Abstract
Transforming growth factor (TGF)-β is a major mediator of kidney fibrosis. In the past decade it was recognized that, besides canonical Smad signaling, many other signaling pathways participate in the process of TGF-β-induced fibrogenesis. One such pathway involves mammalian target of rapamycin complex (mTORC)1. We recently reported that the hypoxia-inducible factor (HIF)-1 is essential for TGF-β-induced collagen expression regardless of ambient oxygen tension. A modulator of HIF expression other than oxygen tension is mTORC1. We therefore sought to evaluate a possible role for mTORC1 activity in TGF-β-induced fibrogenesis. mTORC1 activity was increased in human mesangial cells treated with TGF-β in a TGF-β receptor-dependent manner. Short hairpin (sh)RNA to Smad3 decreased, while overexpression of Smad3 increased, the mTORC1 activity, suggesting that TGF-β stimulation of mTORC1 also requires Smad3. Pretreatment with rapamycin or shRNA for a regulatory molecule of mTORC1, Raptor, reduced TGF-β-induced COL1A2-luc activity and collagen I protein expression. mTORC1 inhibition also prevented the TGF-β-stimulated increase in both hypoxia-responsive element (HRE) activity and HIF-1α protein expression, while activation of mTORC1 by active Rheb increased basal but not TGF-β-induced HRE activity. shRNA to Smad3 reduced HRE activity, while overexpression of Smad3 increased HIF-1α protein expression and activity in an mTORC1-dependent manner. Lastly, overexpression of HIF-1α bypassed the inhibitory effect of mTORC1 blockade on collagen expression. These results suggest that Smad3/mTORC1 interaction to promote HIF-1 expression is a key step in normoxic kidney fibrogenesis.
Keywords: TGF-β, Smad3, mTOR, rapamycin, fibrosis, HIF, collagen
end-stage kidney disease is a major cause of morbidity and mortality, with increasing prevalence during recent decades (16). A striking, common feature of end-stage kidney disease is fibrosis of both the glomerulus and the tubulo-interstitial compartment of the kidney, a complex process involving multiple cell types and a broad variety of mediators. Studies have defined a pivotal role for transforming growth factor (TGF)-β in fibrosis in multiple organ systems, including in the kidney (5, 53, 67). TGF-β is a pleiotropic cytokine that regulates cell growth, differentiation, proliferation, and migration. It increases collagen production in models of diabetic nephropathy and glomerulosclerosis (56). TGF-β signals via type I (TβRI) and type II (TβRII) receptors, a transmembrane serine/threonine kinase complex that ultimately phosphorylates the receptor-activated Smads (R-Smads), which include Smad2 and Smad3 (47). The R-Smads then heteromultimerize with Smad4 and translocate to the nucleus to regulate TGF-β target genes, including type-I collagen (65). Studies from our laboratory and by others show that multiple additional signaling pathways are modulated by TGF-β and are required for fibrogenesis induced by this growth factor (44). These pathways include, among others, the p38 and c-Jun NH2-terminal kinase (JNK; Ref. 27), the ERK mitogen-activated protein kinase (MAPK; Ref. 18), and the phosphatidylinositide 3-kinase-Akt (PI3K/AKT)-mammalian target of rapamycin (mTOR) pathways (15, 21, 33, 34, 51).
In recent years multiple studies have shown an important role for mTOR in kidney disease progression (22, 37). mTOR is the protein kinase component of two distinct complexes with unique activation processes and target proteins (3, 36, 41). mTOR complex 1 (mTORC1) includes Raptor, mLST8, Daptor, and PRAS40 and is a major target of the phosphatidylinositol (3,4,5)-triphosphate kinase (PIP3)/AKT pathway. mTORC1 regulates protein synthesis and cell growth, and autophagy by phosphorylating its two major targets, S6 kinase 1 (S6K1) and 4E related protein (4ERP). In addition, mTORC1 activates several transcription factors including HIF-1α, peroxisome proliferator-activated receptor (PPAR)-γ, and PPAR-γ coactivator-1α (PGC-1α), that regulate genes controlling angiogenesis, mitochondrial function and adipogenesis, among many others (40). mTOR complex 2 (mTORC2) includes Rictor, mLST8, Daptor, and mSIN; its activation process is poorly understood and its main targets include AKT itself and protein kinase Cα (PKCα; Ref. 46).
While most studies relate mTOR signaling to cell hypertrophy contributing to kidney fibrosis, several reports have shown that mTORC1 and mTORC2 are activated by TGF-β and that this activation is essential for its profibrotic effects on collagen production (11, 34, 49, 57). Furthermore, glomerular mTORC1 activity was increased in several disease models and this activation seemed to be correlated with the severity of the pathological process (4, 17, 23, 63). In other studies, pharmacologic and genetic manipulation that decreased mTORC1 activity was shown to delay disease progression and prevent glomerular fibrotic changes (4, 31, 50, 66, 69). In contrast, clinical data suggest that rapamycin may have deleterious effect in patients with advanced kidney disease (6).
Recently, we reported that HIF-1α participates in normoxic fibrogenic signaling by TGF-β (1). Here, we present data suggesting that mTORC1 is activated by TGF-β in human glomerular mesangial cells in a Smad3 COOH-terminal phosphorylation-dependent fashion and that this activation has a major role in the process of normoxic HIF activation by TGF-β. We also found that mTORC1 activity is important for collagen expression induced by TGF-β and this effect is at least partly mediated by HIF activity, since overexpression of HIF-1α overcame the effect of mTORC1 inhibition on TGF-β-induced collagen expression. These data suggest a TGF-β-Smad3-mTOR-HIF signaling cascade in normoxic mesangial cell fibrogenesis.
MATERIALS AND METHODS
Materials.
Active recombinant TGF-β1 (R&D Systems, Minneapolis, MN) was reconstituted as a 4-μg/ml stock solution in 4 mM HCl with 1 mg/ml bovine serum albumin. Antibodies were purchased from the following vendors: goat anti-mouse IgG-horseradish peroxidase (HRP) and mouse anti-goat IgG-HRP antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); anti-rabbit IgG-HRP from Promega (Madison, WI); mouse anti-β-actin from Sigma (St. Louis, MO); mouse anti-HIF-1α from BD Lifesciences (Franklin Lakes, NJ); and rabbit phospho-Akt (Ser-473), raptor, rictor, phospho-Smad3 (Ser 423/425), Smad3, phospho-S6 kinase (Thr-389), S6 kinase, phospho S6 ribosomal protein (S6RP) (Ser-235/236), S6RP, and Akt antibodies were purchased from Cell Signaling Technology (Danvers, MA). Goat anti-type I collagen antibody was obtained from SouthernBiotech (Madison, WI).
The TβRI ALK5 inhibitor SB431542 was purchased from Calbiochem/EMD (La Jolla, CA). Wortmannin and rapamycin were purchased from Santa-Cruz Biotechnology. Puromycin was purchased from Sigma and used at 3 μg/ml.
Plasmid constructs.
The −378COL1A2-Luc construct containing the sequence 378 bp of the α2 (I) collagen promoter and 58 bp of the transcribed sequence fused to the luciferase reporter gene was constructed as previously described (48). The hypoxia-responsive element (HRE)-Luc construct (trimeric units of HRE sequences cloned into the pGL2 basic vector) was a gift from J. M. Leiden (7). Wild-type (WT) Smad3 and dominant negative (DN) Smad3A in pEXL vector were obtained from H. F. Lodish and X. Liu (38). A nondegradable HIF-1α mutant (68) (Addgene plasmid 18955), where proline residues 402 and 564 are substituted with alanines was a kind gift from W. Kaelin. Myc-tagged S6K1 (14) was a gift of J. Chen (Addgene plasmid 26610), and active Rheb in which Asparagine at the 153 was changed to threonine (61) was a gift from F. Tamanoi (Addgene plasmid 19997). These last three plasmids were obtained through Addgene (http://www.addgene.org). pcDNA3 vector was purchased from Invitrogen (Carlsbad, CA). shRNA for Smad3, Raptor, and pGIPZ containing scrambled shRNA were purchased from Thermo Scientific (Waltham, MA).
Cell culture.
Primary human mesangial cells (HMC) were isolated from the normal margin of nephrectomized kidney as previously described (54) and used for experiments at passages 6–8. Transformed human mesangial cells (tHMC) were a gift from A. Sorokin (59). HMC were grown in DMEM/F-12 supplemented with 10% heat-inactivated cosmic calf serum (CCS), glutamine, sodium pyruvate, penicillin-streptomycin, insulin, and HEPES buffer. tHMC were grown in DMEM/F-12 supplemented with 10% heat-inactivated FBS, glutamine, penicillin-streptomycin, amphotericin B, insulin, and HEPES buffer. Human embryonic kidney cells (HEK) were grown in DMEM supplemented with 10% heat-inactivated FBS, glutamine, penicillin-streptomycin, insulin, and HEPES buffer.
Stable Smad3 knockdown cell line.
To generate stable knockdown cell lines, pGIPZ clones (RHS4349, empty vector; V2LHS 251359, smad3) were obtained from Thermo Scientific. These constructs were first subjected to CaPO4 transfection for lentiviral packaging in HEK293 FT cells (Invitrogen) using psPAX2 and pMD2.G according to the manufacturer's protocol. tHMC cells were incubated with viral supernatants, and after 48 h, puromycin was added in a concentration previously determined to result in 100% cell death of noninfected tHMC cells to select infected cells. Once the cells reached confluence, they were frozen or used for experiments up to passages 4 in the continual presence of puromycin.
Cell treatments.
Cells were changed to medium containing 0.33% serum 24 h before treatment. In experiments involving hypoxia, cells were exposed to normoxia (21% O2-5% CO2) or hypoxia (1.5% O2-5% CO2-93.5% N2), carried out in a glove-box hypoxia chamber (Coy Laboratory Products, Grass Lake, MI); in experiments involving inhibitors, cells were treated with 10 μM SB431542, 20 μM, rapamycin, or DMSO as vehicle for 30 min before addition of TGF-β1 or vehicle.
Preparation of cell lysates and Western blot analysis.
Cells were washed twice with ice-cold PBS and lysed on ice in RIPA buffer (50 mM Tris·HCl pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS) containing serum protease and phosphatase inhibitors (Sigma). Cleared cell lysates were subjected to SDS-PAGE (8–10% polyacrylamide gels), transferred onto PVDF membranes (Millipore, Bedford, MA), and probed with antibodies as indicated in Figs. 1–7. Densitometric analysis was performed using the NIH Image 1.61 program for Windows.
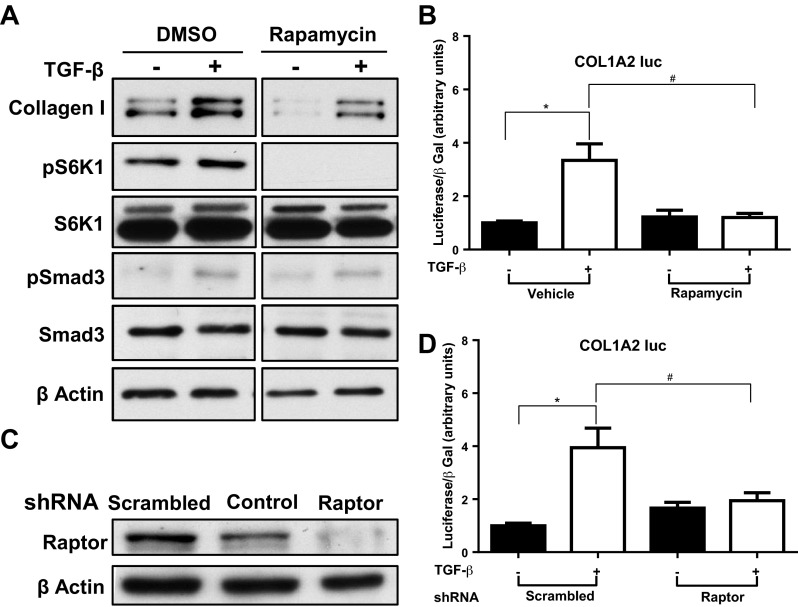
Fig. 1.
Mammalian target of rapamycin complex (mTORC)1 inhibition reduces transforming growth factor (TGF)-β-induced type I collagen expression in human mesangial cells (HMC). A: HMC were treated with mTORC1-specific inhibitor (rapamycin 10 nM) or DMSO for 30 min and then with TGF-β1 (1 ng/ml) or vehicle for 24 h. Whole cell lysate was analyzed by Western blot for type-I collagen. Each experiment was performed at least 3 times, and data from a representative experiment are shown. B: HMC were transfected with the COL1A2 promoter construct and treated in triplicate with rapamycin 10 nM or DMSO for 30 min and then TGF-β1 (1 ng/ml) or vehicle for 24 h. Luciferase assay values corrected for transfection efficiency using β-galactosidase (β-gal) controls, are shown (means ± SE). C: HMC were transduced with lentiviral particles containing short-hairpin (sh)RNA to Raptor or scrambled control, and knockdown of Raptor was evaluated by Western blotting with whole cell lysate harvested after 48-h incubation with the shRNA. D: HMC were cotransfected with the COL1A2 promoter construct and shRNA for Raptor or scrambled shRNA. After 20 h the cells were treated with TGF-β1 (1 ng/ml) or vehicle for 24 h. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE). Results shown in the graphs are the combination of 3 separate experiments (*P <0.05, #P <0.05).
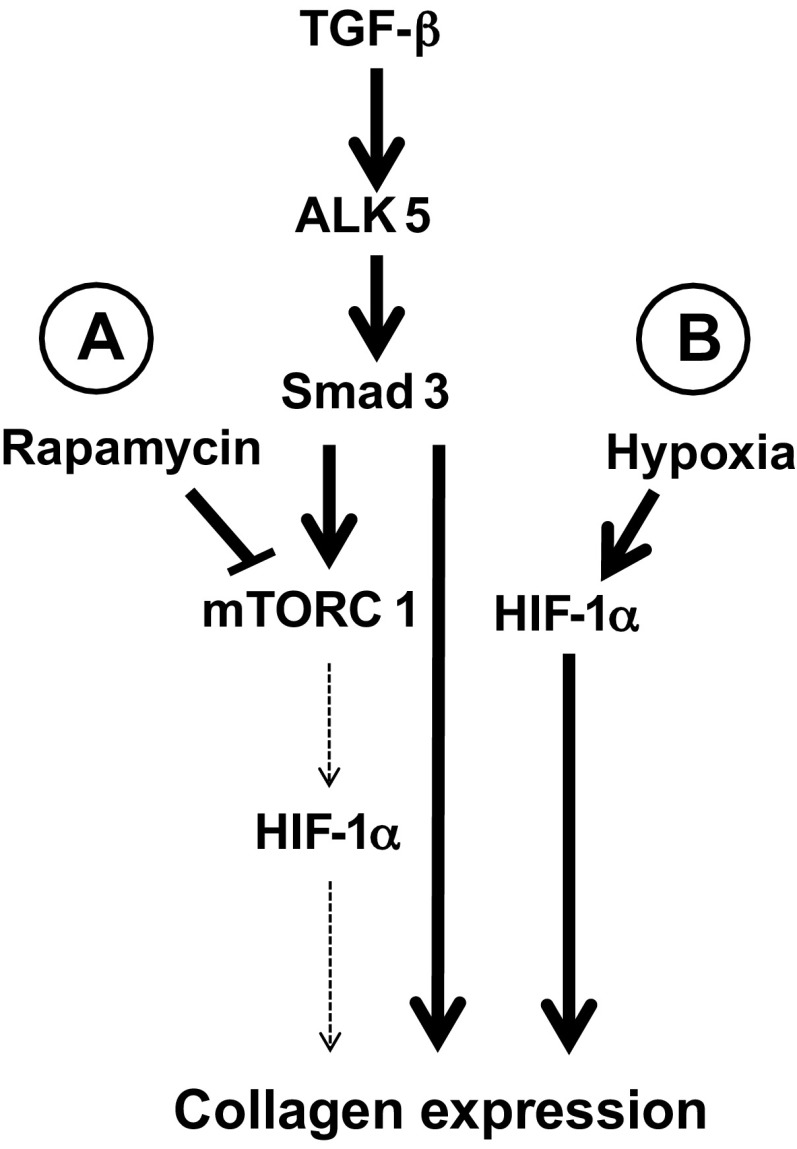
Fig. 7.
Pathways leading to collagen expression. TGF-β induces Smad3 COOH-terminal phosphorylation in an ALK5-dependent fashion. Smad3 translocates to the nucleus and mediates collagen expression in addition to increasing HIF-1α expression in an mTORC1 dependent fashion. Both Smad3 and HIF-1α are required for maximal TGF-β-induced collagen expression. A: under normoxia, mTOR1 inhibition using rapamycin prevents TGF-β-induced HIF-1α expression and reduces collagen expression. B: under hypoxia (or hypoxia mimic), HIF-1α is stabilized in a manner that is independent of mTORC1. As a result, mTORC1 inhibition does not affect TGF-β-induced collagen expression.
Transfection.
HMC were plated in triplicate in six-well plates at 1.2 × 105 cells per well. Eighteen to twenty-four hours later, cells were switched to 1% CCS medium and transfected with 0.5 μg of the indicated constructs along with CMV-SPORT-β-galactosidase (Invitrogen/GIBCO-BRL) as a control for transfection efficiency. Transfection was performed with the X-tremeGENE HP transfection reagent (Roche Applied Science, Indianapolis, IN) according to the company protocol. After 3–20 h of transfection, 1 ng/ml of TGF-β1 or vehicle was added to the culture medium. After a 24-h incubation, cells were harvested with 200 μl of reporter lysis buffer and luciferase and β-galactosidase activities were measured with a commercial kit (Promega), as directed.
Immunoprecipitation.
HMC were transfected with Smad3 along with Myc-tagged S6K1 (0.5 μg each), incubated for 24 h with 0.33% CCS-containing media, and then lysed as described above. Cleared cell lysate was incubated with anti Myc antibody for 12, and 30 μl of protein G-conjugated agarose beads (Invitrogen) were added and incubated for 1 h and then washed three times with RIPA buffer. Precipitated proteins were eluted by addition of 30 μl sample buffer and boiling for 5 min.
Statistics.
All data are expressed as means ± SE. Differences of data between groups were determined by two-way ANOVA, with Bonferroni post hoc tests using Prism 4.0 (www.graphpad.com). Values of P = 0.05 or smaller were considered significant.
RESULTS
mTOR inhibition reduces TGF-β-stimulated type-I collagen expression.
To determine the significance of mTOR activation by TGF-β in kidney fibrogenesis, we evaluated the effect of mTOR inhibition on collagen expression and COL1A2 promoter activity. Treatment with TGF-β increased collagen-I protein expression significantly. Rapamycin, a specific mTORC1 inhibitor, reduced both the unstimulated and stimulated collagen-I expression (Fig. 1A). Smad3 phosphorylation by TGF-β and inhibition of S6K1 phosphorylation by rapamycin confirmed the efficacy of these reagents. In quantification of four individual experiments, stimulation with TGF-β significantly increased collagen-I protein by 1.8-fold (P < 0.001) in vehicle-treated HMC, while in rapamycin-treated cells the increase by TGF-β was not statistically significant (P = 0.29). As we hypothesized that mTORC1 modulates HIF-mediated, TGF-β-induced collagen transcription, we first evaluated collagen promoter-reporter activation by TGF-β. TGF-β induced a threefold increase in COL1A2-Luc reporter activity (Fig. 1B). Rapamycin did not change the unstimulated activity but totally prevented the TGF-β-stimulated activity. To further evaluate the role of mTORC1 in TGF-β-induced collagen expression, we used shRNA for Raptor, an essential component of mTORC1. The efficacy of the shRNA was validated by transducing HMC with lentiviral particles containing Raptor shRNA or scrambled shRNA for 48 h and demonstrating reduced Raptor protein expression by Western blot (Fig. 1C). Whereas stimulation with TGF-β increased COL1A2 promoter activity by fourfold, disruption of mTORC1/Raptor signaling abolished the TGF-β stimulated increase, without affecting basal activity (Fig. 1D).
TGF-β induces mTORC1 activity by an ALK5-dependent mechanism.
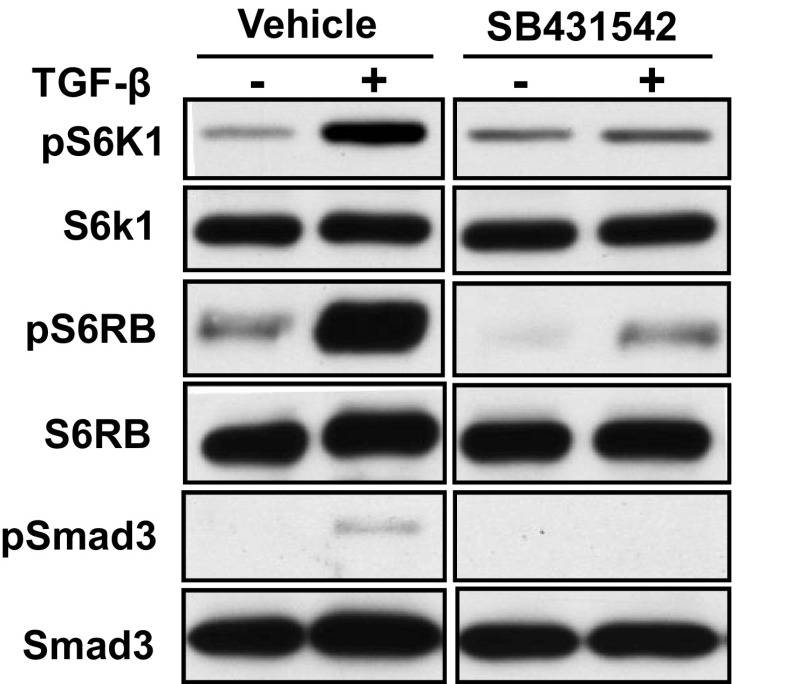
We evaluated the effect of TGF-β stimulation on mTORC1 activity by measuring the phosphorylation of S6K1 and S6 ribosomal protein in response to TGF-β in HMC. S6K1 phosphorylation was significantly increased by treatment with TGF-β (Fig. 2, top). Inhibition of TβRI kinase activity by SB431542 did not affect the unstimulated level but completely prevented the increase with TGF-β stimulation. These results suggest that ALK5/TβRI activity is required for TORC1 activation by TGF-β.
Fig. 2.
TGF-β1 induces mTORC1 activity by a TβRI-dependent mechanism. HMC were treated with the TβRI receptor (ALK5) kinase inhibitor (SB431542; 5 mM) or DMSO for 30 min and then with TGF-β1 (1 ng/ml) or vehicle for 6 h. Whole cell lysate was analyzed by Western blot for phosphorylated S6K1. A set of results from a representative experiment is shown from 3 independent experiments.
TGF-β-induced TORC1 activation requires Smad3 COOH-terminal phosphorylation.
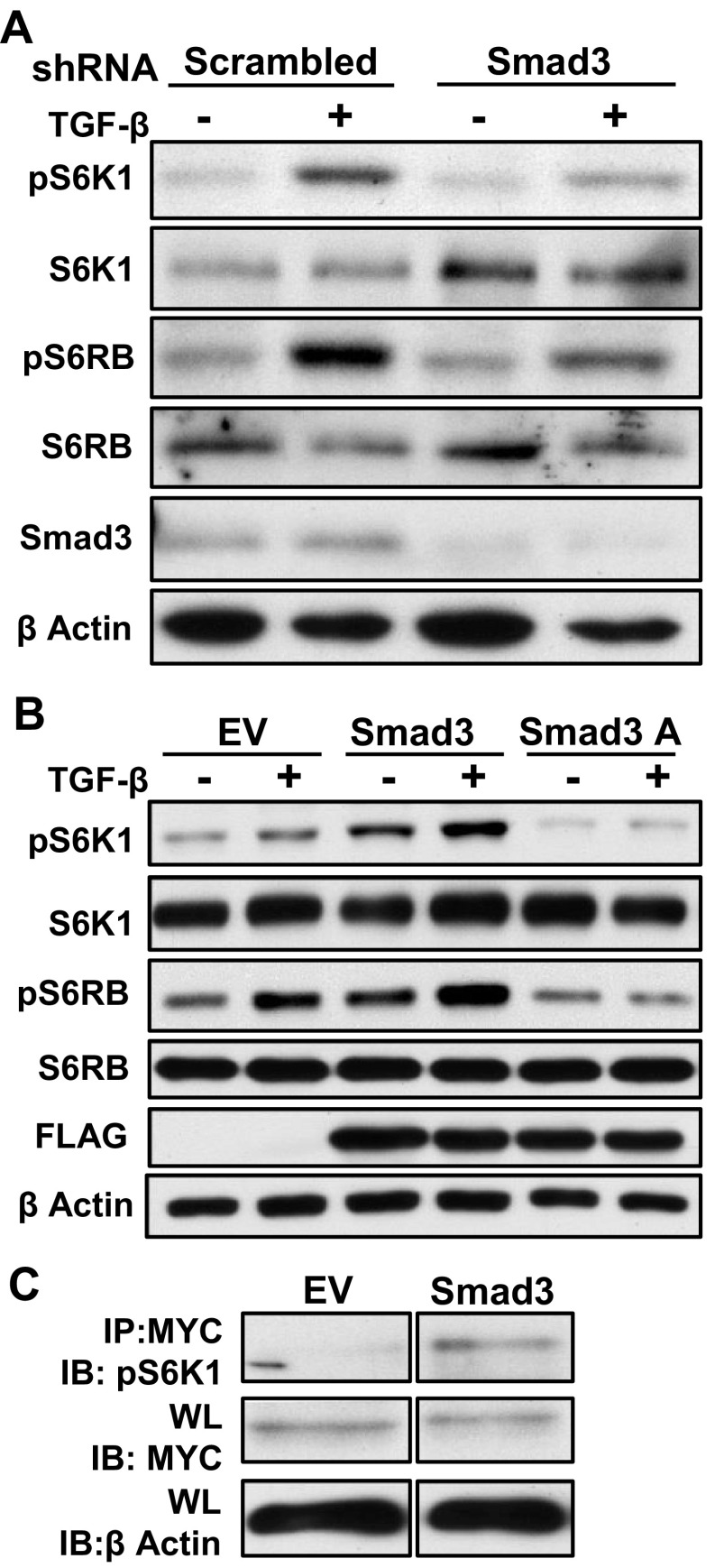
Since TGF-β-dependent mTORC1 activation is inhibited by an ALK5 antagonist, we speculated that Smad3, a main target of TβR that is active in fibrosis (48), is required for mTORC1 activation. To test this hypothesis, we silenced or overexpressed Smad3 in HEK 293 cells. Smad3 levels were reduced by the shRNA for Smad3 compared with the scrambled shRNA, and consequently, TGF-β-induced, but not basal, phosphorylation of S6K1 and S6RP was prevented or reduced (Fig. 3A). As the TβR phosphorylates Smad3 at distinct COOH-terminal sites, we also evaluated whether the COOH-terminal phosphorylation is required for mTORC1 activation by TGF-β. HEK cells were transiently transfected with pEXL empty vector, a construct containing WT Smad3 or a DN Smad3 (Smad3A), in which the COOH-terminal phospho-acceptor serines are replaced with alanine, interfering with TβR activation of endogenous Smad3. Overexpressing WT Smad3 in HEK cells increased S6K1 phosphorylation in both the unstimulated cells and the TGF-β exposed cells. Conversely, overexpression of Smad3A totally abolished TGF-β stimulated S6K1 phosphorylation, without affecting nonstimulated phosphorylation levels (Fig. 3B). To verify these results in primary mesangial cells, in which transfection efficiency is ∼10%, we cotransfected HMC with Myc-tagged S6K1 and pEXL empty vector or WT Smad3 and evaluated S6K1 phosphorylation of the immunoprecipitated, Myc-tagged molecules. As anticipated from the results in HEK cells, S6K1 phosphorylation was also increased by WT Smad3 overexpression in HMC (Fig. 3C). These results imply that TGF-β-induced mTORC1 phosphorylation requires Smad3 COOH-terminal phosphorylation.
Fig. 3.
TGF-β-induced TORC1 activation requires Smad3 COOH-terminal phosphorylation. A: human embryonic kidney (HEK) cells were transfected with shRNA for Smad3 or scrambled shRNA for 24 h and treated with TGF-β1 (1 ng/ml) or vehicle for 2 h. Whole cell lysate was analyzed by Western blot for phosphorylated S6K1 and S6RP. Effective knockdown of Smad3 was confirmed (bottom). B: HEK were transfected with FLAG-tagged wild-type (WT) Smad3, FLAG-tagged dominant negative Smad3 construct (Smad3A), or pEXL empty vector (EV) for 24 h and then treated with TGF-β1 (1 ng/ml) or vehicle for 2 h. Whole cell lysate was analyzed by Western blot for phosphorylated S6K1 and S6RP. Transfection efficiency was evaluated by western blot analysis for FLAG. C: HMC were cotransfected with Myc-tagged S6K1 and WT Smad3 or pEXL EV for 24 h. Cell lysates were immunoprecipitated (IP) with anti Myc antibody and phosphorylated S6K1 was detected by immunobloting (IB). Each experiment was performed at least 3 times, and data from a representative experiment are shown. WL, whole lysate.
TGF-β-induced HIF expression is mTORC1 dependent.
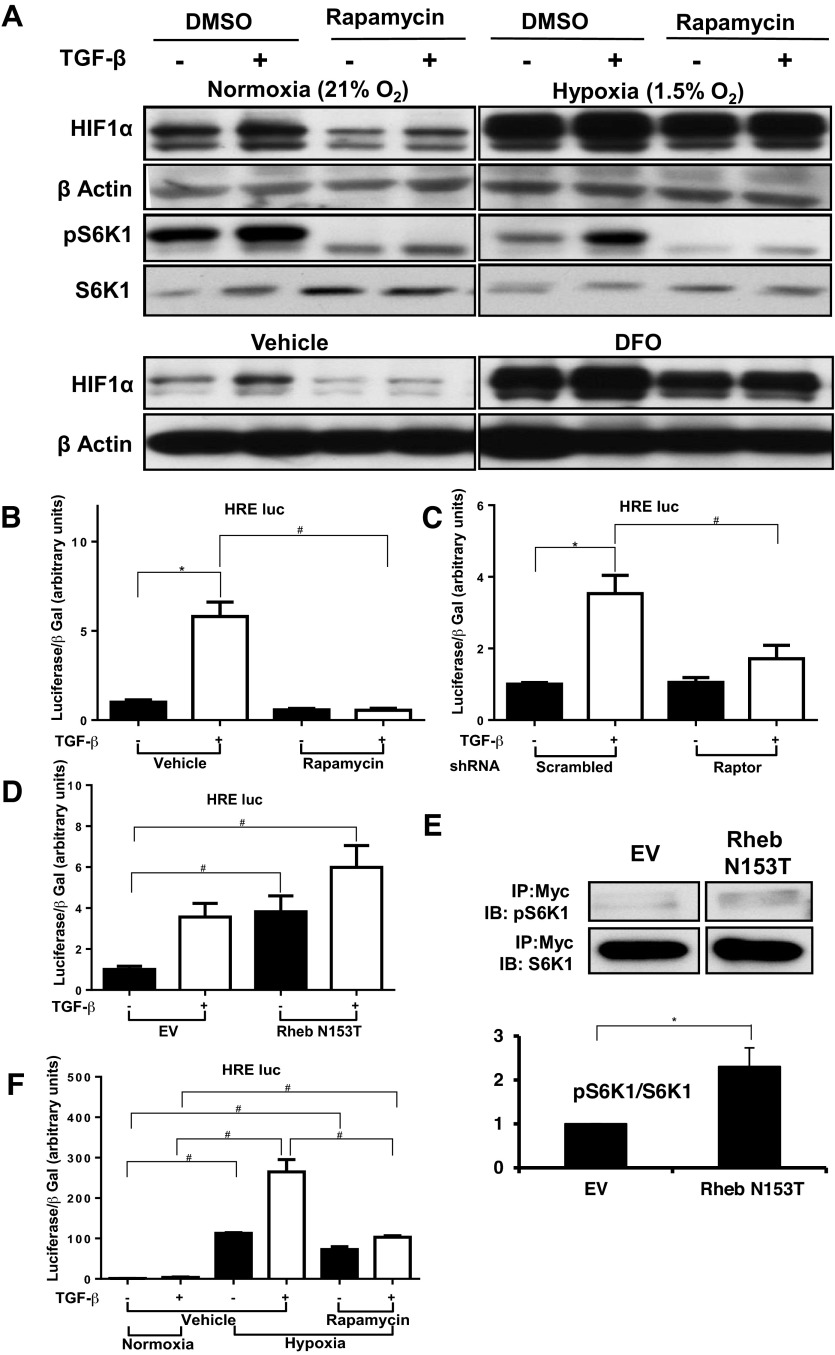
Previous work from our laboratory identified HIF-1 and HIF-2 as important factors in kidney cell type-I collagen expression by a human proximal tubular cell line (2) and by HMC (Hanna C, et al., unpublished observations). Since mTORC1 is known to regulate HIF activity (42), we speculated that the effect of mTORC1 inhibition on collagen expression (Fig. 1A) might be explained by its preventing TGF-β-induced HIF expression. First, we evaluated whether mTORC1 inhibition reduces TGF-β-induced HIF-1α protein expression. In normoxia (21% O2-5% CO2), HIF-1α protein expression was increased by TGF-β treatment (Fig. 4A), but rapamycin totally prevented this phenomenon. In contrast, under hypoxia (1.5% O2-5% CO2) or in the presence of the hypoxia mimetic deferoxamine (DFO), rapamycin did not affect the increased HIF-1α protein expression but still prevented the further increase with TGF-β treatment. Rapamycin also significantly reduced the TGF-β-induced HRE-luc activity in normoxia, which reflects HIF upregulation and subsequent binding to a target gene (Fig. 4B). As anticipated from the effect of rapamycin, disruption of mTORC1 by shRNA targeting Raptor also prevented the TGF-β-induced increases in normoxic HRE-luc activity (Fig. 4C). In HMC transfected with a constitutively active Rheb construct that activates mTORC1 (61), unstimulated HRE-luc activity was fourfold higher than in HMC transfected with an empty vector (Fig. 4D), while the TGF-β-stimulated activity was not significantly changed. The efficacy of the active Rheb was verified by transfecting HMC with the active Rheb as well as Myc-tagged S6K1 and evaluating the phosphorylation of the tagged S6k1S6K1 after immunoprecipitation with anti Myc antibody (Fig. 4E). All of these results suggest that normoxic activation of HIF by TGF-β depends on mTORC1 activation, including Rheb activity. Under hypoxia, the predominant mechanism for HIF upregulation (24) is inactivation of prolyl hydroxylase (PHD) that hydroxylates HIFs and subjects them to degradation. Here, we tested whether the mTORC1 pathway also modulates HIF expression in hypoxia. Hypoxia increased HRE-luc activity 100-fold and TGF-β stimulation further increased it by 2.5-fold (Fig. 4F, middle 2 bars). Rapamycin did not change the effect of hypoxia (67-fold increase) but totally prevented the enhancement by TGF-β either under hypoxia (right 2 bars). These results indicate that mTORC1 plays a role in HIF-1α expression stimulated by TGF-β in normoxia but not in HIF-1α stimulated by hypoxia.
Fig. 4.
TGF-β-induced HIF-1α expression under normoxia is mTORC1 dependent. A: HMC were treated with rapamycin 10 nM or DMSO for 30 min, treated with TGF-β1 (1 ng/ml) or vehicle, and then exposed to hypoxia (1.5% O2-5% CO2) or normoxia (21% O2-5% CO2; top 4 rows) or deferoxamine (DFO; 100 μM) (bottom 2 rows) for 6 h. Whole cell lysate was analyzed by Western blot for HIF-1α nd pS6K1. Each experiment was performed at least 3 times, and data from a representative experiment are shown. B: HMC were transfected with the HIF-response element (HRE)-luc construct and treated in triplicate with rapamycin 10 nM or DMSO for 30 min and then TGF-β1 (1 ng/ml) or vehicle for 24 h. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE). C: HMC were cotransfected with the HRE-luc construct and shRNA for Raptor or with scrambled shRNA. After 20 h the cells were treated with TGF-β1 (1 ng/ml) or vehicle. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE). D: HMC were transfected with the HRE-luc construct and treated in triplicate with rapamycin (10 nM) or DMSO for 30 min, followed by addition of TGF-β1 (1 ng/ml) or vehicle, and then exposed to hypoxia (1.5% O2-5% CO2) or normoxia (21% O2-5% CO2) for 24 h. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls (means ± SE). E: HMC were cotransfected with Myc-tagged S6K1 and construct containing the constitutively active mutant of Rheb (N153T) or pcDNA3 EV for 24 h. Cell lysates were immunoprecipitated with anti-Myc antibody and phosphorylated S6K1was detected by immunobloting. A representative experiment (top) and quantification of 3 experiments (bottom) are shown. F: HMC were cotransfected with the HRE-luc construct and construct containing the constitutively active mutant of Rheb (N153T) or control pcDNA3 EV. Cells were treated with TGF-β1 (1 ng/ml) or vehicle for 24 h in triplicate. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE). Results shown for all graphs represent combination of 3 separate experiments (*P <0.05, #P <0.05).
TGF-β-induced HIF expression is Smad3 and TORC1 dependent.
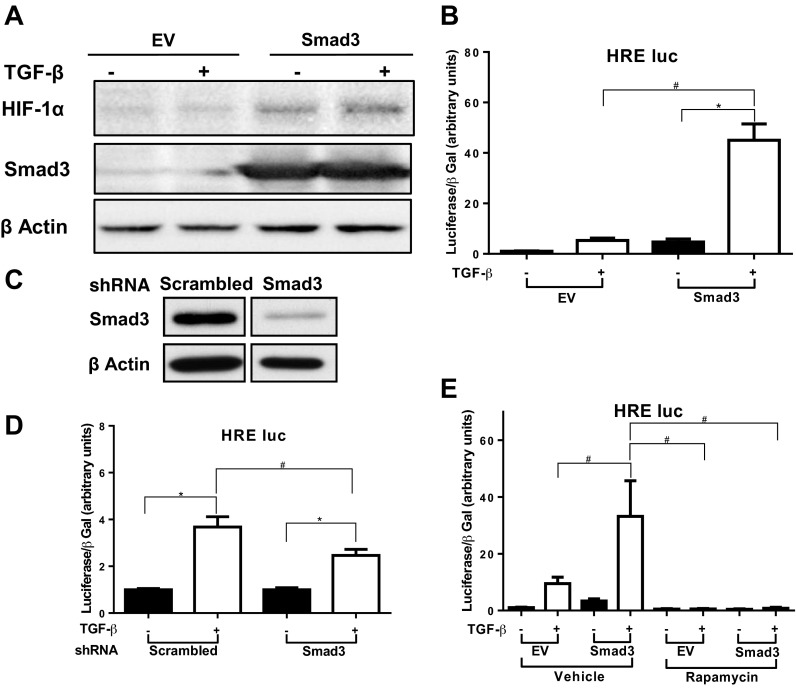
As Smad3 COOH-terminal phosphorylation is needed for TGF-β-induced mTORC1 activity and mTORC1 activity is essential for TGF-β-induced HIF expression and activity, we evaluated the role of Smad3 in mTORC1-mediated HIF activity. In HEK, overexpression of Smad3 increased both unstimulated and TGF-β-induced HIF-α protein expression (Fig. 5A). In a similar fashion, Smad3 overexpression increased HRE activity in both unstimulated and TGF-β-treated HMC (Fig. 5B). To further evaluate roles for Smad3 in HIF activity, we generated tHMC stably transfected with shRNA for Smad3 or scrambled shRNA. Smad3 levels were reduced in the Smad3 shRNA-transfected cells as anticipated (Fig. 5C). TGF-β-induced HRE activity was partially but significantly decreased in these cells (Fig. 5D). To evaluate whether Smad3-induced HIF expression is mTORC1 dependent we overexpressed Smad3 in HMC and treated the cells with rapamycin. Treatment with rapamycin drastically reduced HRE activity both in the empty vector-transfected cells and the cells overexpressing Smad3, with no difference in fold change between the WT and the empty vector-transfected cells (Fig. 5E). These results suggest that Smad3 plays a central role in TGF-β-induced HIF expression in an mTORC1-dependent manner.
Fig. 5.
TGF-β induces increased HIF expression in a Smad3- and TORC1-dependent manner. A: HEK were transfected with FLAG-tagged WT Smad3 or pEXL EV for 24 h and then treated with TGF-β1 (1 ng/ml) or vehicle for 6 h. Whole cell lysate was analyzed by Western blot for HIF-1α. Experiments were repeated 3 times, and sets of results from a representative experiment are shown. B: HMC were cotransfected with the HRE-luc construct and a construct containing WT Smad3 or pcDNA3 EV and then treated with TGF-β1 (1 ng/ml) or vehicle for 24 h. Luciferase assay results, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE). C: transformed human mesangial (tHMC) cells stably expressing scrambled shRNA or shRNA directed against Smad3 lysate were analyzed by Western blot for Smad3. D: Smad3 knockdown tHMC cells or control were transfected with the HRE-luc construct and treated in triplicate with TGF-β1 (1 ng/ml) or vehicle for 24 h. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE). E: HMC were cotransfected with the HRE-luc construct and construct containing WT Smad3 or pEXL EV. Cells were treated with rapamycin (10 nM) or vehicle for 30 min and then with TGF-β1 (1 ng/ml) or vehicle for 24 h. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE). Each luciferase experiment was performed in triplicate. Results shown for all graphs represent combination of 3 separate experiments (*P <0.05, #P <0.05).
HIF-1α overexpression abolishes the effect of mTORC1 inhibition on collagen expression.
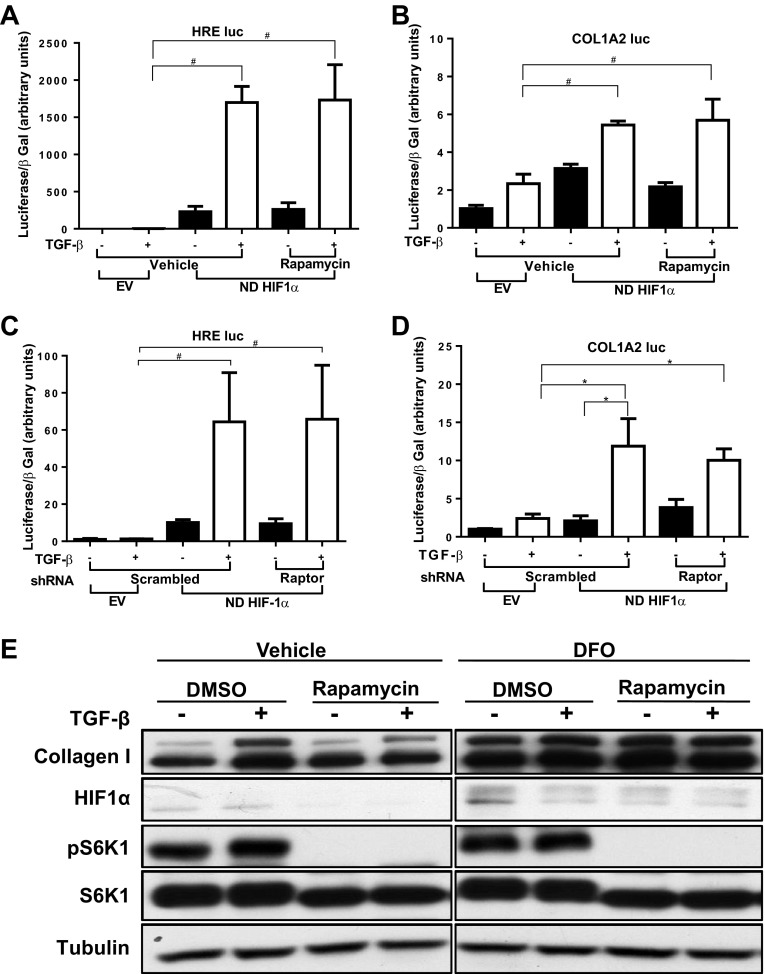
Previous results from our laboratory show that HIF expression and activity are essential for TGF-β-induced collagen production. Inhibition of HIF-1α abolishes the TGF-β-induced COL1A2 promoter activity in HKC (2). Inhibition of HIF-1α or HIF-2α had the same effect in HMC (Hanna C, et al., unpublished observations). As our results show that TGF-β-induced HIF activity is mTORC1 dependent, we evaluated whether overexpression of HIF-1α might bypass the effect of mTOR inhibition on collagen expression in mesangial cells. As anticipated, HRE-luc activity was greatly increased by overexpression of nondegradable (ND) HIF-1α, and rapamycin no longer inhibited this response (Fig. 6A). These changes in HIF activity were reflected by those of COL1A2-luc activity induced by TGF-β (Fig. 6B). We then tested whether the effect of mTORC1 disruption on collagen I expression can be reversed by ND-HIF-1α overexpression. Similar to the chemical inhibition of mTOR, disruption of mTORC1 by shRNA targeting Raptor did not inhibit either HRE activity or COL1A2-luc activation by TGF-β in the presence of ND-HIF-1α (Fig. 6, C and D). Finally, we evaluated the role of mTOR in type-I collagen protein expression by HMC treated with DFO, which prevents HIF degradation by inhibiting prolyl hydroxylase (20). As TGF-β-induced HIF-1α expression peaks within 6 h and returns to baseline after 24 h, HIF-1α was not increased at the time point when collagen protein expression was examined (24 h). Treatment with TGF-β increased collagen I protein expression, and this response was prevented by rapamycin treatment (Fig. 6E). DFO increased both unstimulated and TGF-β-stimulated collagen expression and prevented the effect of mTORC1 inhibition with rapamycin.
Fig. 6.
Inhibitory effect of mTORC1 inhibition on collagen expression can be reversed by HIF-1α overexpression. A and B: HMC were cotransfected with the HRE-luc construct (A) or COL1A2-luc construct (B) and a construct containing nondegradable (ND) HIF-1α or pcDNA3 EV. Cells were treated in triplicate with rapamycin (10 nM) or DMSO for 30 min followed by addition of TGF-β1 (1 ng/ml) or vehicle for 24 h. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE). Results shown are the combination of 2 separate experiments (*P < 0.05, #P < 0.05). C and D: HMC were cotransfected with the HRE-luc construct (C) or COL1A2-luc construct (D), a construct containing nondegradable HIF-1α or pcDNA3 EV, and shRNA for Raptor or control scrambled shRNA. After 20 h the cells were treated with TGF-β1 (1 ng/ml) or vehicle for 24 h. Luciferase assay values, corrected for transfection efficiency using β-galactosidase controls, are shown (means ± SE; *P <0.05, #P <0.05). Each luciferase experiment was performed in triplicate. Results shown are combination of 2 separate experiments (*P < 0.05, #P < 0.05). E: HMC were treated with rapamycin (10 nM) or vehicle for 30 min, then treated with deferoxamine (DFO) 100 μM or vehicle for 30 min, followed by addition of TGF-β1 (1 ng/ml) or vehicle for 24 h. Whole cell lysate was analyzed by Western blot for collagen I. Representative set of immunoblots is shown from 3 separate experiments.
Together, these results suggest that TGF-β activates mTORC1 in an ALK5 and Smad3-dependent manner and this activation is essential for normoxic HIF expression, which plays a major role in TGF-β-induced collagen expression (Fig. 7).
DISCUSSION
HIFs have been implicated in a steadily increasing spectrum of biological and pathological processes. Our previous results showed that TGF-β stimulation causes normoxic expression of HIF-1α and this process is essential for optimal type-I collagen expression in renal tubular epithelial cells (2) and mesangial cells (Hanna C, et al., unpublished observations). As no change in HIF stability (1) or mRNA expression was found, we hypothesized that mTORC1, a known activator of HIF translation, might play a major role in TGF-β-induced normoxic HIF expression and, consequently, collagen production. Indeed, TGF-β stimulation of cultured human mesangial cells increased mTORC1 activity in a Smad3 COOH-terminal phosphorylation-dependent fashion. Further, normoxic, TGF-β-induced HIF-1α activation is Smad3 and mTORC1 dependent and is necessary for collagen production under normoxic conditions. In contrast, hypoxia-induced HIF1α activation does not require mTORC1 activity. Further, overexpression of HIF-1α abolishes the effect of mTORC1 inhibition on TGF-β-induced collagen expression. These results suggest the importance of a TGF-β/Smad3/TORC1/HIF pathway in at least one aspect of renal fibrogenesis. It should be noted that, although there appeared to be an increase in collagen protein expression in Fig. 1A, the average increase from four independent experiments was not statistically significant. On the other hand, collagen promoter activity was fully inhibited. This slight mismatch between collagen transcription and protein expression demonstrates the complexity of TGF-β effects on collagen expression that include, in addition to transcription, RNA stability, RNA translation, and protein stability.
The effect of Smad3 on mTORC1 activity is not surprising since there are known interactions, albeit with varying outcomes, between Smad3 and AKT, a major activator of mTOR (26, 60), and activation of PI3K, an activator of AKT, is required for TGF-β-induced collagen (21) and PAI-1 (12). Furthermore a recent report shows that silencing Smad3 using specific siRNA decreased mTORC1 and mTORC2 activity by preventing the suppression of deptor (10).
Given that TGF-β does not affect HIF-1α stability or mRNA expression, the dependence of TGF-β-induced HIF expression on mTORC1 is not unexpected, as mTOR is known to increase HIF-1α expression, probably through increased translation (35, 43). Although mTORC1 activity is required for TGF-β-induced HIF expression, our results do not rule out other pathways by which Smad3 might increase HIF activity beyond mTORC1 pathway. Activation of mTORC1 by overexpressing active Rheb did not fully increase HRE-luc activity in the absence of TGF-β, suggesting a non-mTORC1 mechanism by which TGF-β activates HIF in addition to the mTORC1 activation described here.
Several reports suggest that HIF has a role in kidney disease progression and fibrosis in kidney fibroblast (45) and such animal disease models as unilateral ureteral obstruction (19), 5/6 renal ablation (28), and angiotensin II infusion (64). Conversely, in other reports HIF was found to be protective in the same disease models of UUO and remnant nephropathy (29, 58). These differences may represent different effect of HIF on different cell types in the kidney, different time frames of the studies, different techniques used to enhance or reduce HIF activity, or different genetic backgrounds of the animal models. Our own published (2) and unpublished results show that both HIF-1 activity and HIF-2 activity may have significant roles in TGF-β-induced collagen expression. Thus preventing TGF-β-induced HIF expression might be of therapeutic benefit. Our present findings suggest that mTORC1 inhibition might be beneficial as it prevents TGF-β-induced HIF expression while basal expression and the response to hypoxia are preserved.
mTORC1 inhibition, by rapamycin or one of its analogs, or by disruption of the complex itself, was shown to be beneficial in several models of kidney disease (8, 17, 22, 23, 30, 32, 39) and in a model of TGF-β-induced peritoneal angiogenesis (55). However, in practice, the use of rapamycin and its analogs in patients with advanced kidney failure has had disappointing results, as the treatment tended to exacerbate proteinuria (6, 13, 25, 62), while treatment at an earlier stage often demonstrated favorable outcome (9, 52). We found in the present study that mTORC1 inhibition can prevent normoxic, TGF-β-induced HIF-1 expression and reduce collagen expression, whereas under hypoxia the reduction in HIF expression is modest and does not affect collagen expression. Collectively, somewhat conflicting data from various studies suggest that vascular disease and thus kidney hypoxia may have an influence on the response to mTORC1 inhibition. To address these questions, animal studies evaluating the effect of different modalities of mTOR inhibition on kidney disease progression should be carefully designed to distinguish events in normoxic conditions from those related to hypoxia as a consequence of the disease progression.
mTORC2 inhibition could affect TGF-β-induced collagen expression in a different manner than mTORC1 inhibition. Rahimi et al. (49) showed that disruption of mTORC2 by shRNA for Rictor reduced TGF-β-induced COL1A2 promoter activity in fibroblasts. Therefore, it also might be informative to compare the specific roles of mTORC1 and mTORC2 in TGF-β-induced collagen expression.
In summary, mTORC1 is activated by TGF-β in a Smad3-dependent manner, and this activation is essential for the normoxic HIF activation that is important in type-I collagen production by mesangial cells. Further research is needed to elucidate the relationships among TGF-β, mTOR, HIF, and collagen expression.
GRANTS
This work was supported by National Institute of Diabetes, Digestive, and Kidney Diseases Grant DK-049362 (to H. W. Schnaper) and National Cancer Institute Grant CA-77816 (to L. C. Platanias)
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.R.-Z., T.H., S.C.H., C.H., L.C.P., and H.W.S. conception and design of research; B.R.-Z. performed experiments; B.R.-Z., T.H., S.C.H., C.H., L.C.P., and H.W.S. analyzed data; B.R.-Z., S.C.H., C.H., L.C.P., and H.W.S. interpreted results of experiments; B.R.-Z. and T.H. prepared figures; B.R.-Z. drafted manuscript; B.R.-Z., T.H., S.C.H., C.H., L.C.P., and H.W.S. approved final version of manuscript; T.H., S.C.H., C.H., L.C.P., and H.W.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We appreciate thoughtful comments from the other members of the Schnaper Laboratory.
REFERENCES
- 1. Basu A, Menicucci G, Maestas J, Das A, McGuire P. Plasminogen activator inhibitor-1 (PAI-1) facilitates retinal angiogenesis in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 50: 4974–4981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1α and TGF-β/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol 300: F898–F905, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beauchamp EM, Platanias LC. The evolution of the TOR pathway and its role in cancer. Oncogene 2012 Dec 17 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4. Bonegio RG, Fuhro R, Wang Z, Valeri CR, Andry C, Salant DJ, Lieberthal W. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J Am Soc Nephrol 16: 2063–2072, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol 13: 2600–2610, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Bumbea V, Kamar N, Ribes D, Esposito L, Modesto A, Guitard J, Nasou G, Durand D, Rostaing L. Long-term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transplant 20: 2517–2523, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275: 25130–25138, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Chen G, Chen H, Wang C, Peng Y, Sun L, Liu H, Liu F. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One 7: e33626, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho ME, Hurley JK, Kopp JB. Sirolimus therapy of focal segmental glomerulosclerosis is associated with nephrotoxicity. Am J Kidney Dis 49: 310–317, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Das F, Ghosh-Choudhury N, Bera A, Dey N, Abboud HE, Kasinath BS, Choudhury GG. Transforming growth factor beta integrates Smad 3 to mechanistic target of rapamycin complexes to arrest deptor abundance for glomerular mesangial cell hypertrophy. J Biol Chem 288: 7756–7768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Das F, Ghosh-Choudhury N, Mahimainathan L, Venkatesan B, Feliers D, Riley DJ, Kasinath BS, Choudhury GG. Raptor-rictor axis in TGFbeta-induced protein synthesis. Cell Signal 20: 409–423, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Das F, Ghosh-Choudhury N, Venkatesan B, Li X, Mahimainathan L, Choudhury GG. Akt kinase targets association of CBP with SMAD 3 to regulate TGFbeta-induced expression of plasminogen activator inhibitor-1. J Cell Physiol 214: 513–527, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Diekmann F, Budde K, Oppenheimer F, Fritsche L, Neumayer HH, Campistol JM. Predictors of success in conversion from calcineurin inhibitor to sirolimus in chronic allograft dysfunction. Am J Transplant 4: 1869–1875, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294: 1942–1945, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Finer G, Schnaper HW, Kanwar YS, Liang X, Lin HY, Hayashida T. Divergent roles of Smad3 and PI3-kinase in murine adriamycin nephropathy indicate distinct mechanisms of proteinuria and fibrogenesis. Kidney Int 82: 525–536, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol 18: 2644–2648, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Godel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mor A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstadt H, Kerjaschki D, Cohen CD, Hall MN, Ruegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest 121: 2197–2209, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-beta1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int 56: 1710–1720, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hirsila M, Koivunen P, Xu L, Seeley T, Kivirikko KI, Myllyharju J. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J 19: 1308–1310, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Hubchak SC, Sparks EE, Hayashida T, Schnaper HW. Rac1 promotes TGF-β-stimulated mesangial cell type I collagen expression through a PI3K/Akt-dependent mechanism. Am J Physiol Renal Physiol 297: F1316–F1323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huber TB, Walz G, Kuehn EW. mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression. Kidney Int 79: 502–511, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Ruegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest 121: 2181–2196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Kamar N, Frimat L, Blancho G, Wolff P, Delahousse M, Rostaing L. Evaluation of the efficacy and safety of a slow conversion from calcineurin inhibitor- to sirolimus-based therapies in maintenance renal-transplant patients presenting with moderate renal insufficiency. Transpl Intl 20: 128–134, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11: 881–889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SI, Kwak JH, Zachariah M, He Y, Wang L, Choi ME. TGF-β-activated kinase 1 and TAK1-binding protein 1 cooperate to mediate TGF-β1-induced MKK3-p38 MAPK activation and stimulation of type I collagen. Am J Physiol Renal Physiol 292: F1471–F1478, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1α in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: F1023–F1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kobayashi H, Gilbert V, Liu Q, Kapitsinou PP, Unger TL, Rha J, Rivella S, Schlondorff D, Haase VH. Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol 188: 5106–5115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kramer S, Wang-Rosenke Y, Scholl V, Binder E, Loof T, Khadzhynov D, Kawachi H, Shimizu F, Diekmann F, Budde K, Neumayer HH, Peters H. Low-dose mTOR inhibition by rapamycin attenuates progression in anti-thy1-induced chronic glomerulosclerosis of the rat. Am J Physiol Renal Physiol 294: F440–F449, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Kurayama R, Ito N, Nishibori Y, Fukuhara D, Akimoto Y, Higashihara E, Ishigaki Y, Sai Y, Miyamoto K, Endou H, Kanai Y, Yan K. Role of amino acid transporter LAT2 in the activation of mTORC1 pathway and the pathogenesis of crescentic glomerulonephritis. Lab Invest 91: 992–1006, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Kurdian M, Herrero-Fresneda I, Lloberas N, Gimenez-Bonafe P, Coria V, Grande MT, Boggia J, Malacrida L, Torras J, Arevalo MA, Gonzalez-Martinez F, Lopez-Novoa JM, Grinyo J, Noboa O. Delayed mTOR inhibition with low dose of everolimus reduces TGFbeta expression, attenuates proteinuria and renal damage in the renal mass reduction model. PLoS One 7: e32516, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-beta-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci 125: 1259–1273, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol 178: 437–451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Land SC, Tee AR. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J Biol Chem 282: 20534–20543, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol 20: 2493–2502, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Liu X, Sun Y, Constantinescu SN, Karam E, Weinberg RA, Lodish HF. Transforming growth factor beta-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci USA 94: 10669–10674, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyo JM. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17: 1395–1404, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189: 1177–1201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10: 457–468, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10: 594–601, 2004 [DOI] [PubMed] [Google Scholar]
- 43. McInturff AM, Cody MJ, Elliott EA, Glenn JW, Rowley JW, Rondina MT, Yost CC. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood 120: 3118–3125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mu Y, Gudey SK, Landstrom M. NonSmad signaling pathways. Cell Tissue Res 347: 11–20, 2012 [DOI] [PubMed] [Google Scholar]
- 45. Norman JT, Clark IM, Garcia PL. Hypoxia promotes fibrogenesis in human renal fibroblasts. Kidney Int 58: 2351–2366, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle 10: 2305–2316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Piek E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-(beta) type I receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells. J Cell Sci 112: 4557–4568, 1999 [DOI] [PubMed] [Google Scholar]
- 48. Poncelet AC, de Caestecker MP, Schnaper HW. The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells. Kidney Int 56: 1354–1365, 1999 [DOI] [PubMed] [Google Scholar]
- 49. Rahimi RA, Andrianifahanana M, Wilkes MC, Edens M, Kottom TJ, Blenis J, Leof EB. Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-beta. Cancer Res 69: 84–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rangan GK, Coombes JD. Renoprotective effects of sirolimus in non-immune initiated focal segmental glomerulosclerosis. Nephrol Dial Transplant 22: 2175–2182, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-beta signaling through a direct interaction with Smad3. Nat Cell Biol 6: 358–365, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Schena FP, Pascoe MD, Alberu J, del Carmen Rial M., Oberbauer R., Brennan DC, Campistol JM, Racusen L, Polinsky MS, Goldberg-Alberts R, Li H, Scarola J, Neylan JF. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 87: 233–242, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-β signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 284: F243–F252, 2003 [DOI] [PubMed] [Google Scholar]
- 54. Schnaper HW, Kopp JB, Poncelet AC, Hubchak SC, Stetler-Stevenson WG, Klotman PE, Kleinman HK. Increased expression of extracellular matrix proteins and decreased expression of matrix proteases after serial passage of glomerular mesangial cells. J Cell Sci 109: 2521–2528, 1996 [DOI] [PubMed] [Google Scholar]
- 55. Sekiguchi Y, Zhang J, Patterson S, Liu L, Hamada C, Tomino Y, Margetts PJ. Rapamycin inhibits transforming growth factor beta-induced peritoneal angiogenesis by blocking the secondary hypoxic response. J Cell Mol Med 16: 1934–1945, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes 44: 1139–1146, 1995 [DOI] [PubMed] [Google Scholar]
- 57. Shegogue D, Trojanowska M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem 279: 23166–23175, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Song YR, You SJ, Lee YM, Chin HJ, Chae DW, Oh YK, Joo KW, Han JS, Na KY. Activation of hypoxia-inducible factor attenuates renal injury in rat remnant kidney. Nephrol Dial Transplant 25: 77–85, 2010 [DOI] [PubMed] [Google Scholar]
- 59. Sraer JD, Delarue F, Hagege J, Feunteun J, Pinet F, Nguyen G, Rondeau E. Stable cell lines of T-SV40 immortalized human glomerular mesangial cells. Kidney Int 49: 267–270, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Suwanabol PA, Seedial SM, Zhang F, Shi X, Si Y, Liu B, Kent KC. TGF-β and Smad3 modulate PI3K/Akt signaling pathway in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 302: H2211–H2219, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Urano J, Sato T, Matsuo T, Otsubo Y, Yamamoto M, Tamanoi F. Point mutations in TOR confer Rheb-independent growth in fission yeast and nutrient-independent mammalian TOR signaling in mammalian cells. Proc Natl Acad Sci USA 104: 3514–3519, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van den Akker JM, Wetzels JF, Hoitsma AJ. Proteinuria following conversion from azathioprine to sirolimus in renal transplant recipients. Kidney Int 70: 1355–1357, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Wang S, Wilkes MC, Leof EB, Hirschberg R. Noncanonical TGF-β pathways, mTORC1 and Abl, in renal interstitial fibrogenesis. Am J Physiol Renal Physiol 298: F142–F149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Z, Tang L, Zhu Q, Yi F, Zhang F, Li PL, Li N. Hypoxia-inducible factor-1alpha contributes to the profibrotic action of angiotensin II in renal medullary interstitial cells. Kidney Int 79: 300–310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wrana JL, Attisano L. The Smad pathway. Cytokine Growth Factor Rev 11: 5–13, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Wu MJ, Wen MC, Chiu YT, Chiou YY, Shu KH, Tang MJ. Rapamycin attenuates unilateral ureteral obstruction-induced renal fibrosis. Kidney Int 69: 2029–2036, 2006 [DOI] [PubMed] [Google Scholar]
- 67. Yamamoto T, Noble NA, Miller DE, Border WA. Sustained expression of TGF-beta 1 underlies development of progressive kidney fibrosis. Kidney Int 45: 916–927, 1994 [DOI] [PubMed] [Google Scholar]
- 68. Yan Q, Bartz S, Mao M, Li L, Kaelin WG., Jr The hypoxia-inducible factor 2 alpha N-terminal and C-terminal transactivation domains cooperate to promote renal tumorigenesis in vivo. Mol Cell Biol 27: 2092–2102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang Y, Wang J, Qin L, Shou Z, Zhao J, Wang H, Chen Y, Chen J. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am J Nephrol 27: 495–502, 2007 [DOI] [PubMed] [Google Scholar]