Abstract
Purpose.
Optokinetic eye movements stabilize gaze by tracking motion of the visual scene during sustained movement of a creature's body. The purpose of this study was to describe vertical and horizontal optokinetic nystagmus (OKN) in nonhuman primates (NHPs) with normal binocular vision, and to compare their responses to NHPs with binocular maldevelopment induced by prism-rearing.
Methods.
Optical strabismus was created in infant macaques (n = 6) by fitting them with prism goggles. The goggles were removed after 3, 6, 9, or 12 weeks to determine the effects of increasing durations of binocular noncorrespondence. Infant NHPs (n = 2) reared wearing plano goggles served as controls. OKN was evoked by horizontal or vertical stripe motion. Eye movements were recorded by using binocular search coils.
Results.
NHPs reared in early infancy under conditions of binocular noncorrespondence for durations of 6 weeks or longer had horizontal OKN responses biased directionally in favor of nasalward motion. NHPs reared with prisms for any duration had vertical OKN responses more biased than normal NHPs in favor of upward motion. Diagonal “crosstalk” during horizontal or vertical OKN (vertical slow phases during horizontal stimulus motion, and vice versa) was present to some degree in all NHPs. However, crosstalk—upward during horizontal OKN and nasalward during vertical OKN—was most pronounced in NHPs reared with prism for durations long enough to induce a permanent esotropic strabismus (longer than 3 weeks).
Conclusions.
With fusion maldevelopment, the OKN pathways retain a nasalward and upward bias. During forward locomotion, optic flow excites temporalward and downward visual motion in each eye. The OKN biases would act in counterbalance. The biases attenuate with emergence of fusion, but may persist and crosstalk when fusion is impeded.
Keywords: optokinetic nystagmus, strabismus, primate
If development of binocular vision is impeded in early infancy, eye tracking remains unbalanced; eye tracking is better for nasalward and upward motion.
Introduction
Optokinetic eye movements stabilize gaze by tracking motion of the visual scene during sustained movement of a creature's body.1 The neural processing and brain pathways that generate horizontal optokinetic nystagmus (OKN) in adult, nonhuman primates (NHPs) have been described in detail.2–5 Less is known about the neural organization of vertical OKN. We also need to understand in greater detail the effects of binocular maldevelopment on OKN, both horizontal and vertical.
Infant NHPs and infant humans have an asymmetry of horizontal OKN when viewing monocularly, favoring nasalward (NW) stimulus motion.6–10 The asymmetry resolves in the first 3 to 6 months of life if the infant has normal binocular experience. Disruption of binocular vision during this critical period—by strabismus, amblyopia or both—causes a persistent nasalward OKN asymmetry.11–18
In NHPs and in humans with normal binocular vision, vertical OKN is asymmetric. Upward (UW) stimulus motion evokes more robust OKN than downward (DW) motion.19–27 In children and adults with strabismus and/or amblyopia, the asymmetry favoring upward OKN may be more pronounced.25,28,29
Behavioral studies have shown that the postnatal development of binocular sensory and motor functions in infant NHPs parallels closely that of infant humans, but on a compressed time scale (i.e., 1 week of monkey development is equivalent to 1 month of human).6,7,30,31 Infant NHPs with strabismus display the same constellation of perceptual and ocular motor abnormalities found in strabismic humans, including defective stereopsis, abnormal vergence, and horizontal OKN asymmetries.14,16,18,32–35 It is unclear, however, what effect strabismus has on vertical OKN, or the interaction between horizontal and vertical OKN in NHPs.
The purpose of this study was to compare vertical and horizontal OKN in normal and strabismic NHPs. The strabismus was induced by rearing normal infant NHPs in prism goggles for varying durations after birth. The prism goggles cause binocular image noncorrespondence, disrupting the development of fusion, stereopsis, eye alignment, and gaze.
Methods
Animals and Goggle Rearing Groups
Eight infant rhesus monkeys were used. Six wore prism goggles to induce optical strabismus; two controls were reared without prisms (plano lens goggles). They were born at the Yerkes Regional Primate Research Center in Atlanta, Georgia, and fitted with goggles on the first day of life. The fitting procedure was modified from the method of Crawford et al.36 as described in detail in our earlier reports.17,18
As listed in Table 1, the experimental animals viewed through an 11.4° base-in prism in the right eye and 11.4° base-down prism in the left eye, precluding fusion by causing combined horizontal-vertical binocular image noncorrespondence (Fig. 1). The prism goggles were worn for durations of 3, 6, 9, or 12 weeks. Once the defined period of goggle-rearing ended the monkeys were transported to Washington University in St. Louis, Missouri. At age 6 months, they were trained to perform visual fixation tasks (without goggles) using fruit juice as a positive feedback reward.37
Table 1.
Visuomotor Findings and Rearing Conditions of the Eight Animals Used in the Study
|
Animal/Sex/Age at Testing, y |
Rearing Conditions |
Eye Alignment |
Latent Nystagmus* |
Horizontal Pursuit/ OKN Asymmetry† |
DVD |
| Controls | |||||
| WE/M/1.5 | 3-wk plano lens | Ortho | No | No | No |
| AY/M/2 | 12-wk plano lens | Ortho | No | No | No |
| Prism reared | |||||
| ZA/F/1.5 | 3-wk prism | Ortho | No | No | No |
| EO/F/1.5 | 6-wk prism | Esot 5°, Hypert 2° | Yes | Yes | Yes |
| WY/F/2 | 6-wk prism | Esot 3°, Hypert 3° | Yes | Yes | Yes |
| YI/M/1.7 | 9-wk prism | Esot 12°, Hypert 5° | Yes | Yes | Yes |
| QE/F/1.5 | 12-wk prism | Esot 10°, Hypert 5° | Yes | Yes | Yes |
| SY/M/1.5 | 12-wk prism | Esot 11°, Hypert 4° | Yes | Yes | Yes |
Esot, esotropia; hypert, hypertropia; ortho, orthophoric.
Yes = repetitive cycles of nasalward slow-phase ≥ 0.2°/s viewing monocularly each eye.
Yes = mean velocity for nasalward pursuit exceeding temporalward by >20% viewing monocularly each eye.
Figure 1.
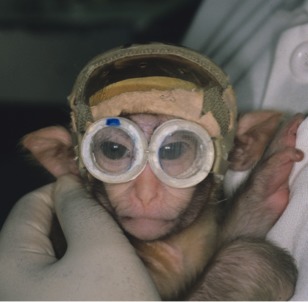
Infant monkey wearing prism goggles to induce binocular image noncorrespondence (optical strabismus).
Cycloplegic refractions revealed a refractive error ≤ +3.00 spherical equivalent in each of the experimental and control animals. Visual acuity was tested in each eye using spatial-sweep visual-evoked potentials (VEPs).38 In the months before coil implantation, eye alignment was assessed using photographs and video recordings (Hirschberg method).39
All procedures were performed in compliance with the Association for Research in Vision and Ophthalmology resolution on the use of animals in research and were approved by the Washington University Animal Care and Use Committee. A minimal number of animals were used. General and local anesthesia as well as postoperative analgesics and aseptic technique were employed for all surgeries. Awake recordings used only positive reinforcement (automated delivery of a bolus of fruit drink) and were not stressful for the animals.
Search Coil/Head Restraint Implantation and Recording Method
Detailed descriptions of the training, surgical and recording methods have been described in a previous report.37 After initial fixation training, scleral search coils were implanted beneath the conjunctiva in both eyes, and a custom-built, polycarbonate head restraint device was attached to the skull. Eye movements were recorded using standard magnetic search coil techniques.37,40 During each recording session, the monkey sat in a primate chair in the middle of field coils and viewed initially a small laser spot (subtending approximately 0.05°) projected onto the back of a translucent screen located 50 cm in front of the animal. The head restraint precluded head movement. Recordings were performed under conditions of binocular and monocular viewing for each animal. Monocular viewing was achieved by positioning an opaque plastic occluder, hinged to the head restraint, in front of either eye. Voltages proportional to horizontal and vertical eye position were digitized at 500 Hz. Eye velocity signals were obtained by passing the eye position signals through a finite impulse response filter (DC to 90 Hz) and differentiated. Angular resolution of the system was approximately 0.05°. Experiments were controlled and the data were acquired and analyzed with the aid of a computer and interactive signal processing software (Spike 2 for Macintosh; Cambridge Electronic Design, Cambridge, UK; and Igor Graphics, Wave Metrics, Lake Oswego, OR).
OKN Stimulus
Large-field vertical and horizontal OKN was evoked under conditions of monocular or binocular viewing using horizontally or vertically oriented, 100% contrast, black and white square-wave gratings (0.1 cyc/deg) back projected onto the tangent screen. The stimulus subtended a visual angle of 90° × 90° horizontally and vertically and moved at a velocity of 30°/s in 60-second trials. The screen was blanked for a period of 90 seconds between all trials, and the animal sat in dim background room illumination to allow dissipation of any OKN after-nystagmus.1 The animals were rewarded for looking at the center of the stimulus screen at the start of each OKN trial (i.e., passive or “stare” OKN) independent of the eye velocity achieved during the trial (active “pursue/look” OKN).41,42
Data Analysis
The beginning and the end points of a slow phase trace were marked using a computer cursor to calculate the peak velocity for each slow phase. Means were obtained from ∼20 slow phase peak velocity epochs measured over the first 25 seconds of the response. Nasalward and upward velocities were denoted as positive; temporalward (TW) and downward were denoted as negative. OKN gain was calculated as the ratio of mean peak eye velocity to target (stripe motion) velocity. Horizontal gain was measured for trials of nasalward versus temporalward stripe motion. Vertical gain was measured for trials of upward versus downward stripe motion. For the majority of trials, viewing was monocular and responses from left versus right eye viewing were pooled. A nasotemporal asymmetry index (NTAI) was calculated as the ratio of average nasalward gain to average temporalward gain; an up-down asymmetry index (UDAI) was calculated as the ratio of upward gain to downward gain. The curved lines for plotted data were calculated and drawn in automated fashion using spline interpolation by the method of piecewise polynomials (Igor Graphics).43 Mean velocities were compared between controls and animals at increasing durations of prism-rearing by one-tailed ANOVA. Significance was defined as P < 0.05.
Results
Strabismus and Visuomotor Signs
Table 1 lists the visuomotor findings of the eight animals (six strabismic, two controls) at the time of testing (a minimum of 1 year after removal of the goggles). The control and the 3-week prism-duration monkeys had normal, orthophoric eye alignment (designated “nonstrabismic 3-week” in the Results that follow). The 6-, 9-, and 12-week monkeys all had constant esotropic (convergent) strabismus. The angle of the horizontal strabismus tended to be larger the longer the duration of the infant prism rearing. As in human patients with early-onset esotropia, the strabismus was concomitant (nonparalytic, of constant angle in different gazes).44 Horizontal and vertical saccadic velocities in the strabismic animals were within the 95% confidence intervals for control monkeys in our laboratory (i.e., no animal had evidence of extraocular muscle paresis).
The strabismic animals also had the constellation of visuomotor signs that typify the infantile strabismus syndrome in children: latent nystagmus (LN, latent/manifest latent or “fusion maldevelopment”); horizontal pursuit asymmetry; and dissociated vertical deviation (DVD).44,45 The hyperdeviations in Table 1 are the magnitude of the DVD averaged across eyes in a given animal. The LN in the strabismic animals was low velocity, evident as linear nasalward and upward slow phases. The highest peak and mean slow phase velocities were recorded in the 12-week animals: less than 1.0°/s nasalward and less than 0.22°/s upward. These values were negligible compared with the OKN stimulus velocity of 30°/s. As discussed in previous reports, the nystagmus represents a minimal, nonzero velocity baseline value that cannot account for asymmetries of smooth pursuit or OKN in strabismic humans or monkeys.46,47 Monocular visual acuity was measured without correction for refractive error using spatial sweep VEPs. Acuity was approximately equal (within 0.2 logMAR) in both eyes of the control and strabismic animals (i.e., no strabismic amblyopia).
Horizontal OKN Asymmetry
Horizontal OKN responses were symmetric in the control monkeys and in the nonstrabismic 3-week monkeys. When viewing monocularly with either eye, average slow-phase peak velocity gains exceeded 0.6 for both nasalward and temporalward directions of stimulus motion. In contrast, the strabismic 6-, 9-, and 12-week monkeys had asymmetric OKN favoring nasalward motion with respect to the viewing eye. Table 2 catalogs mean slow-phase velocities for each animal and each direction of stimulus motion. Figure 2 shows representative OKN eye position records in a control monkey (AY) and the strabismic 9-week animal (YI), viewing with the left eye. The animals had average nasalward (in Figs. 2A, 2B rightward) gains of 0.67 and 0.76. The temporalward gain of the control monkey was 0.65 but that of the strabismic 12-week animal was only 0.27 (Figs. 2C, 2D leftward).
Table 2.
OKN Velocities in Response to Horizontal or Vertical Stimulus Motion
|
Slow-Phase Peak Velocity (mean ± SD) Response to Stimulus Motion |
||||||||
|
NW |
TW |
UW |
DW |
|||||
| Controls | Horizontal | Vertical | Horizontal | Vertical | Horizontal | Vertical | Horizontal | Vertical |
| WE, 3-wk plano | +23.1 ± 5 | +3.0 ± 5 | −22.4 ± 4 | +3.0 ± 4 | −3.3 ± 1 | +25.2 ± 7 | −2.1 ± 2 | −20.0 ± 6 |
| AY, 12-wk plano | +20.3 ± 7 | +4.3 ± 6 | −27.1 ± 3 | +4.2 ± 6 | +3.0 ± 9 | +24.6 ± 9 | +2.1 ± 10 | −20.2 ± 8 |
| Prism reared | ||||||||
| ZA, 3-wk prism, eso | +17.4 ± 3 | +5.0 ± 1 | −18.6 ± 5 | +4.0 ± 2 | +4.2 ± 4 | +24.3 ± 6 | +2.0 ± 3 | −11.0 ± 5* |
| EO, 6-wk prism, eso | +24.3 ± 5 | +6.6 ± 3* | −11.5 ± 6* | +4.7 ± 3 | +5.8 ± 6* | +27.6 ± 6 | +2.2 ± 3 | −8.2 ± 7* |
| WY, 6-wk prism, eso | +22.8 ± 4 | +5.1 ± 4 | −9.0 ± 5* | +4.3 ± 3 | +4.5 ± 6 | +26.9 ± 5 | +2.0 ± 3 | −8.1 ± 7* |
| YI, 9-wk prism, eso | +20.7 ± 3 | +7.7 ± 2* | −9.1 ± 7* | +6.7 ± 4* | +8.5 ± 4* | +26.7 ± 11 | +7.6 ± 9* | −8.3 ± 9* |
| QE, 12-wk prism, eso | +16.9 ± 5 | +6.8 ± 2* | −6.0 ± 4* | +4.4 ± 1 | +7.5 ± 7* | +29.6 ± 7 | +3.1 ± 5 | −5.3 ± 3* |
| SY, 12-wk prism, eso | +27.4 ± 11 | +7.5 ± 1* | −8.1 ± 7* | +4.0 ± 1 | +3.8 ± 2 | +30.7 ± 6 | +3.6 ± 2 | −10.0 ± 6* |
+, nasalward or upward; −, temporalward or downward; eso, esotropic strabismus.
Differs from control values at significance level P < 0.05.
Figure 2.
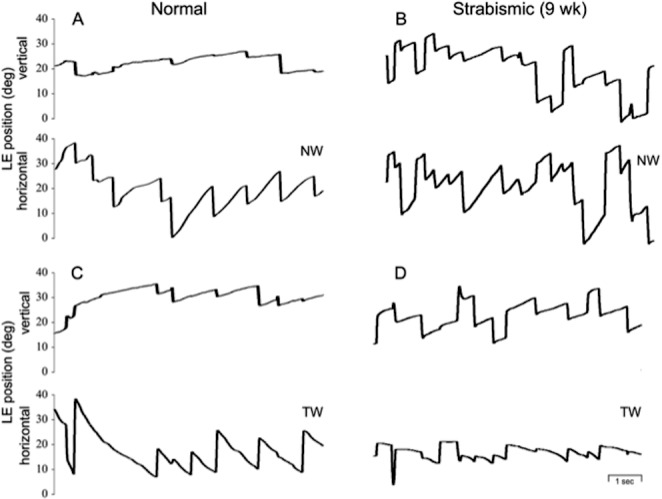
Horizontal OKN trials in normal versus strabismic monkey viewing with the left eye during NW versus TW stimulus motion. In the normal monkey (A, C), horizontal OKN responses were symmetric: slow-phase eye velocity was comparable for NW versus TW stripe motion. In the strabismic 9-week monkey (B, D), OKN was asymmetric: slow-phase eye velocity was robust for NW OKN and weaker for TW OKN. Vertical eye position tracings show upward-directed slow-phase crosstalk, which was minimal in the normal monkey but more pronounced in the strabismic monkey.
The NTAI for these two and the other six monkeys are plotted in Figure 3. An NTAI of 1.0 indicates symmetric OKN: equivalent nasalward versus temporalward gains. An NTAI > 1 indicates asymmetric OKN favoring nasalward motion. The controls (c) and the nonstrabismic 3-week monkeys had NTAI of 0.74 and 0.98, whereas each strabismic monkey had an NTAI of 2.2 or more (ANOVA, P = < 0.05 for temporalward velocities, controls versus each strabismic monkey; Table 2). The largest NTAI (2.7–3.4) were recorded in the strabismic 12-week monkeys (i.e., the animals exposed to the longest duration of binocular noncorrespondence in early infancy). The NTAI also tended to be larger in monkeys with larger angles of esotropic strabismus (Fig. 3). No systematic rightward-leftward asymmetries were evident under conditions of binocular viewing (data not shown).
Figure 3.
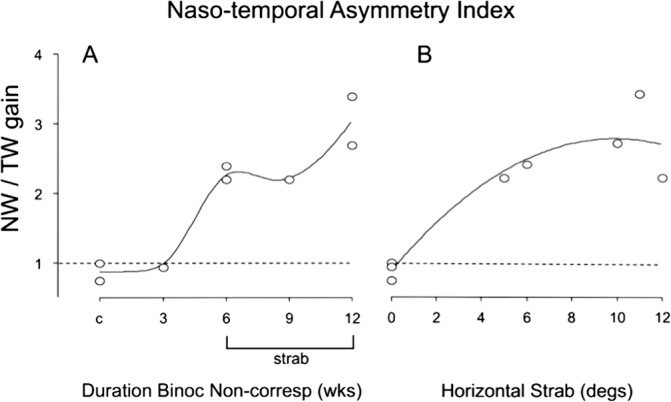
NTAI for the eight monkeys, as a function of duration of prism-rearing (A) and angle of esotropic strabismus (B). Each of the strabismic monkeys (duration of prism rearing: 6–12 weeks) had an NTAI > 2 and the magnitude of the NTAI tended to increase with angle of strabismus. Horizontal dashed line: no asymmetry = 1.
Vertical OKN Asymmetry
Vertical OKN responses were asymmetric in all monkeys, favoring upward stimulus motion. The asymmetry was slight in the two control monkeys and more pronounced in the six monkeys reared with binocular noncorrespondence. Figure 4 shows representative vertical OKN eye position records in a control monkey (WE) and a strabismic 12-week animal (SY), viewing with the left eye. Figure 4A and 4B display responses to upward stripe motion, and Figure 4B and 4C display responses to downward motion. Average slow-phase peak velocity gain for upward motion was 0.8 (25 ± 7°/s) in the control monkey and 1.0 (30 ± 6°/s) in the strabismic monkey; downward gains were 0.67 and 0.34, respectively. Upward peak velocities were comparable in all of the animals. But downward velocities were lower than controls in each of the monkeys reared with binocular noncorrespondence (Table 2; one-tailed ANOVAs, P < 0.05).
Figure 4.
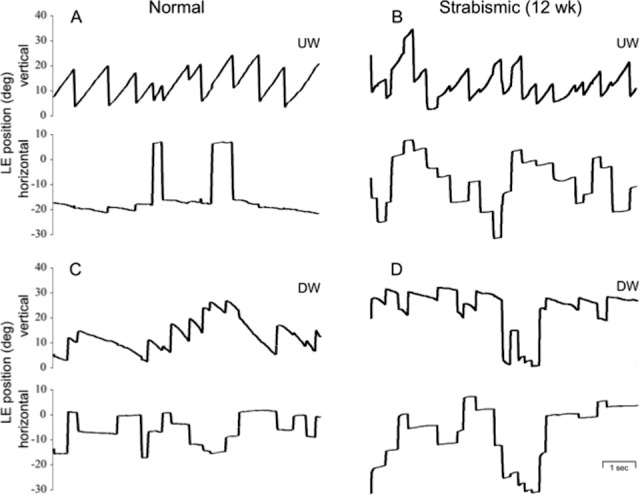
Vertical OKN trials in normal versus strabismic monkey viewing with the left eye during UW versus DW stimulus motion. In the normal monkey (A, C), slow-phase eye velocity was greater for UW OKN and less for DW OKN. In the strabismic 12-week monkey (B, D), the up-down asymmetry was more pronounced. Horizontal eye position tracings show minimal—mainly temporalward—slow-phase crosstalk in the normal monkey. Crosstalk was nasalward during UW and DW OKN in the strabismic monkey.
The UDAIs are plotted in Figure 5. An UDAI > 1 indicates asymmetric OKN favoring upward motion. The controls (c) had UDAI of 1.20 to 1.25, whereas each monkey reared with binocular noncorrespondence had a UDAI of 2.18 or more (range, 2.18–6.5). The largest UDAI (3.0–6.5) were recorded in the strabismic monkeys (i.e., those exposed to binocular noncorrespondence in infancy for 6 weeks or longer). The UDAI tended to be larger in monkeys with larger angles of vertical deviation (Fig. 5). The strabismic 9-week and 12-week NHPs, who had the weakest downward OKN, had occasional epochs of “wrong way” upward slow-phases during trials of downward stripe motion (not shown). No systematic differences were evident across animals when viewing binocularly, other than that gains for either direction of motion were ∼10% higher.
Figure 5.
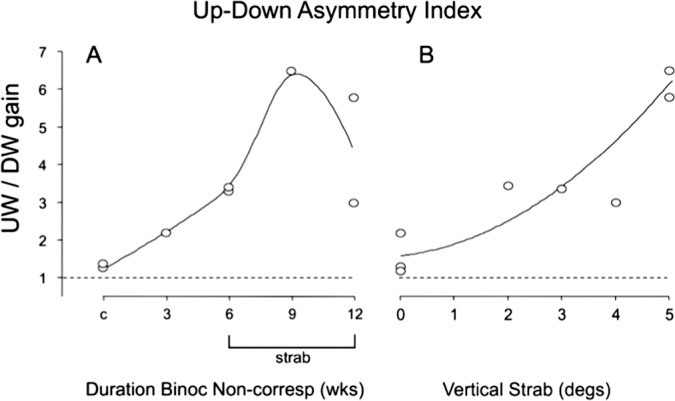
UDAI for the eight monkeys, as a function of duration of prism-rearing (A) and angle of vertical strabismus (B). Each of the prism-reared monkeys had a UDAI > 2, and the permanently strabismic monkeys had an UDAI > 3. UDAI tended to increase with the angle of vertical strabismus. Horizontal dashed line: no asymmetry = 1.
Upward Crosstalk During Horizontal OKN
The eye position records of Figure 2 show an additional, noteworthy feature of the OKN responses. Horizontal stimulus motion evoked a vertical “crosstalk” or diagonal eye movement. The NW and TW slow phases were accompanied by upward slow-phases. The control monkey (Figs. 2A, 2C) had upward velocities that were ∼12% of the horizontal velocities, and the strabismic monkey (Figs. 2B, 2D) upward that were ≥35% of horizontal.
Upward crosstalk was quantified as a percentage: upward velocity divided by the absolute value of horizontal (Fig. 6), so that a positive percentage represents upward crosstalk. For trials of nasalward stimulus motion, upward OKN crosstalk velocities were 13% to 22% of horizontal in control monkeys, versus 25% to 38% in prism-reared monkeys (upward peak velocities in controls: range, 3.0 ± 5 to 4.3 ± 6°/s; in prism-reared, 5.0 ± 1 to 7.7 ± 2°/s). For trials of temporalward motion, upward OKN velocities were 14% to 15% of horizontal in the control versus 22% to 66% in the prism-reared monkeys (Fig. 6). The largest upward crosstalk percentages were recorded in the strabismic monkeys during temporalward OKN. The larger crosstalk was due mainly to smaller denominators (i.e., lower TW eye velocity); temporalward OKN eye velocity was lower than nasalward in each strabismic monkey (Table 2). However, larger numerators (i.e., higher UW eye velocity) were an additional factor contributing to greater crosstalk in the 6-, 9-, and 12-week strabismic monkeys.
Figure 6.
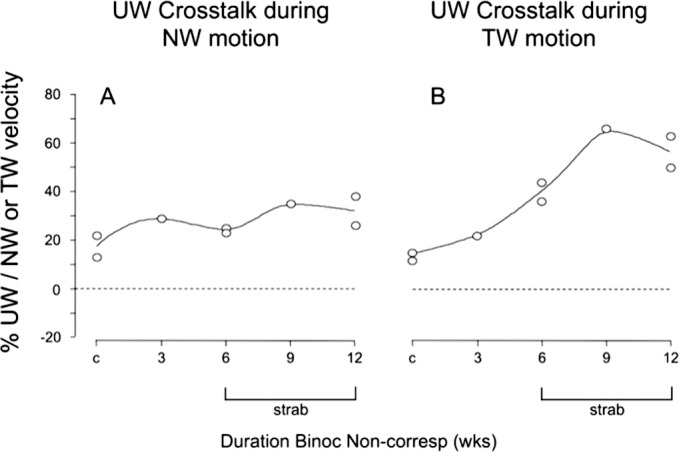
Upward crosstalk during NW (A) versus TW (B) OKN. All monkeys, normal and strabismic, had some degree of upward crosstalk during horizontal OKN. Upward crosstalk was more pronounced for weaker TW OKN in the strabismic animals.
Nasalward Crosstalk During Vertical OKN
The eye position records of the strabismic 12-week monkey in Fig. 4B and 4D show that vertical stimulus motion also evoked diagonal crosstalk. Upward and downward slow-phase OKN was accompanied by nasalward slow-phases. The largest nasalward crosstalk was recorded in the 9- and 12-week strabismic monkeys during downward OKN.
Nasalward crosstalk was quantified as nasalward velocity divided by the absolute value of vertical velocity (Fig. 7), so that positive values represent nasalward crosstalk. The control monkeys had horizontal crosstalk velocities ranging from −13% temporalward during upward OKN, to +10% nasalward during downward OKN. The prism-reared monkeys showed only nasalward crosstalk, ranging from 9% to 81%. During upward OKN, the larger crosstalk percentages in the prism-reared monkeys were due to larger numerators (i.e., higher NW eye velocity), not smaller denominators (i.e., lower UW eye velocity). Horizontal velocities during vertical OKN were largest in the strabismic 6-, 9-, and 12-week monkeys (Table 2, P < 0.05). During downward OKN, the larger nasalward crosstalk percentages in the strabismic 9- and 12-week monkeys were due mainly to smaller denominators (i.e., substantially lower downward OKN velocities).
Figure 7.
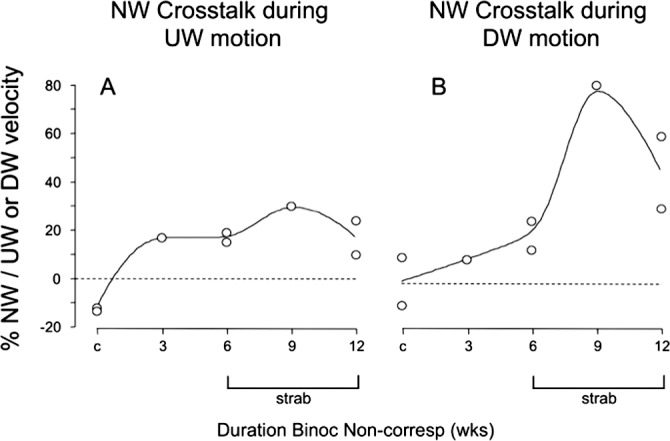
Nasalward crosstalk during UW (A) versus DW (B) OKN. Each of the prism-reared monkeys had some degree of nasalward crosstalk during vertical OKN. Nasalward crosstalk was more pronounced for weaker DW OKN in the strabismic animals.
OKN Crosstalk, Asymmetry Indices, and Directional Bias Polarity
To help clarify the relationship between crosstalk and directional asymmetries of horizontal and vertical OKN in each of the monkeys, we plotted percentage crosstalk as a function of the asymmetry index (Fig. 8). Recall that NTAI > 1 indicates a nasalward bias of horizontal OKN, and a UDAI > 1 indicates an upward bias of vertical OKN. Upward crosstalk increased roughly with increasing NTAI (Pearson product moment correlation, r = 0.69; P = 0.055). Nasalward crosstalk increased systematically with increasing UDAI (Pearson, r = 0.98; P < 0.01).
Figure 8.
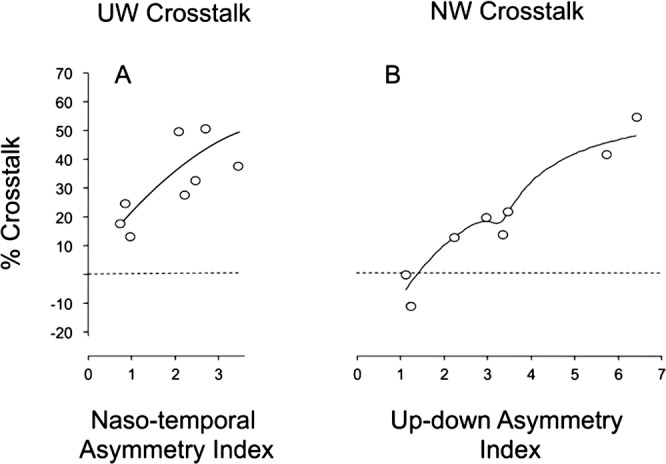
Magnitude of upward (A) or nasalward (B) crosstalk as a function of NTAI or UDAI. Crosstalk tended to increase the greater the asymmetry index.
The diagonal strength of OKN for each of the four directions of stimulus motion tested (nasalward, upward, temporalward, downward) is shown in the Cartesian coordinate graph of Figure 9. Each data point is the peak velocity of an individual OKN slow phase. The four clouds of points are four separate OKN trials, recorded in a control monkey (Fig. 9A) and a strabismic monkey (Fig. 9B) viewing with the left eye. The normal animal has equivalent nasal- and temporalward OKN responses, with a minimal shift upward of the velocity clouds from the zero axis (i.e., minimal upward crosstalk during horizontal OKN). The normal animal's vertical responses are asymmetric, favoring upward OKN. Peak velocity for upward OKN averaged 28 ± 8°/s and downward 22 ± 6°/s, with a minimal shift of each cloud in favor of temporalward crosstalk. The strabismic animal's (Fig. 9B) horizontal OKN responses are biased in favor of nasalward motion. Upward crosstalk is also more pronounced. The strabismic animal's vertical OKN is characterized by a greater bias favoring both upward motion and nasalward crosstalk (upward peak velocity 32 ± 9°/s; downward 9 ± 5°/s).
Figure 9.
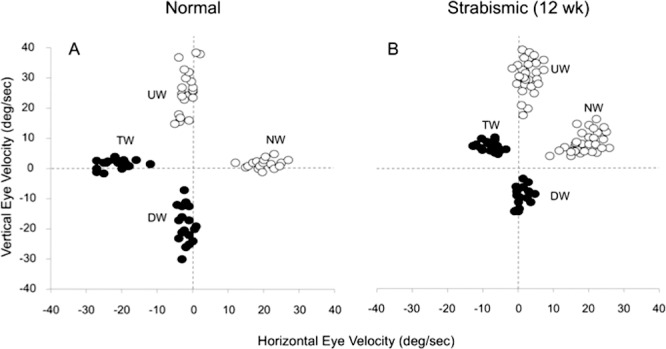
Slow-phase eye velocity for all four directions of stripe motion in a normal (A) versus strabismic (B) monkey. Each data point equals an individual slow-phase during a trial. For horizontal OKN, the normal animal (A) had symmetric NW versus TW slow-phase responses and minimal UW crosstalk. Horizontal OKN in the strabismic monkey (B) was asymmetric, with more robust NW OKN. UW crosstalk during NW versus TW OKN was more pronounced in the strabismic monkey. Vertical OKN in the normal animal (A) was mildly asymmetric, favoring UW motion; horizontal crosstalk was minimal TW. Vertical OKN in the strabismic monkey (B) was more asymmetric, favoring UW motion; horizontal crosstalk was more pronounced NW.
Vector summation of the strength of horizontal OKN, vertical OKN, and diagonal crosstalk for each of the eight animals is shown in the polar plots of Figure 10. Equal strength OKN responses for the four directions of stimulus motion would be represented by arrows at the origin with no length or direction. The two control monkeys had OKN responses biased mildly for upward and temporalward stimulus motion. The six monkeys reared under conditions of binocular noncorrespondence all had more pronounced OKN biases: in each case upward and nasalward. The magnitude of the bias was greatest in the strabismic 6-, 9-, and 12-week animals.
Figure 10.
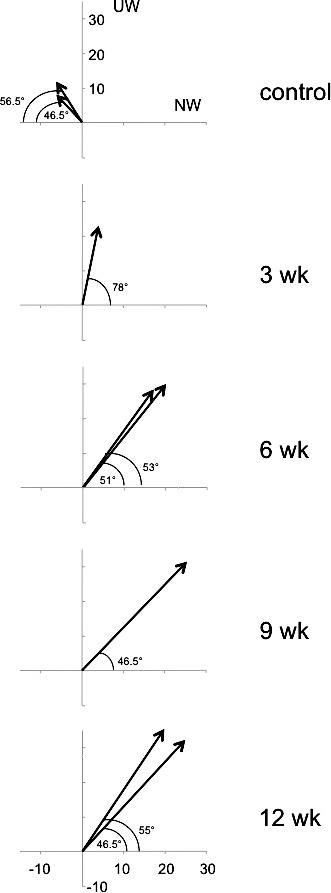
Vector summation of average slow-phase eye velocity for horizontal OKN, vertical OKN and diagonal crosstalk in each of the eight animals. The two control monkeys had OKN responses biased mildly for UW and TW motion. The six prism-reared monkeys had more pronounced OKN biases, UW and NW.
Discussion
The goal of this study was to describe vertical and horizontal OKN in NHPs with normal binocular vision, and to compare their responses with NHPs with binocular maldevelopment induced by prism-rearing. Our results reveal four main findings. First, NHPs reared in early infancy under conditions of binocular noncorrespondence for durations of 6 weeks or longer have horizontal OKN responses that are biased directionally in favor of nasalward motion. Second, NHPs reared with any duration of binocular noncorrespondence have vertical OKN responses that are more biased than normal NHPs in favor of upward motion. Third, diagonal crosstalk during horizontal or vertical OKN is present to some degree in all NHPs. And fourth, diagonal crosstalk—upward during horizontal OKN and nasalward during vertical OKN—is most pronounced in NHPs reared with binocular noncorrespondence long enough to induce a permanent esotropic strabismus (i.e., longer than 3 weeks).
Horizontal OKN Asymmetry
Infant NHPs and infant humans have an asymmetry of horizontal OKN when viewing monocularly, favoring nasalward stimulus motion. The asymmetry resolves in the first 3 to 6 weeks of life in monkeys, and in the first 3 to 6 months of life in humans if the infant has normal binocular experience.6,8–10,48 Disruption of binocular vision during this critical period—by strabismus, amblyopia, or both—causes a persistent nasalward OKN asymmetry.11–15 Our results reinforce these findings. We found a nasalward bias of horizontal OKN in each strabismic monkey. As reported previously,17,18 the magnitude of the nasalward bias (quantified as NTAI) tended to increase with duration of binocular noncorrespondence and angle of strabismus (Fig. 2).
Nasalward biases are a general feature of perceptual and visuomotor behavior in primates before onset of binocular fusion and stereopsis. In the first months of life, monocular VEPs elicited by oscillating grating stimuli (motion VEPs) show a nasotemporal asymmetry.49,50 Monocular preferential-looking testing reveals greater sensitivity to nasalward motion.51,52 Monocular smooth pursuit is biased for nasalward target motion53,54 and fusional vergence is biased toward convergence.55,56
If binocular development in children is impeded by perinatal damage to the cerebral inputs to striate cortex (area V1), or lack of eyeball clarity (i.e., amblyopia from a congenital cataract), the nasalward biases persist and become exaggerated.57,58 Perturbing binocular experience in infant NHPs produces analogous nasalward biases.16–18,34,47,59–62 The common pathophysiology is fusion maldevelopment from lack of corresponding (synchronous, correlated) monocular visual signals.18 Esotropia is the most common form of strabismus in children and the most common form of naturally occurring strabismus in NHPs.63–66 Humans and NHPs with the infantile strabismus (fusion maldevelopment) syndrome have nasotemporal asymmetries of motion VEPs, nasalward slow-phase LN, and nasalward biases of both smooth pursuit and OKN.18,60,66 In NHPs, the nasalward bias increases with duration of exposure to binocular noncorrespondence, and loss of binocular connections in V1.17,18,60,67
Vertical OKN Asymmetry
In normal adult NHPs and normal adult humans, vertical OKN is asymmetric. Upward stimulus motion evokes more robust OKN than downward motion.19–27 An upward bias of vertical OKN in humans has been reported consistently in studies using magnetic search coil eye movement recordings. Conflicting results have been reported when EOG or infrared recording methods are used, which are subject to eyelid interference artifact during vertical eye motion.68 Our results are compatible with these findings. Upward peak velocities in the two normal monkeys were 20% to 25% greater than downward velocities (Fig. 4).
The up-down asymmetry is exaggerated in infancy. In some normal infants, downward OKN is absent altogether.19 Downward OKN gain improves from age 1 month to 1 year, but at age 1 year is still only ∼50% that of normal adults. Children and adults who had infantile-onset strabismus may retain the deficit of downward OKN, as their up-down OKN asymmetries can exceed those of control subjects.25,28,29
Our findings in the strabismic NHPs are similar. Each of the strabismic NHPs had upward OKN gains equivalent to—but downward OKN gains substantially less than—those of the control NHPs. The relative deficit of downward OKN (Figs. 3, 4) was greatest in the NHPs exposed to the longest durations of binocular noncorrespondence.
Crosstalk and Directional Biases of Horizontal-Vertical OKN
Hainline et al.19 used the term “crosstalk” to describe diagonal slow-phase eye movement evoked during strictly horizontal or vertical stimulus motion in healthy human infants: “In many infant records, we saw evidence that OKN in one direction was ‘contaminated' by movements in the orthogonal dimension.” They reported that crosstalk was prominent only during trials of downward OKN; horizontal eye movement averaged 61% of the amplitude of downward eye movement. The infants viewed binocularly, and the crosstalk component was idiosyncratically rightward or leftward. They reported that crosstalk was absent in the OKN records of healthy adults.
Garbutt et al.29 reported horizontal crosstalk during vertical OKN in strabismic adults. Their patients who had the classical stigmata of infantile-onset strabismus (LN and DVD, subjects 1–4) had weak downward OKN and the greatest crosstalk during downward OKN. The direction of the horizontal crosstalk during downward OKN was generally in the same direction as the directional bias during monocular horizontal OKN. They did not find vertical crosstalk during horizontal OKN.
Our findings in strabismic NHPs are in general agreement with these reports in human, albeit we found crosstalk during both horizontal and vertical OKN. The greatest crosstalk occurred during trials that required strabismic animals to generate OKN in the direction of their weakest responses. And the crosstalk component was in the same direction as their directional biases: nasalward and upward. For vertical stimulus motion, strabismic NHPs had trouble generating downward OKN, and the crosstalk was nasalward. For horizontal stimulus motion, strabismic NHPs had trouble generating temporalward OKN, and the crosstalk was upward. The greater crosstalk percentages (Figs. 6–9) were attributable to two factors. First, temporalward and downward velocities were weak in all strabismic animals; and second, cross axis upward and nasalward velocities were larger in four of the strabismic animals.
Why in the presence of strabismus do the tracking pathways retain a nasalward and upward bias? Garbutt et al.29 suggested that these represent a counterbalance to optic flow. During forward locomotion, optic flow excites temporalward and downward visual motion (the ground being closer and more textured than the sky). The smooth tracking system would counter this flow by weighting for nasalward and upward motion. The weighting may be less necessary when binocular fusion stabilizes gaze. If fusion fails to develop, the immature weighting persists.
Crosstalk Movements of the Viewing Eye Versus Cross-Axis Movements of the Nonviewing Eye in Strabismic Macaques
The crosstalk we report here occurred in the viewing eye. Stimulus motion was strictly horizontal or strictly vertical, yet the viewing eye's response deviated from the strictly horizontal or vertical axis. The diagonal crosstalk was not dependent on orbital position of the eye, which changes throughout any given OKN trial. Thus, extraocular muscle paresis, muscle length adaptation, or orbital pulley mechanical factors do not explain the crosstalk response.69 Each strabismic monkey we recorded had a DVD, but DVD in monkeys—as in strabismic humans—does not occur in the fixating (viewing) eye; it is manifest only in the nonfixating (deviated, suppressed, covered) eye.45
Das and colleagues have published an elegant series of studies describing cross-axis movements of the nonfixating eye in macaques with early-onset, nonparalytic (“concomitant”) strabismus similar to our monkeys.70–73 During smooth pursuit and saccade tracking along a strictly horizontal or vertical axis, their monkeys show cross-axis responses in the nonfixating eye. By recording from vertical burst-tonic motoneurons of the brainstem, they documented that firing rates of vertical motoneurons correlated with vertical eye movements whether the movement was appropriate, as in vertical smooth pursuit when the eye was fixating, or whether it was inappropriate, as in a vertical component observed during horizontal smooth pursuit when the eye was nonfixating. The cross-axis movement in the nonfixating eye, while distinct from the fixating eye crosstalk we report, is similar in directional bias. When the fixating eye pursues nasalward target motion, the nonfixating eye moves conjugately temporalward but also cross-axis–upward; when the fixating eye pursues upward, the nonfixating eye moves conjugately upward but also cross-axis–nasalward (see Fig. 6 on page 3863 of Ref. 73).
Pathophysiology of the OKN Biases
Major cerebral areas that process visual motion for generating horizontal pursuit and OKN include V1, the medial temporal (MT), and the medial superior temporal (MST) visual areas.1 These areas in turn project to visuomotor neurons in downstream nuclei of the brainstem, including the nucleus of the optic tract (NOT).4,74,75 Neuroanatomical studies of strabismic NHPs indicate a paucity of binocular connections between the ocular dominance columns of area V1.18,65,67,76,77 Monocular connections are retained. Physiological recordings in strabismic NHP have shown that the V1 deficit of binocularity is passed forward to areas MT and MST.47,61 The responses of MT neurons in these animals are monocular (eye specific). Although MT in strabismic NHPs shows no bias for nasalward motion, the output of MT neurons responsive to temporalward motion appears to be impeded disparately by the lack of binocular connections. Many eye-specific MST neurons have a nasalward smooth tracking preference, a preference not found in the binocular MST neurons of normal primates.61 Findings in NOT of strabismic NHPs are similar; neurons are preponderantly eye specific and have a nasalward bias.34
In both humans and NHPs with early-onset strabismus, vertical pursuit is biased for upward motion.46,47 The slow-phases of LN often have a small, upward component.16,78 It would be useful to link these upward smooth eye movements physiologically to the upward bias of OKN we describe. Yet little is known about vertical tracking pathways in NHPs, normal or strabismic. Area MT contains neurons sensitive to all directions of visual motion and in normal NHPs, MT shows no preference for upward motion.79–81 A small (∼10%) upward bias is evident in MT of strabismic NHPs.47 MST in normal NHPs has an abundance of vertical-smooth-tracking–related neurons, but it remains to be determined—in normal or strabismic NHP—if up-down asymmetries are present. In NOT, there are no vertical pursuit or vertical OKN neurons per se. Nonetheless, NOT neurons, like those of MT or MST, have broad directional tuning.34,82 So there is neuronal modulation during oblique/diagonal directions of visual motion. The modulation reveals a small upward bias of many NOT neurons in both normal and strabismic NHPs.34 Vertical pursuit neurons are located preponderantly within the brainstem nucleus of the posterior commissure.1 The lateral terminal nucleus is also responsive to vertical motion. It will be important to determine if the neurons within these nuclei disclose an upward eye tracking asymmetry.
Acknowledgments
Supported by Grant EY10214 from the National Institutes of Health.
Disclosure: F. Ghasia, None; L. Tychsen, None
References
- 1. Leigh RJ, Zee DS. The Neurology of Eye Movements. New York, NY: Oxford University Press; 1999. [Google Scholar]
- 2. Hoffmann KP. Neural basis for changes of the optokinetic reflex in animals and men with strabismus and amblyopia. In: Lennerstrand G. ed Strabismus and Amblyopia. London: McMillan Press; 1988: 89–98 [Google Scholar]
- 3. Mustari MJ, Fuchs AF. Discharge patterns of neurons in the pretectal nucleus of the optic tract (NOT) in the behaving primate. J Neurophysiol. 1990; 64: 77–90 [DOI] [PubMed] [Google Scholar]
- 4. Hoffmann KP, Distler C, Ilg U. Callosal and superior temporal sulcus contributions to receptive field properties in the macaque monkey's nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract. J Comp Neurol. 1992; 321: 150–162 [DOI] [PubMed] [Google Scholar]
- 5. Mustari MJ, Fuchs AF, Kaneko CRS, Robinson FR. Anatomical connections of the primate pretectal nucleus of the optic tract. J Comp Neurol. 1994; 349: 111–128 [DOI] [PubMed] [Google Scholar]
- 6. Atkinson J. Development of optokinetic nystagmus in the human infant and monkey infant: an analogue to development in kittens. In: Freeman RD. ed Developmental Neurobiology of Vision. New York, NY: Plenum Publishing; 1979: 277–287 [Google Scholar]
- 7. Braddick AA. Development of optokinetic nystagmus in the human infant and monkey infant. In: Fisher DF, RA Monty, Senders JW. eds Eye Movements: Cognition and Visual Perception. Hillsdale, NJ: L. Erlbaum Associates; 1981. [Google Scholar]
- 8. Naegele JR, Held R. The postnatal development of monocular optokinetic nystagmus in infants. Vision Res. 1982; 22: 341–346 [DOI] [PubMed] [Google Scholar]
- 9. Mohn G. The development of binocular and monocular optokinetic nystagmus in human infants. Invest Ophthalmol Vis Sci. 1989. (suppl 3): 30–49 [Google Scholar]
- 10. Distler C, Vital-Durand F, Korte R, Korbmacher H, Hoffmann KP. Development of the optokinetic system in macaque monkeys. Vision Res. 1999; 39: 3909–3919 [DOI] [PubMed] [Google Scholar]
- 11. Schor CM. Subcortical binocular suppression affects the development of latent and optokinetic nystagmus. Am J Optom Physiol Optics. 1983; 60: 481–502 [DOI] [PubMed] [Google Scholar]
- 12. Sparks D, Marys L, Gurski M, Hickey T. Long- and short-term monocular deprivation in the rhesus monkey: effects on visual fields and optokinetic nystagmus. J Neurosci. 1986; 6: 1771–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demer JL, von Noorden GK. Optokinetic asymmetry in esotropia. J Pedriatr Ophthalmol Strabismus. 1988; 25: 286–292 [DOI] [PubMed] [Google Scholar]
- 14. Tychsen L, Leibole M, Drake D. Comparison of latent nystagmus and nasotemporal asymmetries of optokinetic nystagmus in adult humans and macaque monkeys who have infantile strabismus. Strabismus. 1996; 4: 171–177 [DOI] [PubMed] [Google Scholar]
- 15. Schor CM, Levi DM. Disturbances of small-field horizontal and vertical optokinetic nystagmus in amblyopia. Invest Ophthalmol Vis Sci. 1980; 19: 668–683 [PubMed] [Google Scholar]
- 16. Tusa RJ, Mustari MJ, Burrows AF, Fuchs AF. Gaze-stabilizing deficits and latent nystagmus in monkeys with brief, early-onset visual deprivation: eye movement recordings. J Neurophysiol. 2001; 86: 651–661 [DOI] [PubMed] [Google Scholar]
- 17. Wong AMF, Foeller P, Bradley D, Burkhalter A, Tychsen L. Early versus delayed repair of infantile strabismus in macaque monkeys: I. Ocular motor effects. J AAPOS. 2003; 7: 200–209 [DOI] [PubMed] [Google Scholar]
- 18. Tychsen L. Causing and curing infantile esotropia in primates: the role of de-correlated binocular input (an American Ophthalmological Society Thesis). Trans Am Ophthalmol Soc. 2007; 105: 564–593 [PMC free article] [PubMed] [Google Scholar]
- 19. Hainline L, Lemerise E, Abramov I, Turkel J. Orientational asymmetries in small-field optokinetic nystagmus in human infants. Behav Brain Res. 1984; 13: 217–230 [DOI] [PubMed] [Google Scholar]
- 20. Wei G, Lafortune SH, Ireland DJ, Jell RM. Stimulus velocity dependence of human vertical optokinetic nystagmus and afternystagmus. J Vestib Res. 1992; 2: 99–106 [PubMed] [Google Scholar]
- 21. Pasik P, Pasik T, Valciukas JA, Bender MB. Vertical optokinetic nystagmus in the split-brain monkey. Exp Neurol. 1971; 30: 162–171 [DOI] [PubMed] [Google Scholar]
- 22. Murasugi CM, Howard IP. Up-down asymmetry in human vertical optokinetic nystagmus and afternystagmus: contributions of the central and peripheral retinae. Exp Brain Res. 1989; 77: 183–192 [DOI] [PubMed] [Google Scholar]
- 23. Clement G. A review of the effects of space flight on the asymmetry of vertical optokinetic and vestibulo-ocular reflexes. J Vestib Res. 2003; 13: 255–263 [PubMed] [Google Scholar]
- 24. van den Berg AV, Collewign H. Directional asymmetries of human optokinetic nystagmus. Exp Brain Res. 1988; 70: 597–604 [DOI] [PubMed] [Google Scholar]
- 25. Valmaggia C, Proudlock F, Gottlob I. Look and stare optokinetic nystagmus in healthy subjects and in patients with no measurable binocularity. A prospective study. Klin Monbl Augenheilkd. 2005; 222: 196–201 [DOI] [PubMed] [Google Scholar]
- 26. Matsuo V, Cohen B. Vertical optokinetic nystagmus and vestibular nystagmus in the monkey: up-down asymmetry and effects of gravity. Exp Brain Res. 1984; 53: 197–216 [DOI] [PubMed] [Google Scholar]
- 27. Takahashi M, Sakurai S, Kanzaki J. Horizontal and vertical optokinetic nystagmus in man. ORL J Otorhinolaryngol Relat Spec. 1978; 40: 43–52 [DOI] [PubMed] [Google Scholar]
- 28. Tychsen L, Hurtig RR, Thalacker J. Impaired downward smooth pursuit in infantile strabismus. Invest Ophthalmol Vis Sci. 1984; 25 (suppl 3): 74 [Google Scholar]
- 29. Garbutt S, Han Y, Kumar AN, Harwood M, Harris CM, Leigh RJ. Vertical optokinetic nystagmus and saccades in normal human subjects. Invest Ophthalmol Vis Sci. 2003; 44: 3833–3841 [DOI] [PubMed] [Google Scholar]
- 30. Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Ann Rev Neurosci. 1985; 8: 495–546 [DOI] [PubMed] [Google Scholar]
- 31. O'Dell C, Boothe RG. The development of stereoacuity in infant rhesus monkeys. Vision Res. 1997; 37: 2675–2684 [DOI] [PubMed] [Google Scholar]
- 32. Tychsen L, Yildirim C, Anteby I, Boothe R, Burkhalter A. Macaque monkey as an ocular motor and neuroanatomic model of human infantile strabismus. In: Lennerstrand G, Ygge J. eds Advances in Strabismus Research: Basic and Clinical Aspects. London: Wenner-Gren International Series, Portland Press Ltd.; 2000: 103–119 [Google Scholar]
- 33. Yildirim C, Tychsen L. Disjunctive optokinetic nystagmus in a naturally esotropic macaque monkey: interactions between nasotemporal asymmetries of versional eye movement and convergence. Ophthalmic Res. 2000; 32: 172–180 [DOI] [PubMed] [Google Scholar]
- 34. Mustari MJ, Tusa RJ, Burrows AF, Fuchs AF, Livingston CA. Gaze-stabilizing deficits and latent nystagmus in monkeys with early-onset visual deprivation: Role of the pretectal NOT. J Neurophysiol. 2001; 86: 662–675 [DOI] [PubMed] [Google Scholar]
- 35. Tychsen L, Scott C. Maldevelopment of convergence eye movements in macaque monkeys with small and large-angle infantile esotropia. Invest Ophthalmol Vis Sci. 2003; 44: 3358–3368 [DOI] [PubMed] [Google Scholar]
- 36. Crawford ML, Harwerth RS, Smith EL, von Noorden GK. Loss of stereopsis in monkeys following prismatic binocular dissociation during infancy. Behav Brain Res. 1996; 79: 207–218 [DOI] [PubMed] [Google Scholar]
- 37. Foeller P, Tychsen L. Eye movement training and recording in alert macaque monkeys: 1. Operant visual conditioning 2. Magnetic search coil and head restraint surgical implantation 3. Calibration and recording. Strabismus. 2002; 10: 5–22 [DOI] [PubMed] [Google Scholar]
- 38. Norcia AM, Tyler CW. Spatial frequency sweep VEP: visual acuity during the first year of life. Vision Res. 1985; 25: 1399–1408 [DOI] [PubMed] [Google Scholar]
- 39. Quick MW, Boothe RG. A photographic technique for measuring horizontal and vertical eye alignment throughout the field of gaze. Invest Ophthalmol Vis Sci. 1992; 33: 234–246 [PubMed] [Google Scholar]
- 40. Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966; 21: 1068–1070 [DOI] [PubMed] [Google Scholar]
- 41. Honrubia V, Downey WL, Mitchell DP, Ward PH. Experimental studies on optokinetic nystagmus. II. Normal humans. Acta Otolaryngol. 1968; 65: 441–448 [DOI] [PubMed] [Google Scholar]
- 42. Yee R, Baloh R, Honrubia V. Pathophysiology of optokinetic nystagmus. In: Honrubia V, Brazier MH. eds Nystagmus and Vertigo. London: Academic Press; 1982: 251–275 [Google Scholar]
- 43. Subbotin YN. Spline interpolation. Encyclopedia of Mathematics. 2001. Available at: http://www.encyclopediaofmath.org/index.php/Spline_interpolation. Accessed September 30, 2013 [Google Scholar]
- 44. Tychsen L. Infantile esotropia: Current neurophysiologic concepts. In: Rosenbaum AL, Santiago AP. eds Clinical Strabismus Management. Philadelphia, PA: WB Saunders; 1999: 117–138 [Google Scholar]
- 45. Tychsen L. Latent Nystagmus and Dissociated Vertical-Horizontal Deviation. In: CS Hoyt, Taylor D. eds Pediatric Ophthalmology and Strabismus. London: Elsevier Science Ltd.; 2012: 901–908 [Google Scholar]
- 46. Tychsen L, Lisberger SG. Maldevelopment of visual motion processing in humans who had strabismus with onset in infancy. J Neurosci. 1986; 6: 2495–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kiorpes L, Walton PJ, O'Keefe LP, Movshon JA, Lisberger SG. Effects of artificial early-onset strabismus on pursuit eye movements and on neuronal responses in area MT of macaque monkeys. J Neurosci. 1996; 16: 6537–6553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Atkinson J, Braddick O. Development of optokinetic nystagmus in the human infant and monkey infant. In: Fisher DF, RA Monty, Senders JW. eds Eye Movements: Cognition and Visual Perception. Hillsdale, NJ: Lawrence Erlbaum Associates; 1981: 53–64 [Google Scholar]
- 49. Norcia AM, Garcia H, Humphry R, Holmes A, Hamer RD, Orel-Bixler D. Anomalous motion VEPs in infants and in infantile esotropia. Invest Ophthalmol Vis Sci. 1991; 32: 436–439 [PubMed] [Google Scholar]
- 50. Wilson JR, Noyd WW, Aiyer AD, Norcia AM, Mustari MJ, Boothe RG. Asymmetric responses in cortical visually evoked potentials to motion are not derived from eye movements. Invest Ophthalmol Vis Sci. 1999; 40: 2435–2439 [PubMed] [Google Scholar]
- 51. Bosworth RG, Birch EE. Motion detection in normal infants and young patients with infantile esotropia. Vision Res. 2005; 45: 1557–1567 [DOI] [PubMed] [Google Scholar]
- 52. Bosworth RG, Birch EE. Direction-of-motion detection and motion VEP asymmetries in normal children and children with infantile esotropia. Invest Ophthalmol Vis Sci. 2007; 48: 5523–5531 [DOI] [PubMed] [Google Scholar]
- 53. Jacobs M, Harris C, Taylor D. The development of eye movements in infancy. In: Lennerstrand G. ed Update on Strabismus and Pediatric Ophthalmology. Proceedings of the Joint ISA and AAPO&S Meeting, Vancouver, Canada. Boca Raton, FL: CRC Press; 1994: 140–143 [Google Scholar]
- 54. Tychsen L. Critical periods for development of visual acuity. Depth perception and eye tracking. In: Bailey Jr DB, Bruer JT, Symons FJ, Lichtman JW. eds Critical Thinking About Critical Periods. Baltimore, MD: Paul H; Brookes Publishing Co.; 2001: 67–80 [Google Scholar]
- 55. Horwood A, Williams B. Does neonatal ocular misalignment predict later abnormality? Eye. 2001; 15: 485–491 [DOI] [PubMed] [Google Scholar]
- 56. Horwood AM, Riddell PM. Can misalignments in typical infants be used as a model for infantile esotropia? Invest Ophthalmol Vis Sci. 2004; 45: 714–720 [DOI] [PubMed] [Google Scholar]
- 57. Khanna S, Sharma A, Inder T, Tychsen L. Prevalence of the ocular motor signs of the infantile strabismus complex in children with and without cerebral visual pathway white matter injury. Invest Ophthalmol Vis Sci. 2009; 49: 1209 [Google Scholar]
- 58. Tychsen L. Visual cortex mechanisms of strabismus: development and maldevelopment. In: Lorenz B, Brodsky MC. eds Pediatric Ophthalmology, Neuro-Ophthalmology, Genetics. Berlin Heidelberg: Springer-Verlag; 2010: 41–57 [Google Scholar]
- 59. Tusa RJ, Mustari MJ, Das VE, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann NY Acad Sci. 2002; 956: 346–360 [DOI] [PubMed] [Google Scholar]
- 60. Tychsen L, Wong AMF, Foeller P, Bradley D. Early versus delayed repair of infantile strabismus in macaque monkeys: II. Effects on motion visually evoked responses. Invest Ophthalmol Vis Sci. 2004; 45: 821–827 [DOI] [PubMed] [Google Scholar]
- 61. Mustari MJ, Ono S, Vitorello KC. How disturbed visual processing early in life leads to disorders of gaze-holding and smooth pursuit. Prog Brain Res. 2008; 171: 487–495 [DOI] [PubMed] [Google Scholar]
- 62. Hasany A, Wong A, Foeller P, Bradley D, Tychsen L. Duration of binocular decorrelation in infancy predicts the severity of nasotemporal pursuit asymmetries in strabismic macaque monkeys. 2008; 156: 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kiorpes L, Boothe RG. Naturally occurring strabismus in monkeys (Macaca nemestrina). Invest Ophthalmol Vis Sci. 1981; 20: 257–263 [PubMed] [Google Scholar]
- 64. Kiorpes L, Boothe RG, Carlson MR, Alfi D. Frequency of naturally occurring strabismus in monkeys. J Pedriatr Ophthalmol Strabismus. 1985; 22: 60–64 [DOI] [PubMed] [Google Scholar]
- 65. Tychsen L, Richards M, Wong A, et al. Spectrum of infantile esotropia in primates: behavior, brains and orbits. J AAPOS. 2008; 12: 375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tychsen L. Why do Humans develop strabismus?. In: CS Hoyt, Taylor D. eds Pediatric Ophthalmology and Strabismus. London: Elsevier Science Ltd.; 2012: 756–763 [Google Scholar]
- 67. Richards M, Tychsen L, Burkhalter A, Foeller P, Bradley D, Wong AMF. Early versus delayed correction of infantile strabismus in macaque monkeys: effects on horizontal binocular connections in the striate cortex. Neuro-Ophthalmology. 2007; 31: 171–174 [Google Scholar]
- 68. Knapp CM, Proudlock FA, Gottlob I. OKN asymmetry in human subjects: a literature review. Strabismus. 2013; 21: 37–49 [DOI] [PubMed] [Google Scholar]
- 69. Narasimhan A, Tychsen L, Poukens V, Demer JL. Horizontal rectus muscle anatomy in naturally and artificially strabismic monkeys. Invest Ophthalmol Vis Sci. 2007; 48: 2576–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Das VE, Fu LN, Mustari MJ, Tusa RJ. Incomitance in monkeys with strabismus. Strabismus. 2005; 13: 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Das VE, Mustari MJ. Correlation of cross-axis eye movements and motoneuron activity in non-human primates with “A” pattern strabismus. Invest Ophthalmol Vis Sci. 2007; 48: 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Joshi AC, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2011; 52: 6697–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Das VE. Responses of cells in the midbrain near-response area in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2012; 53: 3858–3864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fuchs AF, Mustari MJ. The optokinetic response in primates and its possible neuronal substrate. In: SA Miles, Wallman J. eds Visual Motion and its Role in the Stabilization of Gaze. New York, NY: Elsevier; 1993: 343–369 [PubMed] [Google Scholar]
- 75. Mustari MJ, Burrows A, Livingston C. Response properties of small-field pretectal nucleus of the optic tract neurons during smooth pursuit eye movements. Soc Neurosci. 1996; 22: 419 [Google Scholar]
- 76. Tychsen L, Burkhalter A. Neuroanatomic abnormalities of primary visual cortex in macaque monkeys with infantile esotropia: preliminary results. J Pediatr Ophthalmol Strabismus. 1995; 32: 323–328 [DOI] [PubMed] [Google Scholar]
- 77. Tychsen L, Wong AMF, Burkhalter A. Paucity of horizontal connections for binocular vision in V1 of naturally-strabismic macaques: cytochrome-oxidase compartment specificity. J Comp Neurol. 2004; 474: 261–275 [DOI] [PubMed] [Google Scholar]
- 78. Richards M, Wong A, Foeller P, Bradley D, Tychsen L. Duration of binocular decorrelation predicts the severity of latent (fusion maldevelopment) nystagmus in strabismic macaque monkeys. Invest Ophthalmol Vis Sci. 2008; 49: 1872–1878 [DOI] [PubMed] [Google Scholar]
- 79. Van Essen DC, Maunsell JHR, Bixby JL. The middle temporal visual area in the macaque: myeloarchitecture, connections, functional properties and topographic organization. J Comp Neurol. 1981; 199: 293–326 [DOI] [PubMed] [Google Scholar]
- 80. Maunsell JHR, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983; 49: 1127–1147 [DOI] [PubMed] [Google Scholar]
- 81. Albright TD. Directions and orientation selectivity of neurons in visual area MT of the macaque. J Neuro. 1984; 52: 1106–1128 [DOI] [PubMed] [Google Scholar]
- 82. Mustari MJ, Fuchs AF. Response properties of single units in the lateral terminal nucleus of the accessory optic system in the behaving primate. J Neurophysiol. 1989; 61: 1207–1220 [DOI] [PubMed] [Google Scholar]


