Abstract
Asymptomatic mouth floor enlargements may be observed in edentulous patients. These masses, which protrude from the mouth floor, may complicate the fitting of dentures and require surgery. Whether this "entity" may be considered an anatomical variation of the mouth floor or represent specific alterations in the sublingual gland is not known.
Objective
The aim of this work is to investigate the morphological and morphometric aspects of the sublingual glands of edentulous patients with mouth floor enlargements and compare the glands of these patients with the sublingual glands of human cadavers.
Material and Methods
Microscopic evaluation was performed on human sublingual glands from edentulous patients with mouth floor enlargements (n=20) and edentulous cadavers (n=20). The patients and cadavers were of similar ages. The data were compared using Mann-Whitney U, Fisher's exact and Student's t tests (p<0.05).
Results
Acinar atrophy, duct-like structures, mononuclear infiltrates, replacement of parenchyma with fibrous/adipose tissue, mucous extravasation and oncocytosis were similar between the groups (p>0.05). Only the variables "autolysis" and "congested blood vessels" presented statistical difference between groups (p=0.014; p=0.043). The morphometric study revealed that the volume densities of acini, ducts, stroma and adipose tissue were similar between the groups (p>0.05).
Conclusion
The microscopic characteristics of the sublingual glands in mouth floor enlargements in edentulous patients correspond to characteristics associated with the normal aging process. The glands are not pathological and represent an age-related alteration that occurs with or without the presence of the mouth floor enlargements.
Keywords: Salivary glands, Sublingual gland, Morphology, Anatomy
INTRODUCTION
Asymptomatic mouth floor enlargements related to the sublingual glands (MFERSG) are observed in inferior edentulous and partially edentulous patients2,6. These masses, which protrude from the mouth floor and are covered by a normal-appearing mucosa, may complicate the fitting of dentures and require pre-prosthetic surgery6,8 (Figure 1).
Figure 1.
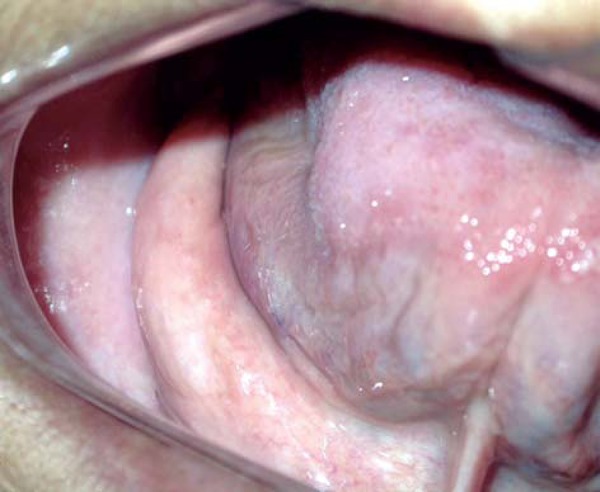
Mouth floor enlargement in a 62-year-old woman
The aetiology of these enlargements is not known, and the terminology is often unsuitable. Some of the names used to describe these enlargements include adenomatoid serous hyperplasia15, swelling or hypertrophy of the sublingual gland3, mouth floor enlargement related to sublingual glands in edentulous and partially edentulous patients5 and idiopathic hyperplasia of the sublingual gland in edentulous or partially edentulous patients6. Campos2 (1996) initially described these swellings as pathological entities and denoted them as "hyperplasia of the sublingual glands" related to the absence of posterior teeth. The aging process may also contribute to the etiopathogenesis of MFERSG6.
Iwaki Filho, et al.6 (2006) microscopically analysed specimens that were surgically removed from the enlarged mouth floors of 19 patients. These enlargements exhibited acinar atrophy, an increase in the number of duct-like structures and substitution of the glandular parenchyma with fat and connective tissue. Azevedo, et al.1 (2005) and Moreira, et al.9 (2006) identified similar characteristics as a result of the physiological aging process in human sublingual glands that were obtained from necropsies. Moreira, et al.9 (2006) identified a decrease in the total volume of the sublingual glands during aging, which conflicts with the apparent volume increase of the swellings.
Microscopic comparisons between the glands of MFERSG patients and those of inferior edentulous individuals without the enlargements may explain the aetiology of these enlargements. Whether this "entity" may be considered an anatomical variation of the mouth floor after teeth loss or represent specific macroscopic and microscopic alterations in the sublingual gland is not known. Therefore this study examined sublingual glands in MFERSG patients and compared them with glands from edentulous cadavers of similar ages who did not present the enlargements.
MATERIAL AND METHODS
The Human Research Ethics Committee of the Bauru School of Dentistry - University of São Paulo (process nº 010/2006) approved this study, and informed written consent in full accordance with ethical principles was obtained from each patient. The São Paulo Death Verification Service - University of São Paulo, where the glands of cadavers were obtained, also approved the research. All experiments followed the guidelines of the Helsinki Declaration.
The sample set included 20 human sublingual glands that were obtained from surgical treatments MFERSG patients6 (MFERSG group). These patients were edentulous or partially edentulous and were referred by the prosthodontist because they presented difficulties in fitting the mandibular dentures. Analysis of the their medical history revealed only systemic diseases as anemia and arterial hypertension. All patients had clinically normal parotid and submandibular glands, which were submitted to examination, palpation and milking. Physical aspects of the saliva such as volume, color and fluidity were absolutely normal6. The MFERSG patients underwent surgical excision under local anaesthesia during pre-prosthetic procedures. The specimens containing mucosa of the mouth floor and most part of the sublingual glands were dissected by divulsion6.
Twenty other human sublingual glands were obtained from edentulous cadavers during autopsies (Control group). Individuals with the following systemic disorders or base diseases were excluded from the study, using the methods and the exclusion criteria of Azevedo, et al.1 (2005): lymphoma, leukemia, mucoviscidosis, rheumatic diseases, Sjögren's syndrome, obesity, cachexia, diabetes mellitus, alcoholic cirrhosis, collagen diseases, history of head or neck cancer-related surgery, and history of cytotoxic drug administration or previous radiotherapy of the head or neck during the last 3 months and macroscopic autolysis. Data regarding the systemic involvement and cause of death were obtained from the familiar and autopsy reports. The main causes of death included pulmonary edema, bronchopneumonia, acute myocardial infarction, cerebral vascular accident, ischemic heart disease, cerebral edema and congestive heart failure. The interval between the time of death and autopsy ranged from 6:05 to 92:55 h, with a mean of 16:46 h. The cadaver glands were completely removed with a portion of the overlying mucosa of the mouth floor.
Specimens from both groups were age- and gender-matched when feasible. The age of both groups ranged from 40 to 79 years (±59.5 years). Only inferior-posterior edentulous cadavers were included because the enlargements are limited to edentulous individuals2,6. However, the presence of clinical mouth enlargements in this group was not detected. The specimens were fixed in 10% formalin and processed using routine procedures. The slides were stained with hematoxylin-eosin (H.E.), and a single pathology expert of the Bauru School of Dentistry performed the microscopic examinations.
Morphological study
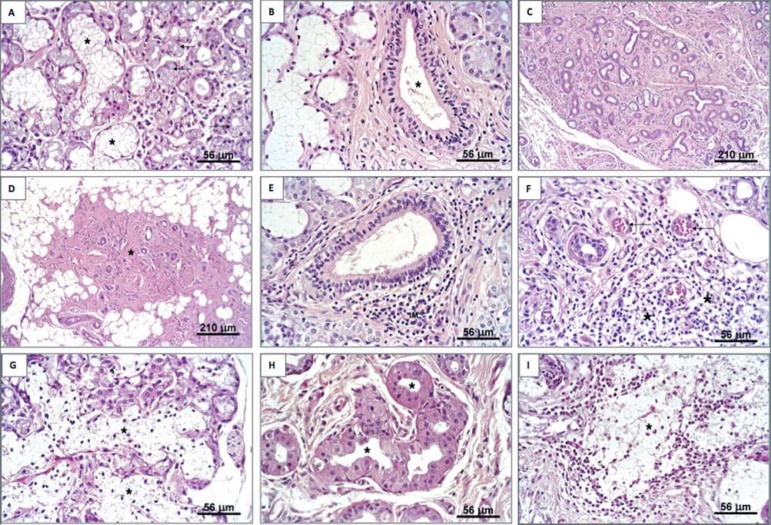
The criteria for the morphological analysis were established in a previous study1 and the same pathologist performed all examinations. Only three slices (anterior, middle and posterior) were processed for histology regardless of the gland size. The slices were scored using the following criteria: 1- acinar atrophy; 2- duct-like structures; 3- mononuclear infiltration; 4- replacement of parenchyma by fibrous tissue; 5- replacement of parenchyma by adipose tissue; 6- oncocytosis; 7- congested blood vessels; 8- acinar autolysis; and 9- mucous extravasation (Figure 2).
Figure 2.
Sublingual glands of cadavers and MFERSG patients (hematoxylin & eosin staining). a: Normal lobule (*) and atrophic acini (arrows). b: True duct (excretory interlobular). c: Intense acinar atrophy, presence of duct-like structures and intense substitution of the parenchyma by fibrous tissue. d: intense replacement with fibrous and adipose tissue containing a few traces of parenchyma (*). e: Periductal focal mononuclear infiltrate (IM). f: Diffuse mononuclear infiltrate (*) and congested blood vessels. g: Acinar autolysis (*). h: Oncocytosis (*) in ductal cells. i: Mucous extravasation (*) MFERSG=mouth floor enlargements related to the sublingual glands
In this study, "acinar atrophy" was defined as a decrease in the size of the acinar cells and/or the number of acini (Figure 2a). Many authors1,2,5,9,13,16,17 prefer to denote doubtful structures as "duct-like structures" because there is no guarantee that some of these structures are atrophic acini or ducts. We considered duct-like structures those with shrunken seromucous cells, granule depletion and widened lumens that are adjacent to ductal epithelial cells and resemble intralobular non-striated ducts (Figure 2c), which is consistent with the characterization used in our previous study1. We excluded true ducts, which were easily recognized in glands with normal parenchyma from the variable "duct-like structures" because true ducts exhibited a dilated lumen lined by cuboidal cells with central nuclei and an eosinophilic cytoplasm (Figure 2e). The distinction between a duct and a duct-like structure was impossible to discern in glands with advanced atrophy. True ducts were excluded only when the distinction was clear. Therefore, only ambiguous structures were included in the morphological study.
A degree of severity of the alterations in the glands was attributed to the variables. The microscopic findings were classified as discrete when they were observed in up to 1/3 of the section. The findings were moderate when 1/3-2/3 of the section was involved and intense when more than 2/3 of the section was affected. The severity degree was determined from the three slices of each gland to describe the microscopic variables that globally represented the gland. Scores reflected the microscopic characteristics and ranged from 0 to 3: 0- absent; 1- discrete; 2- moderate; and 3- intense1.
The scores of the three slices of each gland were submitted to a conversion scale to produce one representative score for each variable in each gland. The scores of the three slices from each gland were added to produce one value that varied from 0 (if all the initial scores were 0 - absent) to 9 (if all of the 3 initial scores were 3 - intense). This value was submitted to the following conversion scale: the final score was 0 if the sum was 0; 1 if the sum was 1-3; 2 if the sum was 4-6; and 3 if the sum was 7-9.
Morphometrical study
A microscope with a 40x objective and an 8x Zeiss Kpl eyepiece containing a Zeiss II integration grid with 10 parallel lines and 100 points that were symmetrically distributed over a quadrangular area was used for these evaluations. We selected 51 histological fields per gland by systematic randomization19, and the points (Pi) that coincided with the images of the following components were quantified: acini (serous, mucous or mixed), ducts and duct-like structures, stroma (connective tissue, blood vessels, septa, cells, nerves and inflammatory infiltrates), adipose tissue (quantified separately because of the frequent substitution of the parenchyma) and others (artefacts, points in the void and tissues not related to the gland, e.g. mouth floor epithelium)1,9. The total number of points (PT) was also obtained. Volume density (VVi) was calculated using the equation VVi=Pi/PT, and expressed as a percentage or a fraction of one9.
Statistical analysis
The results were analysed using the Sigma Stat JadelTM Scientific software for Windows (Jadel Corporation, Chicago, IL, USA). The variables with scores between 0 and 3 in the morphological study were analysed using the Mann-Whitney U test. Percentage values were also calculated. The values for both groups in the morphometric study were analysed using Student's t-test. The level of significance was set at 5% for all tests.
RESULTS
Morphological study
The results of the morphological analysis of both groups are detailed in Table 1. Only the autolysis and congested blood vessels variables were significantly different between groups (p=0.014 and p=0.043, respectively). Most of the glands (95%) from the MFERSG group and 65% of the glands from the Control group exhibited congested blood vessels (Figure 2f). Half (50%) of the glands from the MFERSG group exhibited moderate or intense degrees of acinar autolysis, and 80% of the glands from the Control group exhibited this characteristic (Figure 2g).
Table 1.
Group comparisons derived from the morphologic analysis
| Variable | MFERSG group | Control group | p |
| present (%) | present (%) | ||
| Acinar atrophy | 95 | 85 | 0.758 |
| Focai infiltrates | 65 | 65 | 1.000 |
| Diffuse infiltrates | 50 | 30 | 0.142 |
| Fibrous tissue | 65 | 70 | 0.547 |
| Adipose tissue | 55 | 45 | 0.547 |
| Oncocytosis | 50 | 50 | 0.883 |
| Autolysis | 50 | 80 | 0.014* |
| Focal mucous extravasation | 75 | 45 | 0.192 |
| Diffuse mucous extravasation | 10 | 15 | 0.758 |
| Congested blood vessels | 95 | 65 | 0.043* |
| Ducts/duct-like structures | 85 | 85 | 0.192 |
Mann-Whitney U Test (p<0.05);
Statistically significant; MFERSG= mouth floor enlargments related to the sublingual glands
Acinar atrophy was observed in both groups (Figure 2a). Atrophy was present in 95% of the glands in the MFERSG group, with discrete, moderate and intense degrees in 55, 25 and 15% of the glands, respectively. Atrophy was present in 85% of the glands in the Control group, with discrete, moderate and intense degrees observed in 30, 40 and 15% of the glands, respectively. No significant difference in acinar atrophy was observed between the groups (p=0.758) (Table 1). The presence of duct-like structures was observed in 85% of the sublingual glands from both groups (p=1.000) (Figures 2c and Table 1).
The mononuclear infiltrates consisted primarily of lymphocytes, with few plasma cells and macrophages. No polymorphonuclear infiltrates were observed in any of the analysed specimens. Discrete or moderate focal infiltrates were observed in 65% of the glands in both groups (p=1.000) (Figure 2e). Diffuse infiltrates were observed in 50 and 30% of the glands in the MFERSG and Control groups, respectively (p=0.142) (Figure 2f and Table 1).
Moderate or intense replacement of the glandular parenchyma with fibrous tissue was observed in 65 and 70% of the sublingual glands in the MFERSG and Control groups, respectively (p=0.547) (Figure 2c, 2d and Table 1). The replacement of the glandular parenchyma with adipose tissue was observed in 55 and 45% of the glands in the MFERSG and Control groups, respectively (p=0.547) (Figure 2d and Table 1).
No gland exhibited a severe degree of oncocytosis, but discrete oncocytosis (Figure 2h) was observed in approximately 50% of the glands of both groups (p=0.883). Both groups primarily exhibited focal mucous extravasation (Figure 2i), and no significant difference between groups was observed (p=0.192) (Table 1).
Morphometric study
The volume densities of acini, ducts, stroma, adipose tissue, parenchyma (acini and ducts) and total stroma (stroma and adipose tissue) in both groups are presented in Table 2. No statistically significant differences in these variables were observed between the groups.
Table 2.
Means and standard deviations (mean ± sd) of various volume densities in both groups
| Variable | MFERSG group | Control group | p |
| mean ± sd (%) | mean ± sd (%) | ||
| Acini | 33.76±18.84 | 35.07±14.91 | 0.752 |
| Ducts | 8.40±3.36 | 9.62±5.11 | 0.444 |
| Stroma | 42.85±9.25 | 39.27±8.58 | 0.209 |
| Adipose tissue | 14.99±14.86 | 16.03±13.96 | 0.794 |
| Parenchyma | 42.16±18.26 | 44.70±16.74 | 0.649 |
| Total stroma | 57.84±18.26 | 55.30±16.74 | 0.649 |
MFERSG= mouth floor enlargments related to the sublingual glands
DISCUSSION
Our results demonstrated that the morphological patterns in the sublingual glands of edentulous patients with mouth floor enlargements were very similar to those of the sublingual glands of edentulous cadavers without enlargements, despite both groups were age/gender-matched. Macroscopically, the mouth floor of the cadavers' group was normal, despite the absence of posterior teeth. It is not possible to affirm when and why these enlargements appear, even because many edentulous patients do not present this condition.
No differences in acinar atrophy, duct-like structures, distribution of mononuclear infiltrates, replacement of parenchyma with fibrous or adipose tissue, oncocytosis or mucous extravasation were observed between groups. However, the sublingual glands of enlarged mouth floors exhibited more congested blood vessels and less acinar autolysis than the glands from the cadavers.
It is known that autolysis increases proportionally over time during the course of post-mortem changes but it should not occur ex vivo if the material is fixed immediately10. We agree with Nery, et al.10 (2010) who stated that this phenomenon might be a result of surgical dissection trauma during glandular removal. Regarding the variable "congested blood vessels", it was more frequent in MFERSG patients. We believe that it is a result of the greater surgical manipulation that is required in vivo.
Importantly, the ages of both groups were matched in the present investigation and the average age of the entire sample group was 59.5 years, which is representative of an elderly population. Consequently, age-related microscopic changes were detected in the majority of glands from both patients and cadavers. The aging process likely begins with acinar atrophy followed by the presence of duct-like structures and ends with the replacement of the parenchyma with fibrous and/or adipose tissue1. These aging alterations have been demonstrated in the human parotid, submandibular, palatal, labial and sublingual glands3,5,9,12-14,16-18. Azevedo, et al.1 (2005) demonstrated that these microscopic alterations are minimal or absent in the sublingual glands of cadavers under the age of 30 years.
The increasing appearance of duct-like structures with age represents the final process of acinar atrophy, and the distinction between a duct and a duct-like structure can be difficult1,3,5,9,12-14,16,17 due to the generalized regressive processes of the glandular parenchyma. Therefore, authors prefer to denote these structures as "duct-like structures"1,3,5,9,13,16,17. Whether the appearance of these structures and the increase in their number with age are the result of a regressive process that begins with acinar atrophy or whether they are true ducts with a proliferation capacity is not known. Additional research to clarify these issues is required.
No significant differences in the volume densities of acini, ducts, stroma and adipose tissue were observed between the groups in our study. The sublingual glands exhibited a minor quantity of parenchyma in relation to the total stroma volume (stroma and adipose tissue) in both groups. This observation is consistent with that of Moreira, et al.9 (2006) who demonstrated that the volume of acini decreases with age but that the volume of ducts, stroma and adipose tissue increases. These authors also found that the glandular volume decreases, on average, 33.78% during the aging process, which indicates that the sublingual glands are not responsible for the mouth floor enlargements.
Our data demonstrated that the sublingual glands in mouth floor enlargements in edentulous patients were not morphologically and morphometrically different from the glands of edentulous cadavers without enlargements and both sets of glands exhibited age-related changes. Therefore, the aging process cannot explain the etiopathogenesis of the mouth floor enlargements in edentulous individuals. Moreira, et al.9 (2006) showed that sublingual glands do not increase in size with age despite the enlargements. Conversely, the total volume of the glands decreases9. We agree that the intrinsic biologic characteristics of the sublingual glands are not responsible for the enlargements although these glands occupied almost the entire specimen that was submitted for microscopic examination. In both groups, a portion of the mouth floor mucosa was excised during the surgical procedure and, microscopically, this epithelium was normal. We do not believe that the surrounding tissues are responsible for the enlargements. The enlargements may arise from local external factors that are already known, such as the absence of posterior-inferior teeth and the degree of alveolar ridge resorption2,4,6. However, these factors are not sufficient to account for the etiopathogenesis in all cases because many edentulous with severe bone resorption in the alveolar margin do not exhibit MFERSG. Also, the cadavers, who were edentulous or partially edentulous, did not present the swellings clinically. We speculated that the accommodation of soft tissues after teeth loss, which varies according to individual elasticity, associated with the tongue movements, might also contribute to the MFERSG. Therefore, the enlargements may be a clinical manifestation of an adaptation to the current anatomical situation since the placement of a dental prosthesis in the lower posterior region may result in the disappearance of the swelling.
The mouth floor enlargements in edentulous patients are not described in academic books, but surgical interventions are frequent. Commonly, surgeons treat and describe these enlargements as a hyperplasia or a hypertrophy. However, the pathologists often describe the microscopic images as a sialodenitis, which is normally observed in the salivary glands during the aging process1,9. The terms "hyperplasia" and "hypertrophy" are wrong, since they represent, respectively, an abnormal increase in the number of normal cells in normal arrangement in an organ or tissue and an enlargement of an organ or part resulting from an increase in the size of the cells. The sialodenitis represents an infectious or inflammatory disorder of the salivary gland that includes focal infiltrates and can be a relatively frequent finding in salivary glands biopsies11. As well as the present study, an investigation in patients with MFERSG6 showed that all these terms are incorrect. The sublingual glands in the enlargements do not show these kinds of alterations but show microscopic changes compatible with the aging process1,9. We suggest that pathologists provide a descriptive microscopic report that emphasizes the possibility of age-related changes in the sublingual gland in MFERSG patients. The final diagnosis will depend on clinical, surgical and microscopic information.
The microscopic characteristics of these glands rule out the diagnosis of Sjögren Syndrome. First of all, the clinical findings are essential to diagnose this autoimmune disorder and patients or cadavers with this disease were excluded from this study. In this investigation, the mononuclear infiltrate basically consisted of lymphocytes, eventually showing some plasma cells and macrophages. The presence of a discrete, focal and periductal mononuclear infiltrate probably corresponds to IgA-secreting plasma cells and duct-associated lymphoid tissue1. Ductal obstruction in adult life might explain the increase of the mononuclear infiltrate with age. Azevedo, et al.1 (2005) found that the presence of a mononuclear infiltrate was associated with the process of acinar atrophy. The infiltrate became diffuse as the process of parenchymal replacement progressed. When replacement of the parenchyma was complete, the infiltrate disappeared, a finding also reported by Vered, et al.17 (2001).
The nomenclature of these volume alterations in the mouth floors in edentulous patients is variable and not well defined. In this study, we used the same terminology adopted by Iwaki Filho, et al.6 (2006). The term "enlargement" is justified because it is nonspecific and represents an increased volume in the mouth floor but not specifically in the sublingual gland. This term does not refer to a disturbance in cell growth or size. Therefore, we suggest that the best nomenclature for these enlargements is "non-pathological enlargements of the mouth floor in edentulous individuals". Academic books should present these enlargements as normal variations of the anatomy of the mouth floor. Surgical treatment should be indicated only when the enlargement impairs denture fitting.
CONCLUSION
The present study demonstrated that the microscopic characteristics of the sublingual glands in mouth floor enlargements in edentulous patients correspond to characteristics associated with the normal aging process. The sublingual glands do not represent pathological changes; they represent an age-related alteration that occurs with or without the presence of the mouth floor enlargements.
ACKNOWLEDGEMENTS
The authors would like to thank FAPESP (grant 05/60441-4) for financial support. We would also like to thank the professors and employees of the Department of Biological Sciences of the Bauru School of Dentistry and Dr. José Roberto Lauris, Dr. Tânia Mary Cestari and Dr. Carla Ruffeil Moreira for their contributions to this study.
REFERENCES
- 1.Azevedo LR, Damante JH, Lara VS, Lauris JRP. Age-related changes in human sublingual glands: a post mortem study. Arch Oral Biol. 2005;50(6):565–574. doi: 10.1016/j.archoralbio.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Campos LA. Hyperplasia of the sublingual glands in adult patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(5):584–585. doi: 10.1016/s1079-2104(96)80052-7. [DOI] [PubMed] [Google Scholar]
- 3.Dayan D, Vered M, Paz T, Buchner A. Aging of human palatal salivary glands: a histomorphometric study. Exp Gerontol. 2000;35(1):85–93. doi: 10.1016/s0531-5565(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 4.Domaneschi C, Mauricio AR, Modolo F, Migliari AD. Idiopathic hyperplasia of the sublingual glands in totally or partially edentulous individuals. Oral Surg Oral Med Oral Pathol Oral Radiol Oral Endod. 2007;103(3):374–377. doi: 10.1016/j.tripleo.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Drummond JR, Chisholm DM. A qualitative and quantitative study of the ageing human labial salivary glands. Arch Oral Biol. 1984;29(2):151–155. doi: 10.1016/0003-9969(84)90120-1. [DOI] [PubMed] [Google Scholar]
- 6.Iwaki L, Filho, Damante JH, Consolaro A, Bonachela WC, Damante CA. Mouth floor enlargements related to the sublingual glands in edentulous or partially edentulous patients: a microscopic study. J Appl Oral Sci. 2006;14(4):264–269. doi: 10.1590/S1678-77572006000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandel L, Romao M. Sublingual salivary gland enlargement. NY State Dent J. 2004;70(7):24–27. [PubMed] [Google Scholar]
- 8.McCord JF, Grant AA. Identification of complete denture problems: a summary. Br Dent J. 2000;189:128–134. doi: 10.1038/sj.bdj.4800703. [DOI] [PubMed] [Google Scholar]
- 9.Moreira CR, Azevedo LR, Lauris JRP, Taga R, Damante JH. Quantitative age-related differences in human sublingual gland. Arch Oral Biol. 2006;51(11):960–966. doi: 10.1016/j.archoralbio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Nery LR, Moreira CR, Cestari TM, Taga R, Damante JH. Post-mortem acinar autolysis in rat sublingual gland: a morphometric study. J Appl Oral Sci. 2010;18(5):509–514. doi: 10.1590/S1678-77572010000500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radfar L, Kleiner DE, Fox PC, Pillemer SR. Prevalence and clinical significance of lymphocytic foci in minor salivary glands of healthy volunteers. Arthritis Rheum. 2002;47(5):520–524. doi: 10.1002/art.10668. [DOI] [PubMed] [Google Scholar]
- 12.Scott J. A morphometric study of age changes in the histology of the ducts of human submandibular salivary glands. Arch Oral Biol. 1977;22(4):243–249. doi: 10.1016/0003-9969(77)90109-1. [DOI] [PubMed] [Google Scholar]
- 13.Scott J. Qualitative and quantitative observations on the histology of human labial salivary glands obtained post mortem. J Biol Buccale. 1980;8(3):187–200. [PubMed] [Google Scholar]
- 14.Scott J, Flower EA, Burns J. A quantitative study of histological changes in the human parotid gland occurring with adult age. J Oral Pathol Med. 1987;16(10):505–510. doi: 10.1111/j.1600-0714.1987.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 15.Tagawa S, Inui M, Mori A, Seki Y, Murata T, Tagawa T. Adenomatoid serous hyperplasia of sublingual gland: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(4):437–440. doi: 10.1016/s1079-2104(96)80311-8. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S, Shinzato K, Nakamura S, Domon T, Yamamoto T, Wakita M. The roles of apoptosis and mitosis in atrophy of the rat sublingual gland. Tissue Cell. 2002;34(5):297–304. doi: 10.1016/s0040816602000034. [DOI] [PubMed] [Google Scholar]
- 17.Vered M, Buchner A, Haimovici E, Hiss Y, Dayan D. Focal lymphocytic infiltration in aging human palatal salivary glands: a comparative study with labial salivary glands. J Oral Pathol Med. 2001;30(1):7–11. doi: 10.1034/j.1600-0714.2001.300102.x. [DOI] [PubMed] [Google Scholar]
- 18.Waterhouse JP, Chisholm DM, Winter RB, Patel M, Yale RS. Replacement of functional parenchymal cells by fat and connective tissue in human submandibular salivary glands: an age-related change. J Oral Pathol Med. 1973;2(1):16–27. doi: 10.1111/j.1600-0714.1973.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 19.Weibel ER. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]