Abstract
Objective
To verify the influence of radiopaque fillers on an epoxy resin-based sealer.
Material and Methods
Experimental sealers were formulated by adding 20%, 40%, 60%, 80%, 100% and 120% of calcium tungstate, ytterbium trifluoride or barium sulphate by weight to an epoxy-resin-base. Setting time, flow, film thickness, radiopacity, sorption, solubility, pH and push-out bond strength were evaluated.
Results
The setting time ranged from 373 to 612.66 min, the flow varied from 13.81±0.49 to 22.49±0.37 mm, and the film thickness ranged from 16.67±5.77 to 33.33±11.54 µm. The lowest pH was 5.47±0.53, and the highest was 6.99±0.03. Radiopacity varied from 0.38±0.04 to 2.57±0.21 mmAl and increased with the amount of filler. Calcium tungstate sealers had a higher sorption and solubility than other sealers. There was no significant difference in the push-out bond strength among the fillers at the 120% concentration.
Conclusion
The inorganic fillers evaluated and their concentrations affect the physicochemical properties of an epoxy resin-based root canal sealer.
Keywords: Tungsten, Ytterbium, Barium, Root canal filling materials
INTRODUCTION
The success of endodontic therapy depends on a complete cleaning and three-dimensional filling of the root canal system. For ideal obturation of the canal, sealers must have certain characteristics, such as high radiopacity, flowability, adhesion to canal walls, reliable working time, biocompatibility and low solubility. Although no cement has all of these properties, some sealers, such as zinc oxide-eugenol-based, calcium hydroxide-based, glass-ionomer and resin-based, are clinically acceptable and widely used23,30. Despite this, epoxy resin-based cement presents suitable physicochemical properties2,7 and satisfactory clinical outcomes13. One of the most important properties of endodontic sealers is radiopacity. Cement radiopacity enhances diagnostic procedures and facilitates the diagnosis in Endodontics. To reach an acceptable radiopacity, inorganic fillers are added to endodontic sealers. Furthermore, these fillers improve the physical, chemical and mechanical properties of the sealer, such as viscosity and film thickness.
The radiopacity of dental materials depends on the composition of the materials (e.g., radiopaque agents) and the filler concentration. X-rays are either absorbed and/or scattered by material to produce radiographic images. Adequate radiopacity in images is necessary to avoid overtreatment and false-positive results and to obtain the proper clinical diagnosis. The chemical structures of agents that produce radiopacity in dental materials should be considered to obtain an acceptable material because the atomic number, density and size of fillers influence the radiopacity3. Elements with a high atomic number can absorb or reflect more X-rays leading to increased radiopacity1. Besides the effects on cement radiolucency, the inorganic fillers could affect the polymerization shrinkage11, degree of conversion4, polymerization rate11 rheological properties4 and water absorption14 when added to resins.
Calcium tungstate (CaWO4) and barium sulphate (BaSO4) are already used as fillers in commercial sealers with epoxy-resin and gutta-percha cones12,27, providing high level of radiopacity27. Ytterbium trifluoride (YbF3) contains ytterbium, which is in the lanthanide series with a high atomic number leading to a high opacity to X-rays. In addition, this filler is very translucent, which is a good optical characteristic for dental materials. Ytterbium trifluoride has already been incorporated in methacrylate-based cements5 and has shown favourable results for an endodontic sealer. However, additions of these radiopaquing fillers on epoxy resin-based root canal sealers properties were barely studied.
The purpose of this study was to evaluate the influence of the addition of different inorganic fillers on the physicochemical properties of an epoxy-resin based root canal sealer.
MATERIAL AND METHODS
Endodontic cements were prepared using an epoxy resin and inorganic fillers. The resin is composed of a bisphenol-A (Araldite® LY 1564, Huntsman Advanced Materials Química Brasil Ltda, Taboão da Serra, SP, Brazil) and a cycloaliphatic amine (Aradur 2963, Huntsman Advanced Materials Química Brasil Ltda, Taboão da Serra, SP, Brazil), base and catalyst, respectively. Base and catalyst were added at 2:1 ratio by weight. Calcium tungstate (CaWO4) (American Elements, Los Angeles, CA, USA), ytterbium trifluoride (YbF3) (American Elements, Los Angeles, CA, USA) and barium sulphate (BaSO4) (Labsynth Produtos para Laboratório Ltda, Diadema, SP, Brazil) at 20%, 40%, 60%, 80%, 100% and 120%, in weight, were added to the amount of epoxy resin base. Colloidal silica nanoparticles (7 nm) (Aerosil 380, Evonik Industries AG, Hanau, Hesse, Germany) were added at 0.5% by weight to adjust the viscosity of cements. Base, catalyst and inorganic fillers were manually mixed for 90 s on a glass plate until obtaining a homogeneous paste, checked visually. A control group without radiopaquing agent filler was included. The sealers were subjected to laboratory tests to characterize selected physicochemical properties, as following.
Particle size
The particle size distribution of each filler was assessed using a laser diffraction particle size analyser (CILAS 1180 Particle-Size-Analyzer, Compagnie Industrielle des Lasers, Orleans, Loiret, France).
The setting time of the experimental sealers was analysed according to ISO 6876:200115. The experimental sealer was placed in a silicone matrix that had an internal diameter of 10 mm and a height of 1 mm. An indenter with a mass of 100±0.5 g and a flat end with a diameter of 2.0±0.1 mm was placed vertically on the horizontal surface of the sealer for 2 s. The surface was then visually evaluated for indentations. Indentations were repeated every 30 min until no indentation was observed, and at this moment the setting time was recorded. The mean value of three measurements for each group was recorded for the setting time of the material.
Flow
The flow test was conducted according to ISO 6876:200115. A total of 0.5 ml of each experimental sealer was placed on a glass plate (40x40x5 mm) using a graduated 1.5 ml syringe. Another plate with a mass of 20±2 g and a load of 100 g was applied to the top of the material for 180±5 s after mixing. Ten minutes after mixing, the load was removed, and the major and minor diameters of the compressed material were measured using a digital caliper. The results were recorded if both measurements were consistent to within 1 mm. If the major and minor diameter discs were not uniformly circular or did not agree within a range of 1 mm, the test was repeated. For each experimental group, the test was conducted three times and the mean value was recorded for the flow.
Film thickness
The film thickness was evaluated according to ISO 6876:200115. Two glass plates that were 5 mm and 10 mm thick were placed together and their combined thickness was measured. A total of 0.5 mL of experimental sealer was placed at the centre of one of the plates, and a second plate was placed on top of the material. A 150 N load was applied vertically on the top of the glass plate 180±5 s after mixing. Ten minutes after the start of mixing, the thickness of the two glass plates and the sealer film was measured using a digital caliper. The difference in the thickness of the two glass plates, with and without sealer, was the film thickness of the experimental sealer. The mean value of three measurements for each sealer was recorded for the film thickness of the material.
Radiopacity
The radiopacity of the experimental sealers was tested according to ISO 6876:200115. Five specimens that were 10.0±0.1 mm in diameter and 1.0±0.01 mm thick were produced. Radiographic images were obtained using a phosphor plate digital system (VistaScan, Dürr Dental GmbH & Co. KG, Bietigheim-Bissingen, Baden-Württemberg, Germany) at 70 kV and 8 mA with a 0.2 s exposure time and a 400 mm focus-film distance. One specimen with the same percent filler from each group was positioned with a specimen from the control group for each film; there were a total of four specimens per film. In all images, an aluminium step-wedge was simultaneously exposed with the specimens. The aluminium step-wedge thickness ranged from 0.5 to 9.0 mm in increments of 0.5 mm. The aluminium alloy used was Al 99.12, Fe 0.47, Mg 0.41 and <0.1 of Cu (% by weight) according to ISO 6876. The images were saved in TIFF format and analysed using Photoshop (Adobe Systems Incorporated, San Jose, CA , USA). The means and standard deviations of the grey levels (pixel density) of the aluminium step-wedge and the specimens were obtained in a standardised area of 1.5 mm2 5.
Sorption and solubility
Water sorption and solubility tests were performed according to ISO 4049:200916, except for the specimen dimensions. The specimens had a diameter of 10±0.1 mm and a thickness of 1.0±0.1 mm. The specimens were placed in desiccators containing silica gel at 37ºC. Each specimen was weighed repeatedly at 24 h intervals on an analytical balance (AUW220D, Shimadzu Philippines Manufacturing Inc, Cavite, Calabarzon, Philippines) until a constant mass (m1) was obtained (i.e., until the specimen's decrease in mass was no more than 0.1 mg in a 24 h period). The diameter and thickness were measured with a digital caliper to calculate the volume (V) in mm3. Then, the specimens were placed in a light-free container with distilled water at 37ºC for 7 days. The specimens were removed from the liquid, and each specimen was weighed after being dried slightly. The weight was recorded (m2). The procedures to obtain m1 were repeated to obtain m3. Water sorption and solubility in micrograms per cubic millimetre were calculated according to a previous study6.
pH
The pH of the sorption and solubility water was evaluated using a digital pHmeter (pH 21, Hanna Instruments, São Paulo, SP, Brazil). Five measurements were performed per group.
Push-out bond strength
Thirty bovine incisors were sectioned transversely 15 mm from the apex, the pulp tissue was removed and the chemo-mechanical preparation was performed. Teeth were divided into 3 groups, and the canals were obturated by lateral condensation technique with gutta-percha points and the 120% concentration of calcium tungstate, ytterbium trifluoride and barium sulphate experimental cements. The roots were stored at 100% humidity and 37ºC for 7 days. Subsequently, the roots were sectioned transversely into 7 slices that were approximately 0.7 mm thick using a low speed disc (Isomet, Buehler Ltd, Lake Bluff, IL, USA) with constant water cooling. The internal diameter of canal of each slice was measured with a digital caliper (Digimess, 100.174BL, Digimess Instrumentos de Precisão Ltda, São Paulo, SP, Brazil) and the contact area between the filling and dentin of each slice was calculated. Each slice was placed with the apical side up on a mechanical testing machine (DL-2000, EMIC Equipamentos e Sistemas de Ensaio Ltda, São José dos Pinhais, PR, Brazil). A force was placed on the shutter towards the apical-neck using a 500 N load cell and a cross-head speed of 1 mm/min with a 1 mm diameter cylindrical device. The bond strength (MPa) was obtained by dividing the required force (N) to displace the filling material by the adhesive area (mm2).
Statistical analysis
Data normality was checked by the Kolmogorov-Smirnov test. Differences among filler compositions and control group were detected using ANOVA and the Tukey post-hoc test. Linear regression was performed to determine the influence of filler composition on radiopacity. A significance level of 5% was used for analysis.
RESULTS
The mean particle sizes of calcium tungstate, ytterbium trifluoride and barium sulphate were 17.79 µm, 14.37 µm and 4.86 µm, respectively. Table 1 shows the setting time, flow, film thickness, sorption, solubility and water pH of experimental sealers. The setting time of experimental sealers ranged from 373 to 612.66±4.71 min, the flow of experimental sealers ranged from 13.81±0.49 to 22.49±0.37 mm and the film thickness ranged from 16.67±5.77 to 33.33±11.54 µm. The pH of experimental sealers ranged from 5.47±0.53 to 6.99±0.03.
Table 1.
Means ± standard deviation for the setting time, flow, film thickness and pH of experimental sealers
| Filler concentration | ||||||
| 20% | 40% | 60% | 80% | 100% | 120% | |
| setting time (min) | ||||||
| CaWO4 | 451.66±4.71 | 430±8.17 | 453±8.17 | 509±0 | 501±0 | 448.66±11.79 |
| YbF3 | 612.66±4.71 | 570±7.07 | 546.66±4.71 | 373±0 | 388±0 | 378±0 |
| BaSO4 | 468.66±6.13 | 478.66±6.13 | 486.66±6.24 | 451.66±10.21 | 384.66±2.36 | 398.33±10.27 |
| flow (mm) | ||||||
| CaWO4 | 20.26±0.51 | 18.92±2.16 | 19.28±0.57 | 18.10±1.28 | 15.02±1.81 | 15.85±0.25 |
| YbF3 | 20.93±0.17 | 18.41±0.35 | 18.50±0.92 | 15.50±0.38 | 13.81±0.49 | 14.59±0.70 |
| BaSO4 | 22.49±0.37 | 21.36±0.37 | 19.43±0.21 | 17.96±0.21 | 18.08±1.21 | 17.69±0.17 |
| fllm thickness (pm) | ||||||
| CaWO4 | 23.33±5.77 | 30±0 | 26.67±5.77 | 26.67±5.77 | 33.33±5.77 | 23.33±11.55 |
| YbF3 | 16.67±5.77 | 26.67±5.77 | 33.33±5.77 | 30±0 | 30±0 | 33.33±5.77 |
| BaSO4 | 33.33±11.54 | 23.33±5.77 | 46.67±5.77 | 20±0 | 16.67±5.77 | 20±0 |
| pH | ||||||
| CaWO4 | 6.31±0.13 | 6.69±0.08 | 6.66±0.08 | 6.82±0.08 | 6.84±0.05 | 6.99±0.03 |
| YbF3 | 6.74±0.08 | 5.47±0.53 | 5.69±0.30 | 6.11±0.22 | 6.25±0.21 | 6.31±0.21 |
| BaSO4 | 6.28±0.09 | 6.27±0.10 | 6.37±0.12 | 6.40±0.10 | 6.45±0.16 | 6.51±0.19 |
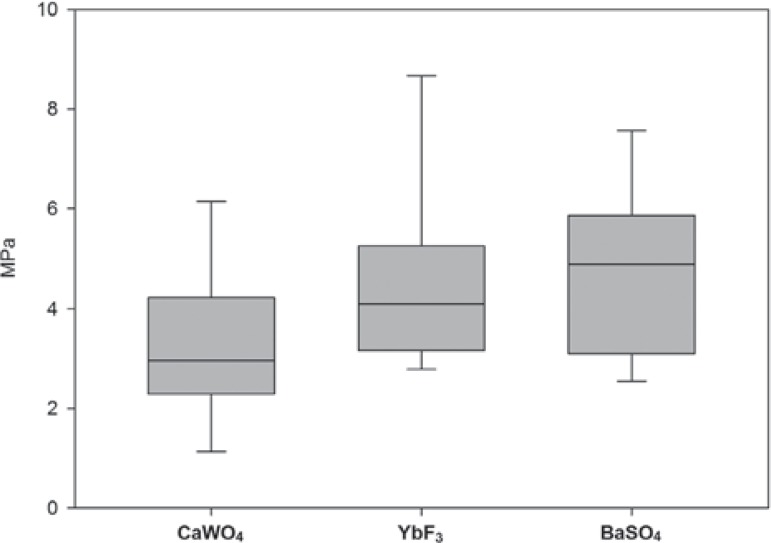
Radiopacity ranged from 0.38±0.04 to 2.57±0.21 mmAl (Table 2) and increased with the amount of filler (CaWO4 r2=0.996, YbF3 r2=0.983, BaSO4 r2=0.994; p<0.05). Table 3 show the water sorption and water solubility of experimental groups compared to the controls. The push-out bond strengths for groups containing 120% of filler were 3.26±1.34 MPa (CaWO4), 4.48±1.66 MPa (YbF3) and 4.73±1.53 MPa (BaSO4) and there was no significant difference between the experimental groups as shown in Figure 1.
Table 2.
Means ± standard deviation for the radiopacity (mmAl) of experimental sealers*
| Filler concentration | ||||||
| 20% | 40% | 60% | 80% | 100% | 120% | |
| CaWO4 | 0.44±0.04 | 0.91±0.06 | 1.24±0.15 | 1.56±0.14 | 1.98±0.10 | 2.43±0.05 |
| YbF3 | 0.38±0.04 | 0.76±0.20 | 1.47±0.37 | 1.63±0.11 | 2.07±0.05 | 2.57±0.21 |
| BaSO4 | 0.44±0.06 | 0.63±0.21 | 1.01±0.10 | 1.29±0.09 | 1.62±0.16 | 2.00±0.07 |
Radiopacity increases with the amount of the filler (CaWO4 r2=0.996, YbF3 r2=0.983, BaSO4 r2=0.994; p<0.05)
Table 3.
Means±standard deviation for the water sorption and solubility of experimental sealers
| 20% | 40% | 60% | 80% | 100% | 120% | |
| Sorption (pg/mm3) | ||||||
| CaWO4 | 145.57±2.81* | 182.08±12.15* | 167.53±12.09* | 236.25±22.46* | 183.21±6.35* | 200.72±3.67* |
| YbF3 | 69.24±6.54 | 79.14±5.10* | 80.10±1.93* | 62.36±4.18 | 67.75±5.97 | 70.01±7.06 |
| BaSO4 | 60.84±7.21 | 54.52±2.67 | 51.73±2.41* | 52.36±2.48* | 51.26±5.87* | 45.20±11.26* |
| Solubility (µg/mm3) | ||||||
| CaWO4 | 105.18±10.45* | 151.18±25.53* | 157.14±10.96* | 180.79±31.50* | 200.10±11.92* | 264.95±11.63* |
| YbF3 | 15.42±1.83* | 14.00±3.08* | 9.96±0.64 | 8.16±1.12 | 10.71±1.61 | 11.83±1.93 |
| BaSO4 | 10.44±2.58 | 10.84±2.17 | 10.02±1.10 | 13.16±0.68 | 13.15±0.92 | 8.11±8.7 |
Statistical difference (p<0.05) against control group. Water soprtion and solubility of control group are 64.28±3.79 μg/mm3 and 9.93±2.20 μg/mm3, respectively
Figure 1.
Push-out bond strength (MPa) of the groups with 120% filler by weight. There are no differences between groups (p>0.05)
DISCUSSION
Improved physical properties of materials and diagnoses could lead to a higher success rate for root canal treatments. The addition of radiopaque fillers to root canal sealers could interfere with the physical and mechanical properties of the sealers4,20. In this study, the concentration of the filler influenced the setting time, flow, film thickness, radiopacity, sorption and solubility of root canal sealers.
The setting time of endodontic sealers should be long enough to allow the filling of the entire root canal system without displacement of the obturation material and the formation of gaps to avoid subsequent bacterial leakage, which could decrease the longevity of the treatment. In this study, the setting time of the sealers ranged from approximately 6 to 10 h. There is no standard for the setting time of endodontic sealers according to ISO 6876. However, the setting time of experimental sealers was suitable to permit root canal filling within an adequate time.
The ability to penetrate accessory canals, dentinal tubules and constitute a thin layer between core materials (e.g. guta-percha points), in order to fill the smallest voids and prevent fluid percolation through the root canal system, is a concern in Endodontics, and this ability is related to flow and film thickness of sealers. All groups present flow nearest to 20 mm and film thickness under 50 µm, which are within the parameters outlined by ISO 6876. The flow and film thickness are directly influenced by the composition of the sealer, temperature and relative humidity. The literature presents different flow and film thickness values for commercial materials26,28. The flow of the other two commercial resin-based sealers also had flow and film thickness values lower than those outlined in ISO 6876. In addition, the flow of the sealer cannot be too high due to a possible periapical extrusion, which could compromise apical healing and lead to decreased tooth longevity24. The film thickness of experimental sealers was similar to widely used commercial sealers, which have film thicknesses of approximately 50 µm10.
The addition of radiopaque agents to root canal filling materials should ideally enable their visualisation and assessment on a radiograph without altering their chemical properties. The high atomic numbers of ytterbium (z=70), barium (z=56) and tungsten (z=74) could explain the increase in radiopacity as the amount of filler increases. Elements with high atomic numbers can absorb more X-rays, leading to a radiopaque image1,21. Experimental groups containing 120% radiopaque agent by weight were more radiopaque than 2 mm of aluminium, which allowed the sealer to be feasibly identified on a radiograph.
In this study, the sorption and solubility values of experimental sealers were influenced by the addition of radiopaque filler. Calcium tungstate groups had higher sorption and solubility values than other groups. These results may be explained by the calcium tungstate particle size, which has a higher mean diameter than ytterbium trifluoride and barium sulphate. The increased particle size causes the CaWO4 particles to be more soluble in water even though the solubility coefficient of calcium tungstate (2.39 mg/100 ml) is lower than those of ytterbium trifluoride (5.77 mg/100 ml) and barium sulphate (0.24 mg/100 ml). Water sorption and solubility have a significant influence on the mechanical properties and degradation of endodontic sealers. Sealers degrade over time as a result of the sorption/solubility process, which could promote resin/filler lixiviation9 and consequently cause porosities on obturation mass. The ISO 6876 details the normalisation of root canal sealing materials but does not consider resin-based material. For this reason, in this study we adopted ISO 4049, a standard for polymer-based filling and restorative and luting materials, even though it is not specific for root canal filling materials. According to ISO 4049, the water sorption of resin-based material cannot be higher than 40 µg/mm3 and the water solubility must be up to 7.5 µg/mm3. The values obtained from sealers containing different fillers, especially the calcium tungstate sealers, do not meet these standards. However, the ytterbium trifluoride and barium sulphate sealers had sorption and solubility values that met ISO 4049 standards. The matrix properties of root canal sealers are important features that predict the solubility of cements. The literature presents a wide range of solubility data for different compositions of sealers showing that the epoxy matrix is more resistant to water diffusion than other matrices, such as ionomeric and methacrylate, and water-based cements7. Furthermore, the process of periapical repair requires favourable conditions, such as the absence of microorganisms and an adequate pH8. Root canal sealers that are in close contact with periapical tissues could interfere with the periapical repair. Lixiviation of sealers as result of solubility could also cause changes in the pH of the periapical environment. An alkaline or neutral pH provides the best conditions for the healing process. All sealers tested in this study had a pH that was close to neutral.
The fluid leakage at root canal sealers interface is a concern to root canal filling longevity. To analyze the dentin-sealer interface, push-out bond strength test showed a good correlation with microleakage elsewhere22,29. In this study, the push-out bond strengths were tested for the groups with high radiopacity (120% filler by weight), and no significant differences were observed. The mean values of bond strength agreed with studies that evaluated commercial epoxy-based root canal sealers17. Theoretically, resin-based root canal sealers could have micromechanical retention with dentin substrate leading to a more stable interface that prevents degradation over time.
Despite the fact of being an in vitro study, the present study clearly identified differences in properties as function of filler type and concentration. The Dental Materials field has the obligation of research development of new materials and provide the experimental explanation of mechanisms involved in phenomena4,5,18-20,25. Furthermore, identifying these differences between fillers provides data to clinicians to take more evidence-based decisions regarding the acquisition of root canal sealers.
CONCLUSION
The inorganic fillers evaluated and their concentrations affect the physicochemical properties of an epoxy resin-based root canal sealer. Ytterbium trifluoride and barium sulphate at 120% of concentration showed adequate properties to be used as fillers at epoxy resin-based root canal sealers.
REFERENCES
- 1.Aoyagi Y, Takahashi H, Iwasaki N, Honda E, Kurabayashi T. Radiopacity of experimental composite resins containing radiopaque materials. Dent Mater J. 2005;24:315–320. doi: 10.4012/dmj.24.315. [DOI] [PubMed] [Google Scholar]
- 2.Bernardes RA, Amorim Campelo A, Junior DS, Pereira LO, Duarte MA, Moraes IG, et al. Evaluation of the flow rate of 3 endodontic sealers: Sealer 26, AH Plus, and MTA Obtura. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e47–e49. doi: 10.1016/j.tripleo.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Bowen RL, Cleek GW. X-ray-opaque reinforcing fillers for composite materials. J Dent Res. 1969;48:79–82. doi: 10.1177/00220345690480013101. [DOI] [PubMed] [Google Scholar]
- 4.Collares FM, Leitune VC, Rostirolla FV, Trommer RM, Bergmann CP, Samuel SMW. Nanostructured hydroxyapatite as filler for methacrylate-based root canal sealers. Int Endod J. 2012;45:63–67. doi: 10.1111/j.1365-2591.2011.01948.x. [DOI] [PubMed] [Google Scholar]
- 5.Collares FM, Ogliari FA, Lima GS, Fontanella VR, Piva E, Samuel SM. Ytterbium trifluoride as a radiopaque agent for dental cements. Int Endod J. 2010;43:792–797. doi: 10.1111/j.1365-2591.2010.01746.x. [DOI] [PubMed] [Google Scholar]
- 6.Collares FM, Ogliari FA, Zanchi CH, Petzhold CL, Piva E, Samuel SM. Influence of 2-hydroxyethyl methacrylate concentration on polymer network of adhesive resin. J Adhes Dent. 2011;13:125–129. doi: 10.3290/j.jad.a18781. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly A, Sword J, Nishitani Y, Yoshiyama M, Agee K, Tay FR, et al. Water sorption and solubility of methacrylate resin-based root canal sealers. J Endod. 2007;33:990–994. doi: 10.1016/j.joen.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Estrela C, Sydney GB, Bammann LL, Felippe O., Júnior Mechanism of action of calcium and hydroxyl ions of calcium hydroxide on tissue and bacteria. Braz Dent J. 1995;6:85–90. [PubMed] [Google Scholar]
- 9.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymernetworks. Dent Mater. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Gambarini G, Testarelli L, Pongione G, Gerosa R, Gagliani M. Radiographic and rheological properties of a new endodontic sealer. Aust Endod J. 2006;32:31–34. doi: 10.1111/j.1747-4477.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonçalves F, Azevedo CL, Ferracane JL, Braga RR. BisGMA/ TEGDMA ratio and filler content effects on shrinkage stress. Dent Mater. 2011;27:520–526. doi: 10.1016/j.dental.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Gurgel-Filho ED, Andrade Feitosa JP, Teixeira FB, Monteiro de Paula RC, Araújo Silva JB, Souza-Filho FJ. Chemical and X-ray analyses of five brands of dental gutta-percha cone. Int Endod J. 2003;36:302–307. doi: 10.1046/j.1365-2591.2003.00653.x. [DOI] [PubMed] [Google Scholar]
- 13.Hale R, Gatti R, Glickman GN, Opperman LA. Comparative analysis of carrier-based obturation and lateral compaction: a retrospective clinical outcomes study. Int J Dent. 2012;2012:954675–954675. doi: 10.1155/2012/954675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu SH, Chen RS, Chang YL, Chen MH, Cheng KC, Su WF. Biphenyl liquid crystalline epoxy resin as a low-shrinkage resin-based dental restorative nanocomposite. Acta Biomater. 2012;8:4151–4161. doi: 10.1016/j.actbio.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 15.International Organization for Standardization . ISO 6876: Dental root canal sealing materials. Geneva: ISO; 2001. [Google Scholar]
- 16.International Organization for Standardization . ISO 4049: Dentistry - Polymer-based restorative materials. Geneva: ISO; 2009. [Google Scholar]
- 17.Lawson MS, Loushine B, Mai S, Weller RN, Pashley DH, Tay FR, et al. Resistance of a 4-META-containing, methacrylate-based sealer to dislocation in root canals. J Endod. 2008;34:833–837. doi: 10.1016/j.joen.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Leitune VC, Collares FM, Takimi A, Lima GB, Petzhold CL, Bergmann CP, et al. Niobium pentoxide as a novel filler for dental adhesive resin. J Dent. 2013;41:106–113. doi: 10.1016/j.jdent.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Leitune VC, Collares FM, Trommer RM, Andrioli DG, Bergmann CP, Samuel SM. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J Dent. 2013;41:321–327. doi: 10.1016/j.jdent.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Leitune VC, Takimi A, Collares FM, Santos PD, Provenzi C, Bergmann CP, et al. Niobium pentoxide as a new filler for methacrylate-based root canal sealers. Int Endod J. 2013;46:205–210. doi: 10.1111/j.1365-2591.2012.02107.x. [DOI] [PubMed] [Google Scholar]
- 21.Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–576. [Google Scholar]
- 22.Neelakantan P, Subbarao C, Subbarao CV, De-Deus G, Zehnder M. The impact of root dentine conditioning on sealing ability and push-out bond strength of an epoxy resin root canal sealer. Int Endod J. 2011;44:491–498. doi: 10.1111/j.1365-2591.2010.01848.x. [DOI] [PubMed] [Google Scholar]
- 23.Ng YL, Mann V, Gulabivala K. Outcome of secondary root canal treatment: a systematic review of the literature. Int Endod J. 2008;41:1026–1046. doi: 10.1111/j.1365-2591.2008.01484.x. [DOI] [PubMed] [Google Scholar]
- 24.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of non-surgical root canal treatment: part 2: tooth survival. Int Endod J. 2011;44:610–625. doi: 10.1111/j.1365-2591.2011.01873.x. [DOI] [PubMed] [Google Scholar]
- 25.Portella FF, Santos PD, Lima GB, Leitune VC, Petzhold CL, Collares FM, et al. Synthesis and characterization of a glycerol salicylate resin for bioactive root canal sealers. Int Endod J. 2013 doi: 10.1111/iej.12149. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Resende LM, Rached-Junior FJ, Versiani MA, Souza-Gabriel AE, Miranda CE, Silva-Sousa YT, et al. A comparative study of physicochemical properties of AH Plus, Epiphany, and Epiphany SE root canal sealers. Int Endod J. 2009;42:785–793. doi: 10.1111/j.1365-2591.2009.01584.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanomaru-Fllho M, Tanomaru JM, Leonardo MR, Sllva LA. Periapical repair after root canal filling with different root canal sealers. Braz Dent J. 2009;20:389–395. doi: 10.1590/s0103-64402009000500006. [DOI] [PubMed] [Google Scholar]
- 28.Verslanl MA, Carvalho-Junlor JR, Padilha MI, Lacey S, Pascon EA, Sousa-Neto MD. A comparatlve study of physlcochemlcal propertles of AH Plus and Eplphany root canal sealants. Int Endod J. 2006;39:464–471. doi: 10.1111/j.1365-2591.2006.01105.x. [DOI] [PubMed] [Google Scholar]
- 29.Zlcarl F, Couthlno E, De Munck J, Poltevln A, Scottl R, Naert I, et al. Bonding effectiveness and sealing ability of fiber-post bondlng. Dent Mater. 2008;24:967–977. doi: 10.1016/j.dental.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Zmener O, Pameljer CH. Cllnlcal and radlographlc evaluatlon of a resln-based root canal sealer: an elght-year update. J Endod. 2010;36:1311–1314. doi: 10.1016/j.joen.2010.04.020. [DOI] [PubMed] [Google Scholar]