962, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng 7: 223–253, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Jordan JE, Zhao ZQ, Vinten-Johansen J. The role of neutrophils in myocardial ischemia-reperfusion injury. Cardiovasc Res 43: 860–878, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Kumar D, Jugdutt BI. Apoptosis and oxidants in the heart. J Lab Clin Med 142: 288–297, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Lee CC. The circadian clock and tumor suppression by Mammalian period genes. Methods Enzymol 393: 852–861, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Mankani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect Immun 74: 4750–4756, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopaschuk GD, Jaswal JS. A role for period 2 in cardioprotection. Cell Metab 16: 2–4, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Neblina F, Toledo AH, Toledo-Pereyra LH. Molecular biology of apoptosis in ischemia and reperfusion. J Invest Surg 18: 335–350, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Maulik N. Reactive oxygen species drives myocardial angiogenesis? Antioxid Redox Signal 8: 2161–2168, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Miura T, Tanno M, Sato T. Mitochondrial kinase signalling pathways in myocardial protection from ischaemia/reperfusion-induced necrosis. Cardiovasc Res 88: 7–15, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94: 1543–1553, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Reimer KA, Jennings RB. The “wavefront phenomenon” of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest 40: 633–644, 1979 [PubMed] [Google Scholar]
- 32.Seal JB, Gewertz BL. Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg 19: 572–584, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Traverse JH. Of mice and men: the quest to determine a circadian basis for myocardial protection in ischemia/reperfusion injury. Circ Res 112: e115–e117, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Vandervelde S, van Amerongen MJ, Tio RA, Petersen AH, van Luyn MJ, Harmsen MC. Increased inflammatory response and neovascularization in reperfused vs. non-reperfused murine myocardial infarction. Cardiovasc Pathol 15: 83–90, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Virag JA, Dries JL, Easton PR, Friesland AM, DeAntonio JH, Chintalgattu V, Cozzi E, Lehmann BD, Ding JM, Lust RM. Attenuation of myocardial injury in mice with functional deletion of the circadian rhythm gene mPer2. Am J Physiol Heart Circ Physiol 298: H1088–H1095, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virag JAI, Lust RM. Coronary artery ligation and intramyocardial injection in a murine model of infarction. J Vis Exp e2581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wojtovich AP, Nadtochiy SM, Brookes PS, Nehrke K. Ischemic preconditioning: the role of mitochondria and aging. Exp Gerontol 47: 1–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation 101: 1019–1026, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Young ME, Bray MS. Potential role for peripheral circadian clock dyssynchrony in the pathogenesis of cardiovascular dysfunction. Sleep Med 8: 656–667, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]