Abstract
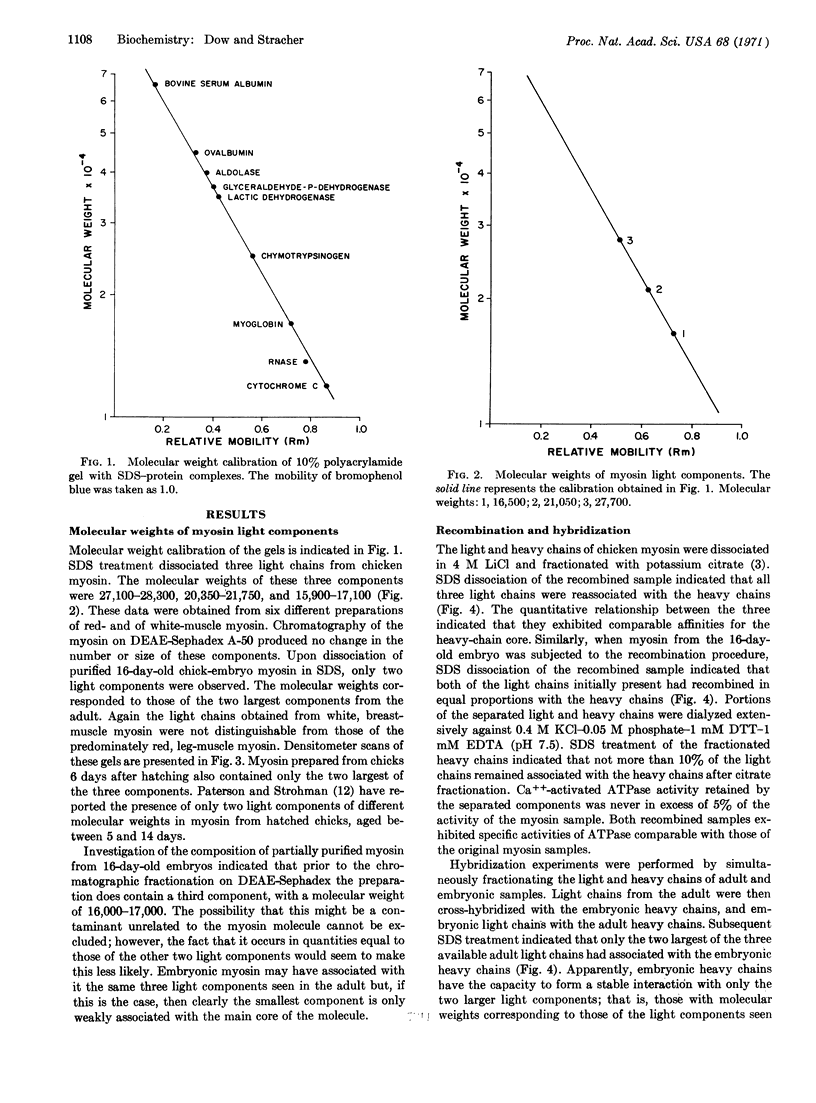
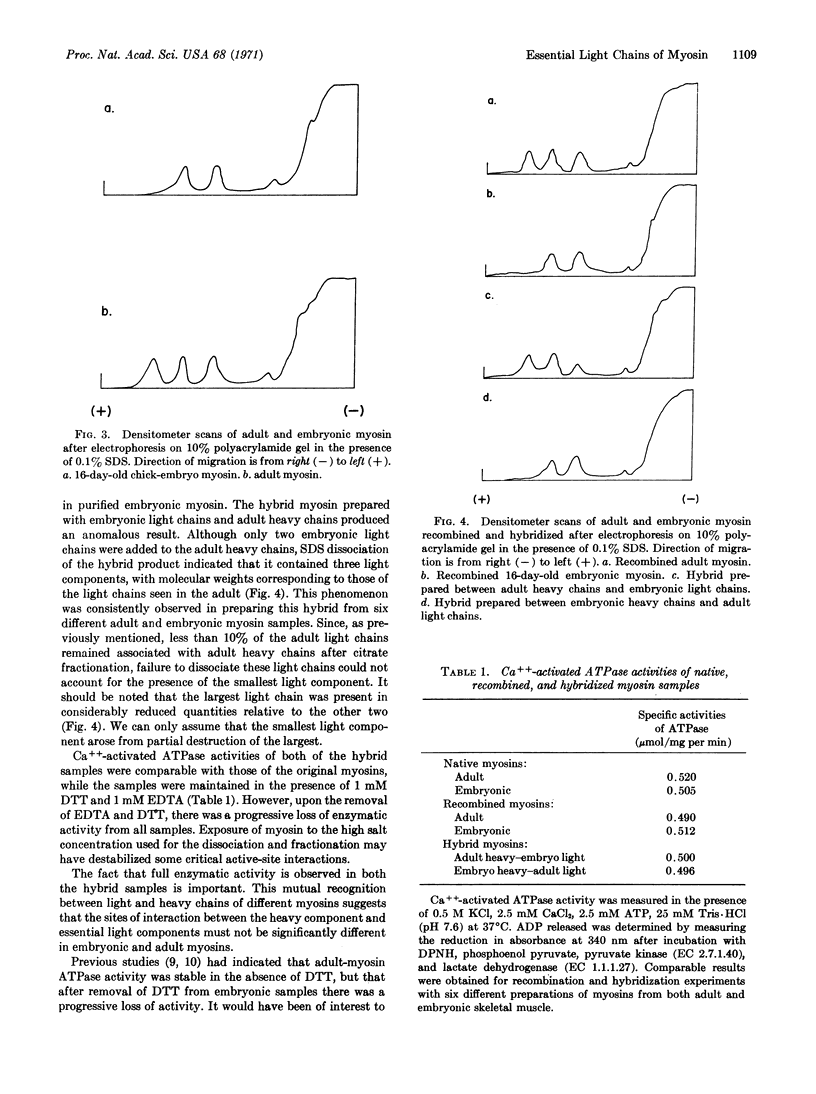
The molecular weights of light chains associated with adult and embryonic chick myosin have been determined by polyacrylamide gel electrophoresis in the presence of 0.1% sodium dodecyl sulfate. Adult muscle myosin contains three light chains with molecular weights averaging 27,700, 21,000, and 16,500, while the embryonic form contains only the two largest of these three. Recombination and hybridization experiments have been performed with these samples. The data clearly demonstrate that only two light chains are required for the expression of the full ATPase activity of myosin. The third light chain consistently is associated with adult myosin, but definitive evidence for its role is lacking.
Keywords: gel electrophoresis, molecular weights, chicken, chick embryo
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dreizen P., Gershman L. C. Relationship of structure to function in myosin. II. Salt denaturation and recombination experiments. Biochemistry. 1970 Apr 14;9(8):1688–1693. doi: 10.1021/bi00810a006. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Gazith J., Himmelfarb S., Harrington W. F. Studies on the subunit structure of myosin. J Biol Chem. 1970 Jan 10;245(1):15–22. [PubMed] [Google Scholar]
- Gershman L. C., Dreizen P. Relationship of structure to function in myosin. I. Subunit dissociation in concentrated salt solutions. Biochemistry. 1970 Apr 14;9(8):1677–1687. doi: 10.1021/bi00810a005. [DOI] [PubMed] [Google Scholar]
- Gershman L. C., Stracher A., Dreizen P. Subunit structure of myosin. 3. A proposed model for rabbit skeletal myosin. J Biol Chem. 1969 May 25;244(10):2726–2736. [PubMed] [Google Scholar]
- Morales M. F. Conformation and displacement in muscle contraction. Proc Natl Acad Sci U S A. 1970 Oct;67(2):572–578. doi: 10.1073/pnas.67.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin structure as revealed by simultaneous electrophoresis of heavy and light subunits. Biochemistry. 1970 Oct 13;9(21):4094–4105. doi: 10.1021/bi00823a010. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V., Stone D. Electrophoretic study of the small subunits of the myosin molecule. Biochem J. 1969 Jul;113(3):28P–29P. doi: 10.1042/bj1130028pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Binding of dodecyl sulfate to proteins at high binding ratios. Possible implications for the state of proteins in biological membranes. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1002–1007. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Cooke P. H. In vitro synthesis of light and heavy polypeptide chains of myosin. Biochem Biophys Res Commun. 1970 Nov 25;41(4):918–925. doi: 10.1016/0006-291x(70)90171-3. [DOI] [PubMed] [Google Scholar]
- Stracher A. Evidence for the involvement of light chains in the biological functioning of myosin. Biochem Biophys Res Commun. 1969 May 22;35(4):519–525. doi: 10.1016/0006-291x(69)90377-5. [DOI] [PubMed] [Google Scholar]
- Trotta P. P., Dreizen P., Stracher A. Studies on subfragment-I, a biologically active fragment of myosin. Proc Natl Acad Sci U S A. 1968 Oct;61(2):659–666. doi: 10.1073/pnas.61.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G. Light chains of myosin. Nature. 1969 Sep 27;223(5213):1362–1364. doi: 10.1038/2231362a0. [DOI] [PubMed] [Google Scholar]



