Abstract
The collecting duct (CD) is a major renal site for the hormonal regulation of Na homeostasis and is critical for systemic arterial blood pressure control. Our previous studies demonstrated that the endothelin-1 gene (edn1) is an early response gene to the action of aldosterone. Because aldosterone and endothelin-1 (ET-1) have opposing actions on Na reabsorption (JNa) in the kidney, we postulated that stimulation of ET-1 by aldosterone acts as a negative feedback mechanism, acting locally within the CD. Aldosterone is known to increase JNa in the CD, in part, by stimulating the epithelial Na channel (ENaC). In contrast, ET-1 increases Na and water excretion through its binding to receptors in the CD. To date, direct measurement of the quantitative effect of ET-1 on transepithelial JNa in the isolated in vitro microperfused mouse CD has not been determined. We observed that the CD exhibits substantial JNa in male and female mice that is regulated, in part, by a benzamil-sensitive pathway, presumably ENaC. ENaC-mediated JNa is greater in the cortical CD (CCD) than in the outer medullary CD (OMCD); however, benzamil-insensitive JNa is present in the CCD and not in the OMCD. In the presence of ET-1, ENaC-mediated JNa is significantly inhibited. Blockade of either ETA or ETB receptor restored JNa to control rates; however, only ETA receptor blockade restored a benzamil-sensitive component of JNa. We conclude 1) Na reabsorption is mediated by ENaC in the CCD and OMCD and also by an ENaC-independent mechanism in the CCD; and 2) ET-1 inhibits JNa in the CCD through both ETA and ETB receptor-mediated pathways.
Keywords: sodium reabsorption, kidney, mineralocorticoid, collecting duct, endothelin-1, aldosterone
the corticosteroid hormone aldosterone stimulates sodium (Na) reabsorption in the collecting duct (CD) via the concerted action of a luminal membrane benzamil-sensitive epithelial Na channel (ENaC) and a basolateral Na-K-ATPase. Endothelin-1 (ET-1) production is stimulated by aldosterone and glucocorticoid in the CD (10, 25, 26) and is an important regulator of systemic blood pressure and volume homeostasis. See Kohan et al. (13) for review.
ET-1 mediates its actions by binding to ETA and ETB receptors, although the relative expression and localization of these receptors in the CD are still under investigation. The ETB receptor binds ET-1, ET-2, and ET-3 with similar affinity; however, the ETA receptor has the greatest affinity for ET-1. ETB expression in the CD has been clearly demonstrated with the greatest levels in the inner medullary CD (IMCD) where it mediates antihypertensive effects. ETA expression has been reported by immunohistochemistry in the rat distal tubule and cortical CD (CCD) with faint staining in the IMCD (32), in the rat CD (21), and in the basal infoldings of the rat CD with the strongest staining in the IMCD (33). In binding assay experiments, ETA receptors were found in the rat and rabbit IMCD (6).
CD principal cell-specific knockout (KO) of either ET-1 (CD ET-1 KO) or the ETB receptor (CD ETB KO) in mice caused a significant increase in blood pressure that was greater on a high-salt diet, whereas CD ETA receptor KO (CD ETA KO) mice were normotensive. Of note, the blood pressures of the CD ET-1 KO were greater than those of the CD ETB KO. However, the combined KO of both ET receptors (CD ETA/B KO) had blood pressures similar to the CD ET-1 KO but greater than those of the CD ETB KO, suggesting that ETA and ETB may have a combined hypotensive effect (9). In fact, renal medullary infusion of ET-1 in ETB receptor-deficient female rats caused Na excretion that could be blocked by ETA receptor antagonism (19). Recent in vivo studies on the role of ETA receptors in mediating the effects of ET-1 in the renal medulla are reviewed in Nakano and Pollock (18). ET receptors can form homo- and heterodimers that have different functional and pharmacological profiles (1), which reflects the complexity of the role of the ET-1 receptors in blood pressure regulation.
ET-1 inhibited Na-K-ATPase activity in isolated rabbit IMCD cells (34), AVP-stimulated chloride and fluid absorption in the CCD of deoxycorticosterone-treated rats (29), and ENaC activity in split-open rat and mouse CCD (2, 3). The effect of ET-1 directly on Na transport has not been measured at the level of the isolated, microperfused CD. The purpose of this study was to definitively test the effects of ET-1 on ENaC-mediated (benzamil-sensitive) Na transport by directly measuring lumen-to-bath isotopic 22Na reabsorption (JNa) using in vitro microperfusion. First, we characterize ENaC-mediated JNa in the CCD and OMCD and confirmed that the CCD also has the capacity to reabsorb Na through a benzamil-insensitive pathway. We observed that ET-1 decreased Na reabsorption in the CCD, and we evaluated the role of ET-1 receptors in mediating this response. The current findings are consistent with a model of ET-1 downregulation of Na reabsorption through mechanisms involving both ET receptors.
METHODS
Animals.
All animal use was in compliance with the American Physiological Society's Guiding Principles in the Care and Use of Laboratory Animals, and animal use protocols were approved by the North Florida/South Georgia Veterans Administration Institutional Animal Care and Use Committee. Wild-type (WT; C57BL/6J) mice between 8 and 23 wk (mean age 13.8 ± 0.4) were fed a normal laboratory chow containing 0.2% Na, 0.5% K (Teklad 2018, Madison, WI) and allowed free access to water. Mice were euthanized by intraperitoneal injection of Na-pentobarbital (120 mg/kg) and cervical dislocation.
CD isolation and in vitro microperfusion.
Standard techniques (4, 35) with the following modifications were used. The dissection, luminal perfusion, and bath solution were (in mM) 100 NaCl, 5 KCl, 25 NaHCO3, 1.5 Na2HPO4, 10 NaCH3CO2, 1.0 MgCl2, 8 glucose, 5 alanine, 1.2 CaCl2, and 1.5 mannitol gassed with 95% O2-5% CO2 to pH 7.4. Both kidneys were quickly removed, and ∼1-mm coronal slices were placed in a chilled Petri dish containing the dissection solution. A single CD segment was hand dissected at 4°C within 60 min, transferred to a thermostatically controlled channel style perfusion chamber, and cannulated as previously described (16, 17). The perfusion flow rate (Vo) was maintained at 2.0–4.0 nl/min (mean flow rate 2.61 ± 0.04). The bath solution was exchanged at a constant flow rate of 1.0 ml/min and maintained at 37 ± 2°C. The luminal perfusion solution contained 50 μCi of [methox-3H] inulin exhaustively dialyzed by the method of Shafer et al. (23) and 5 μCi 22Na as NaCl. Bath collections were taken in tandem with perfusate collections to control for mechanical leak. Any collection with >5% leak was rejected. After an initial equilibration period of 30 min, period 1 samples of the effluent were collected into a constant volume pipette, which was transferred into a scintillation vial for counting. The luminal solution was then exchanged (mock during time control experiments), and a second 30-min equilibration phase followed before the period 2 collections. Unidirectional (lumen to bath) 22Na flux (JNa, in pmol·mm−1·min−1) was calculated as previously reported (35).
Chemicals.
Radioisotopes were obtained from Perkin Elmer (Boston, MA). Benzamil hydrochloride, hydrochlorothiazide (HCTZ), ET-1, BQ-123, and BQ-788 were obtained from Sigma Chemical (St. Louis, MO). All other chemicals were obtained from Sigma Chemical or Fisher Scientific.
Statistical analyses.
The results are expressed as means ± SE. One-way or two-way ANOVA (with repeated measures for paired experiments) was used to compare JNa between groups. Differences between groups were considered statistically significant at P < 0.05.
RESULTS
Na reabsorption in the CCD is mediated by benzamil-sensitive and -insensitive mechanisms.
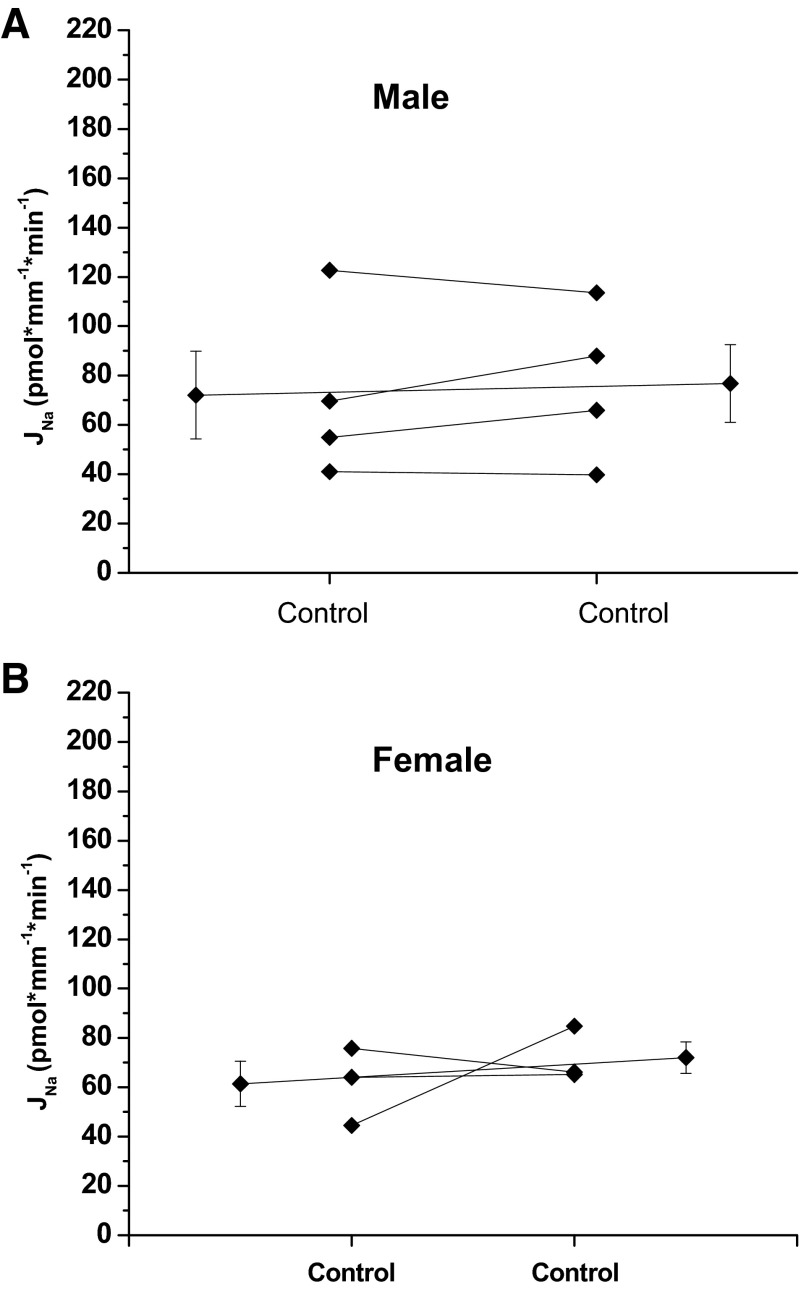
Given the length of time required to ensure equilibration and perform multiple timed collections during each period, time control experiments were performed to determine whether Na reabsorption changed significantly over the duration of the experiment. Gender differences were also evaluated. The rate of Na reabsorption did not change significantly between period 1 and period 2 in either male or female mice (Fig. 1), and male and female mice exhibited similar rates of Na reabsorption.
Fig. 1.
Effect of time on Na reabsorption, JNa, in the cortical collecting duct (CCD) of male and female mice. JNa during period 1 is not different from period 2 in male (A) or female (B) mice (male control 72.1 ± 17.9 vs. control 76.8 ± 15.7, P = NS, n = 4; female control 61.4 ± 9.1 vs. control 72.1 ± 6.4, P = NS, n = 3). Extended line connects the means ± SE for the 2 experimental periods.
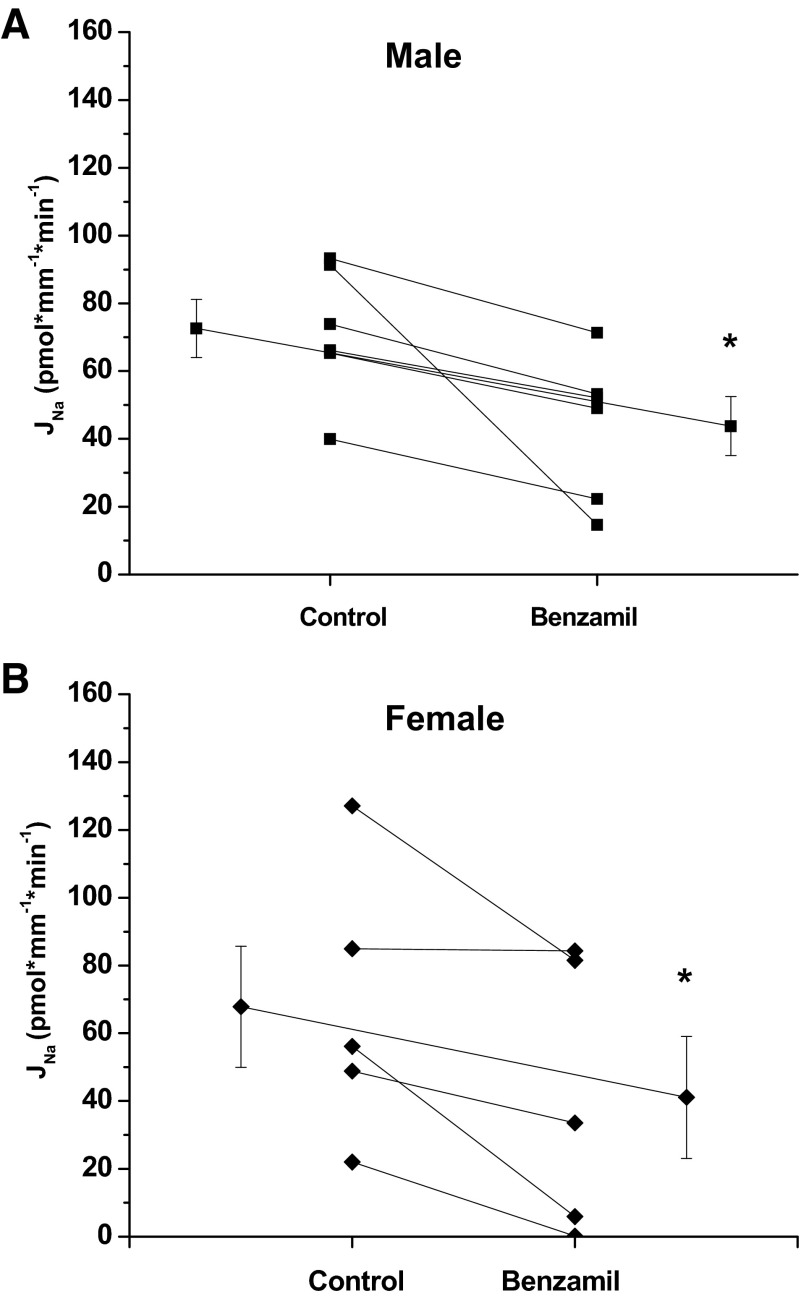
To assess the activity of ENaC, experiments were performed where 1 μM benzamil hydrochloride was present in the perfusate during period 2. The rate of Na reabsorption in both genders was significantly inhibited by benzamil (Fig. 2). Benzamil inhibited Na reabsorption by 39 and 40% in female and males, respectfully. Previous studies have reported Na homeostasis changes during the menstrual cycle, and ENaC is regulated by estrogen and progesterone (5, 8, 11). Chang et al. (5) reported that the female sex steroid hormones affect ENaC subunit mRNA expression, protein translation, and ENaC activity in renal collecting tubule cells, suggesting a role in premenstruation edema. In addition, Nakano and Pollock (19) reported that intramedullary infusion of ET-1 increased urinary Na excretion in female, but not male, sl/sl rats. In an effort to avoid female hormone modulation of ENaC, we performed our assessment of the contribution of ENaC in Na reabsorption and the regulation of this transport by ET-1 strictly in males.
Fig. 2.
Effect of luminal benzamil (1 μm) on Na reabsorption, JNa, in the CCD of male (A) and female (B) mice. Benzamil significantly inhibited JNa in male and female mice (male control 72.6 ± 8.6 vs. benzamil 43.8 ± 8.7, *P < 0.05, n = 6; female control 67.8 ± 17.9 vs. benzamil 41.1 ± 18.0, *P < 0.05, n = 6). Extended line connects the means ± SE for the 2 experimental periods.
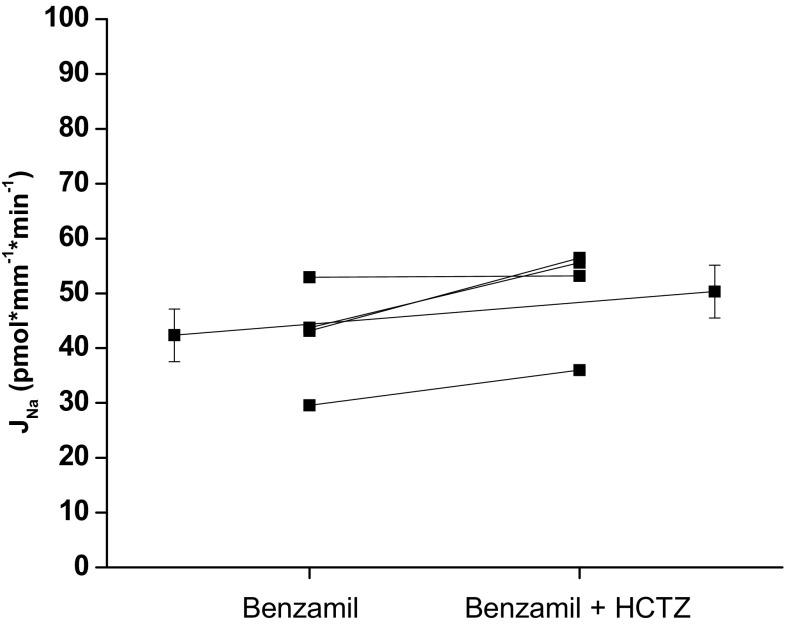
To determine if the benzamil-insensitive transport was mediated by a HCTZ-sensitive mechanism, we measured the effect of HCTZ on Na reabsorption in the presence of benzamil (Fig. 3). HCTZ did not significantly inhibit the residual Na reabsorption.
Fig. 3.
Effect of luminal hydrochlorothiazide (HCTZ; 100 μM) on benzamil-insensitive Na reabsorption, JNa, in the CCD of male mice. HCTZ did not significantly inhibit JNa in male mice (control 42.3 ± 4.8 vs. HCTZ 50.3 ± 4.8, P = NS, n = 4). Extended line connects the means ± SE for the 2 experimental periods.
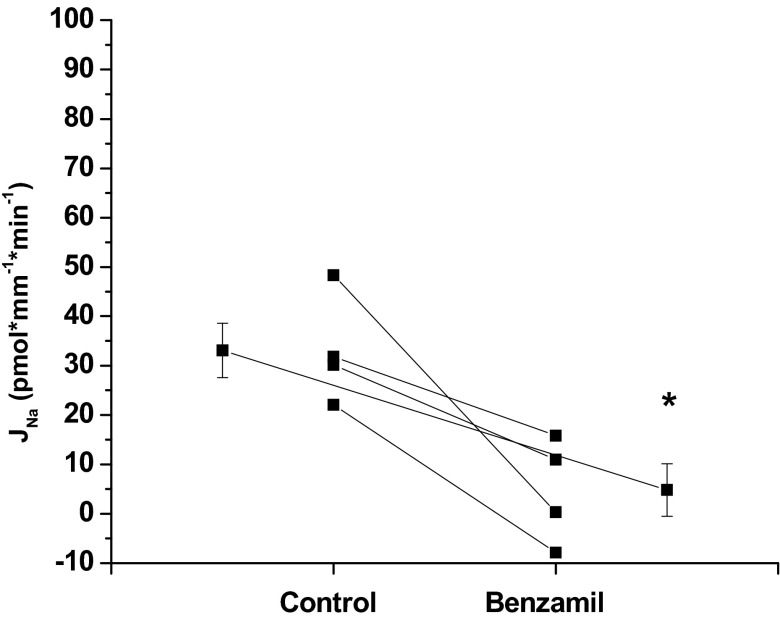
Na reabsorption in the OMCD is mediated entirely by ENaC.
Na reabsorption was heterogeneous along the CD with greater rates in the CCD than in the OMCD. The rate of Na reabsorption in the OMCD was less than half that of the CCD [CCD 72.6 ± 8.6 (n = 6) vs. OMCD 33.1 ± 5.5 (n = 4)]. As shown in Fig. 4, benzamil inhibited the majority of Na reabsorption in the OMCD from male mice.
Fig. 4.
Effect of luminal benzamil (1 μM) on Na reabsorption, JNa, in the OMCD of male mice. Benzamil significantly inhibited JNa in the OMCD of male mice (control 33.1 ± 5.5 vs. benzamil 4.8 ± 5.3; *P < 0.05, n = 4). Extended line connects the means ± SE for the 2 experimental periods.
ET-1 decreases Na reabsorption in the CCD.
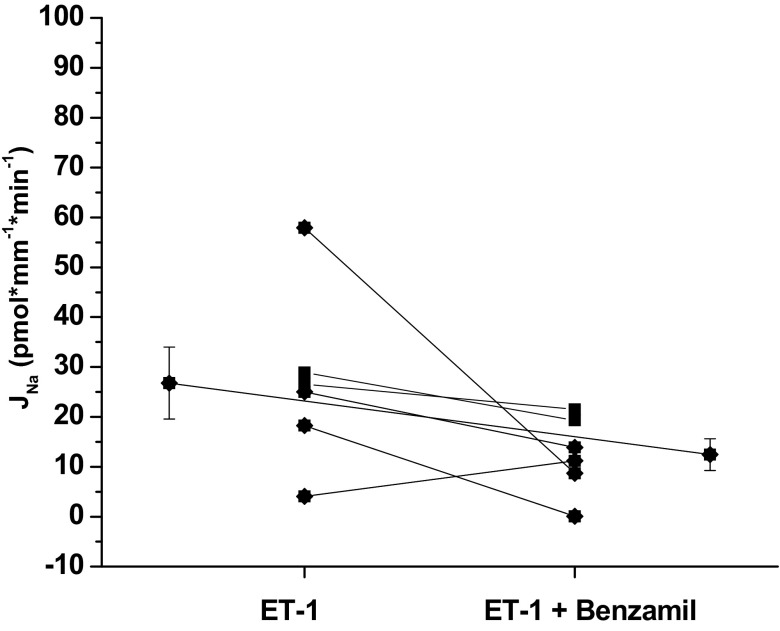
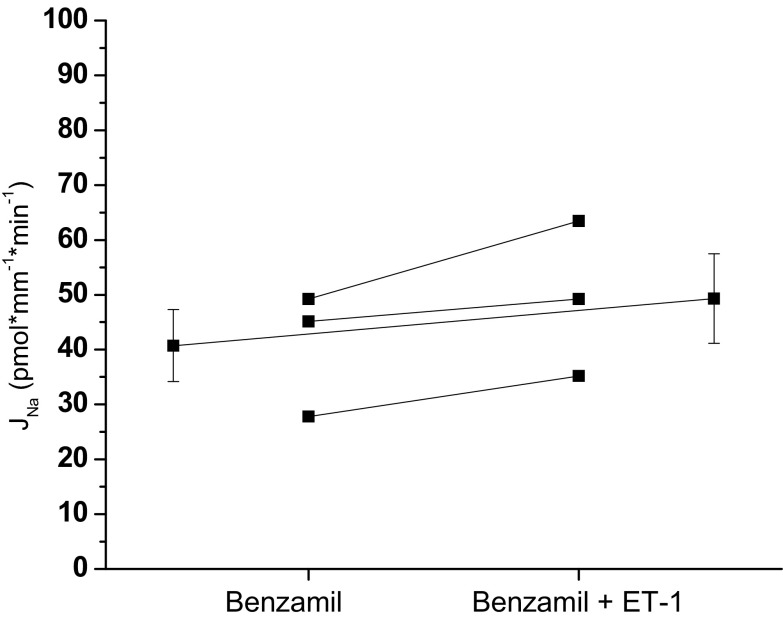
To date, the effect of ET-1 on Na transport has not been evaluated by directly measuring Na in the isolated, microperfused CCD but has been inferred from studies measuring Na-K-ATPase activity in primary cell culture (34), chloride and fluid absorption in the perfused CCD (29), intracellular calcium response in rabbit and mouse CD (14, 20), and Na channel activity in the CCD (2, 3). Our study confirms these previous reports that ET-1 inhibits Na reabsorption. In the presence of ET-1 (20 nM, luminal and basolateral), Na reabsorption was inhibited compared with the control experiments [CCD 72.6 ± 8.6 (n = 6) vs. 26.8 ± 7.2 (n = 6)]. To evaluate the activity of ENaC in the presence of ET-1, benzamil was added to the luminal perfusate during period 2. In the presence of ET-1, the effect of benzamil on Na reabsorption did not reach statistical significance (P = 0.12; Fig. 5). There was a strong inhibitory effect of ET-1 on Na reabsorption in the presence and absence of benzamil (two-way repeated-measures ANOVA, P < 0.05). To determine if ET-1 affected the benzamil-insensitive component of Na reabsorption, we performed paired experiments (Fig. 6). However, ET-1 had no effect on the Na reabsorption in the presence of benzamil.
Fig. 5.
Effect of benzamil in the presence of endothelin-1 (ET-1) on Na reabsorption, JNa, in the CCD of male mice. In the presence of ET-1, benzamil did not significantly inhibit Na reabsorption (ET-1 26.8 ± 7.2 vs. ET-1 + benzamil 12.5 ± 3.2, P = 0.12, n = 6). Extended line connects the means ± SE for the 2 experimental periods.
Fig. 6.
Effect of ET-1 on benzamil-insensitive Na reabsorption, JNa, in the CCD of male mice. In the presence of benzamil, ET-1 did not significantly inhibit Na reabsorption (benzamil 40.7 ± 6.6 vs. benzamil + ET-1 49.3 ± 8.2, P = NS, n = 3). Extended line connects the means ± SE for the 2 experimental periods.
ETA and ETB receptors mediate ET-1 action in the CCD.
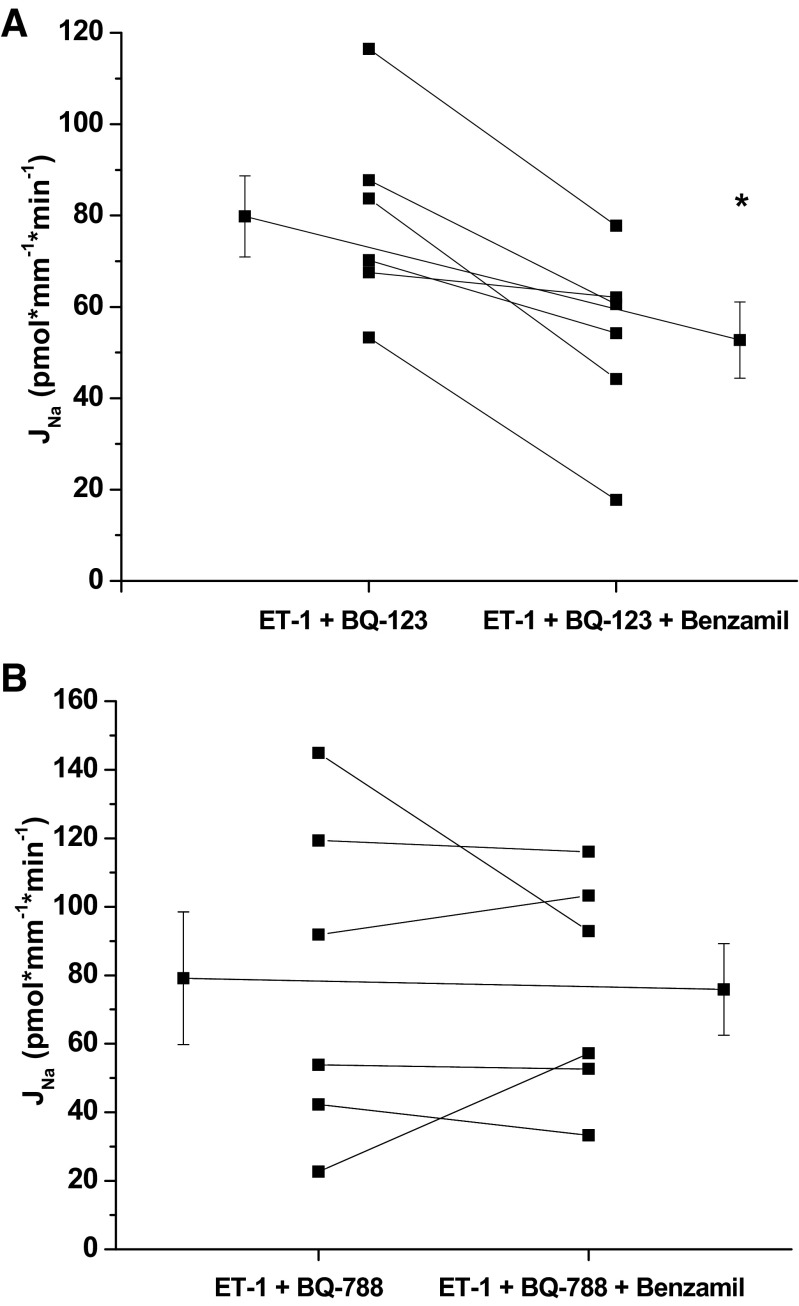
To determine the ET-1 receptor(s) involved in mediating the ET-1 inhibitory effect on Na reabsorption, experiments were performed in the presence of ET-1 receptor inhibitors. We measured the effect of benzamil on Na reabsorption in the presence of ET-1 plus either the ETA receptor inhibitor BQ-123 (1 μM, basolateral) or the ETB receptor inhibitor BQ-788 (1 μM, basolateral; Fig. 7). Both ET-1 receptor inhibitors restored Na reabsorption to basal rates; however, only blockade of the ETA receptor appears to restore Na reabsorption that is benzamil sensitive.
Fig. 7.
Effect of benzamil in the presence of ET-1 and the ETA receptor inhibitor BQ-123 (A) or ETB receptor inhibitor BQ-788 (B) on Na reabsorption in the CCD of male mice. Benzamil significantly inhibited JNa in the presence of ET-1 plus ETA receptor inhibitor BQ-123 (ET-1 + BQ123 control 79.8 ± 8.9 vs. benzamil 52.8 ± 8.3, *P < 0.05, n = 6) but did not inhibit JNa in the presence of ET-1 plus ETB receptor inhibitor BQ-788 (ET-1 + BQ-788 control 79.2 ± 19.4 vs. benzamil 75.9 ± 13.4, P = NS). Extended line connects the means ± SE for the 2 experimental periods.
DISCUSSION
We report that ET-1 inhibits a majority of Na reabsorption in the mouse CCD. This is the first study that directly measures the effect of ET-1 on Na reabsorption in the microperfused CCD. Our data support prior studies of the isolated, perfused rat CCD that reported an inhibitory effect of ET-1 on chloride and water reabsorption (29). Our data strongly support that ET-1 downregulates Na reabsorption via ENaC. When ET-1 was present in the luminal and peritubular solutions, Na reabsorption was reduced by 63% compared with control, and the residual Na transport was <30 pmol·mm −1·min−1. The residual Na transport was not statistically inhibited with luminal benzamil (P = 0.12), suggesting that ENaC activity was small in the presence of ET-1. Direct evidence that ET-1 decreases ENaC activity has been previously reported (2, 3). Using patch-clamp physiology, Bugaj et. al. (2, 3) reported that ET-1 decreased ENaC open probability in split-open rat and mouse CCD.
The current findings suggest that both ET-1 receptors play a role in ET-1 regulation of Na reabsorption. In the absence of ET-1, benzamil inhibited less than half of the total Na flux confirming previous reports (discussed below) that a benzamil-insensitive transport mechanism exists in the CCD. It is unlikely that passive Na diffusion accounts for a significant portion of the residual (benzamil-insensitive) transport in our experiments because in the presence of ET-1 and benzamil, the rate of transport was <15 pmol·mm−1·min−1. More importantly, benzamil fully inhibited Na transport in the OMCD to a value not significantly different from zero confirming that near zero rates can be accurately measured. The isotopic 22Na flux rates reported herein are greater than the reported measurements of net Na flux in the mouse CCD, but these two measurements cannot be equated. When isotopic and net flux for Na are measured in the same tubule, isotopic flux significantly exceeds net Na flux (24). The isotopic flux is similar with other observations for 22Na efflux in the CCD of the rabbit (24).
The emerging concept that B-type intercalated cells (ICs) can absorb NaCl and K extends our understanding of the role of different cell types in the CD in regulating blood pressure. These novel mechanisms are reviewed in Eladari et al. (7). Several studies report that ENaC is not the only significant mechanism for Na reabsorption in the CD. Targeted deletion of the α-subunit of ENaC in the CD inhibited ENaC activity but did not impair Na balance (22). This was interpreted as evidence of significant Na transport mechanisms in more proximal segments but may also indicate the significance of ENaC-independent transport mechanisms in the CD. More directly, two pharmacologically different Na transport mechanisms have been reported in the CCD (28, 30, 31). These include the classic electrogenic, amiloride-sensitive Na transport mechanism mediated by ENaC and an electroneutral benzamil-insensitive, thiazide-sensitive mechanism. Recently, the second mechanism was described by Leviel et al. (15) as a coupling of pendrin-mediated Cl transport with the Na-dependent Cl/HCO3 exchanger NDCBE/Slc4a8 for net reabsorption of NaCl by B-type ICs. In the present study, inhibition of either ETA or ETB receptor fully restored Na reabsorption to the basal rate. ETA blockade restored a benzamil-sensitive component of Na reabsorption presumably ENaC, whereas, ETB blockade restored a benzamil-insensitive Na reabsorptive mechanism. In a previous report, we investigated the frequency of functionally defined ICs in the mouse CCD and determined that B-type ICs comprise ∼50% of the ICs in the middle portion of the CCD and <25% in the inner CCD (16), which is consistent with ultrastructural and immunological studies (12, 27). If B-type IC transporters are responsible for benzamil-insensitive Na transport, then the activity should be diminished in the medullary segments of the CD. Indeed, Na reabsorption in the OMCD was entirely benzamil-sensitive, suggesting that only ENaC is functioning in the OMCD where principal cells and A-type ICs far outnumber any B-type ICs.
To definitively determine whether ET-1 was downregulating Na reabsorption through an ENaC-independent mechanism, we performed paired experiments in which we measured the effect of HCTZ and ET-1 during ENaC-inhibition. Under the conditions of our experiments, we did not observe a HCTZ-sensitive component of Na reabsorption during ENaC-inhibition. Additionally, ET-1 had no effect on the ENaC-independent Na reabsorption. The present data suggest that ET-1 inhibits exclusively a benzamil-sensitive mechanism, presumably ENaC, under the conditions of these experiments, namely mice on a normal Na diet. However, these conclusions do not extend to mice fed a Na-restricted diet. Indeed, on a Na-restricted diet evidence suggests that a Na-driven Cl/HCO3 exchanger (SLC4A8) and a Na-independent Cl/HCO3 exchanger (SLC26A4) function to produce a component of CCD Na absorptive flux (15). Moreover, in our studies when ETA was blocked, a benzamil-sensitive component of Na transport was present and ET-1 binding to the ETB receptor failed to decrease Na reabsorption. Similarly, when ETB was blocked, ET-1 binding to ETA failed to reduce Na transport; however, Na reabsorption was entirely benzamil insensitive. Our data suggest that ET-1 reduces Na reabsorption via a pathway that is sensitive to ETA receptor binding that inhibits ENaC. Only when the ETB receptor is blocked did we see obliteration of ENaC activity, suggesting Na is then reabsorbed by an ENaC-independent mechanism. Previous single-channel studies have reported that either blockade or knockout of the ETB receptor, but not ETA receptor, inhibited or decreased the effects of ET-1 on ENaC activity (3). Furthermore, ET-1 decreased ENaC activity in WT and CD ETA KO mice but not in CD ETB KO and CD ETA/B KO mice strongly signifying that ET-1 inhibition of ENaC is mediated through the ETB receptor in the mouse CD (2). The abrogation of the effect of benzamil on Na reabsorption in the presence of ET-1 plus the ETB blocker (Fig. 8) differs from these previous studies and suggests that the ETA receptor is responsible for the inhibition of ENaC by ET-1 under the conditions of these experiments. Aside from the difference in preparations, a plausible explanation of the difference in results include the experiment design. In the present experiments, the effect of BQ-123 and BQ-788 was examined in the presence of ET-1. In addition, benzamil was used to assess the contribution of ENaC.
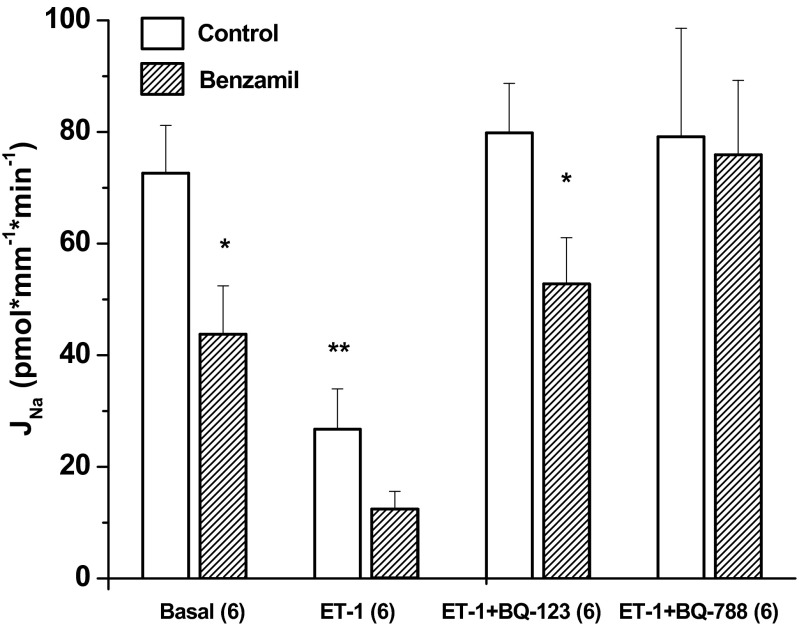
Fig. 8.
ET-1 receptor inhibitors block ET-1 regulation of Na reabsorption, JNa. ET-1 significantly inhibited JNa (basal control 72.6 ± 8.6 vs. ET-1 control 26.8 ± 7.2, P < 0.05, n = 6). Benzamil significantly inhibited JNa in the absence of ET-1 (basal control 72.6 ± 8.6 vs. benzamil 43.8 ± 8.7, P < 0.05, n = 6) and in the presence of ET-1 plus the ETA receptor inhibitor BQ-123 (ET-1 + BQ-123 control 79.8 ± 8.9 vs. benzamil 52.8 ± 8.3, P < 0.05, n = 6). Benzamil did not inhibit JNa in the presence of ET-1 plus the ETB receptor inhibitor BQ-788 (ET-1 + BQ-788 control 79.2 ± 19.4 vs. benzamil 75.9 ± 13.4, P = NS). *P < 0.05, compared with control of the same condition. **P < 0.05, compared with basal control.
However, in studies using the CD ETA/B KO, it has been reported that the ETA and ETB receptors exert a combined hypotensive effect that exceeds that of either receptor alone (9). In our study, ETA blockade fully restored Na reabsorption to basal values and did not appear to affect the benzamil component of Na reabsorption. Thus ET-1 binding to the ETB receptor during ETA blockade does not inhibit ENaC. More importantly, our data imply that the ETA receptor is likely involved in ET-1-regulated, ENaC-mediated Na reabsorption.
In summary, the CD reabsorbs Na through ENaC in the OMCD and through ENaC-dependent and -independent mechanisms in the CCD. ET-1 regulation of Na reabsorption in the CCD involves both ETA and ETB receptors. Future studies should clarify the significance of these transport mechanisms in volume homeostasis and blood pressure control.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-82680.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.J.L., B.D.C., and C.S.W. conception and design of research; I.J.L. performed experiments; I.J.L. and C.S.W. analyzed data; I.J.L., A.K.W., D.E.K., B.D.C., and C.S.W. interpreted results of experiments; I.J.L. prepared figures; I.J.L. drafted manuscript; I.J.L., A.K.W., D.E.K., B.D.C., and C.S.W. edited and revised manuscript; I.J.L., A.K.W., D.E.K., B.D.C., and C.S.W. approved final version of manuscript.
REFERENCES
- 1. Boesen EI. Endothelin ETB receptor heterodimerization: beyond the ETA receptor. Kidney Int 74: 693–694, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bugaj V, Mironova E, Kohan DE, Stockand JD. Collecting duct-specific endothelin B receptor knockout increases ENaC activity. Am J Physiol Cell Physiol 302: C188–C194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol 295: F1063–F1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burg MB, Grantham JJ, Abramow M, Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210: 1293–1298, 1966 [DOI] [PubMed] [Google Scholar]
- 5. Chang CT, Sun CY, Pong CY, Chen YC, Lin GP, Chang TC, Wu MS. Interaction of estrogen and progesterone in the regulation of sodium channels in collecting tubular cells. Chang Gung Med J 30: 305–312, 2007 [PubMed] [Google Scholar]
- 6. Edwards RM, Trizna W. Characterization of 125I-endothelin-1 binding to rat and rabbit renal microvasculature. J Pharmacol Exp Ther 274: 1084–1089, 1995 [PubMed] [Google Scholar]
- 7. Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gambling L, Dunford S, Wilson CA, McArdle HJ, Baines DL. Estrogen and progesterone regulate alpha, beta, and gammaENaC subunit mRNA levels in female rat kidney. Kidney Int 65: 1774–1781, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am J Physiol Renal Physiol 295: F1635–F1640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol 285: F664–F673, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Kienitz T, Allolio B, Strasburger CJ, Quinkler M. Sex-specific regulation of ENaC and androgen receptor in female rat kidney. Horm Metab Res 41: 356–362, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol 10: 1–12, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kurokawa K, Yoshitomi K, Ikeda M, Uchida S, Naruse M, Imai M. Regulation of cortical collecting duct function: effect of endothelin. Am Heart J 125, Suppl: 582–588, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Leviel F, Hubner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lynch IJ, Greenlee MM, Gumz ML, Rudin A, Xia SL, Wingo CS. Heterogeneity of H-K-ATPase-mediated acid secretion along the mouse collecting duct. Am J Physiol Renal Physiol 298: F408–F415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lynch IJ, Rudin A, Xia SL, Stow LR, Shull GE, Weiner ID, Cain BD, Wingo CS. Impaired acid secretion in cortical collecting duct intercalated cells from H-K-ATPase-deficient mice: role of HKα isoforms. Am J Physiol Renal Physiol 294: F621–F627, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Nakano D, Pollock D. New concepts in endothelin control of sodium balance. Clin Exp Pharmacol Physiol 39: 104–110, 2012 [DOI] [PubMed] [Google Scholar]
- 19. Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 53: 324–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naruse M, Uchida S, Ogata E, Kurokawa K. Endothelin 1 increases cell calcium in mouse collecting tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 261: F720–F725, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Omatsu S, Tomoyoshi T. [Immunohistochemical study on endothelin in rat kidney]. Hinyokika Kiyo 43: 109–114, 1997 [PubMed] [Google Scholar]
- 22. Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC. Collecting duct-specific gene inactivation of alphaENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest 112: 554–565, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schafer JA, Troutman SL, Andreoli TE. Volume reabsorption, transepithelial potential differences, and ionic permeability properties in mammalian superficial proximal straight tubules. J Gen Physiol 64: 582–607, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stokes JB. Potassium secretion by cortical collecting tubule: Relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am J Physiol Renal Fluid Electrolyte Physiol 241: F395–F402, 1981 [DOI] [PubMed] [Google Scholar]
- 25. Stow LR, Jacobs ME, Wingo CS, Cain BD. Endothelin-1 gene regulation. FASEB J 25: 16–28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stow LR, Voren GE, Gumz ML, Wingo CS, Cain BD. Dexamethasone stimulates endothelin-1 gene expression in renal collecting duct cells. Steroids 77: 360–366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teng-umnuay P, Verlander JW, Yaun W, Tisher CC, Madsen KM. Identification of distinct subpopulations of intercalated cells in the mouse collecting duct. J Am Soc Nephrol 7: 260–274, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 259: F519–F528, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Tomita K, Nonoguchi H, Terada Y, Marumo F. Effects of ET-1 on water and chloride transport in cortical collecting ducts of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 264: F690–F696, 1993 [DOI] [PubMed] [Google Scholar]
- 30. Tomita K, Pisano JJ, Burg MB, Knepper MA. Effects of vasopressin and bradykinin on anion transport by the rat cortical collecting duct: Evidence for an electroneutral sodium chloride transport pathway. J Clin Invest 77: 136–141, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tomita K, Pisano JJ, Knepper MA. Control of sodium and potassium transport in the cortical collecting duct of the rat: Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest 76: 132–136, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem 54: 1193–203, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Yamamoto T, Suzuki H, Kubo Y, Matsumoto A, Uemura H. Endothelin A receptor-like immunoreactivity on the basal infoldings of rat renal tubules and collecting ducts. Arch Histol Cytol 71: 77–87, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na+-K+-ATPase in intact renal tubular epithelial cells. Am J Physiol Cell Physiol 257: C1101–C1107, 1989 [DOI] [PubMed] [Google Scholar]
- 35. Zhou X, Xia SL, Wingo CS. Chloride transport by the rabbit cortical collecting duct: dependence on H,K-ATPase. J Am Soc Nephrol 9: 2194–2202, 1998 [DOI] [PubMed] [Google Scholar]