Abstract
New discoveries and technological advances in medicine are rapid. The role of technology in the treatment of erectile dysfunction (ED) will be widened and more options will be available in the years to come. These erectile technologies include external penile support devices, penile vibrators, low intensity extracorporeal shockwave, tissue engineering, nanotechnology and endovascular technology. Even for matured treatment modalities for ED, such as vacuum erectile devices and penile implants, there is new scientific information and novel technology available to improve their usage and to stimulate new ideas. We anticipate that erectile technologies may revolutionize ED treatment and in the very near future ED may become a curable condition.
Keywords: erectile dysfunction, technology, erektor, vibrator, low intensity extracorporeal shockwave, nanotechnology, penile implants, temporal CaSO4 penile cast, vacuum erectile device
Introduction
Erectile dysfunction (ED) today affects over 150 million men worldwide [Ayta et al. 1999]. The recognition of ED in literature dates back to 2000 bc, whereas treatment options were only first introduced in the early 1960s [Maggi et al. 2000]. The current treatment for ED is derived from four central options that consist of oral therapy, vacuum erectile device (VED), penile injection or intraurethral suppositories, and penile prosthesis. These treatment modalities are today’s mainstays for treating ED.
However, we now approach an era where new technology in medicine is fast growing and quickly becoming applicable. The role of technology in ED treatment is widening and more options may become available. This review article provides insight and a review of recent technological advances, new medical devices and research in the treatment of ED.
External penile support devices
Recently, a new mechanical device called ‘Erektor’ was introduced. The device is applied externally and no surgical intervention is required. It is marketed to provide length and rigidity to the penile shaft and is worn during sexual intercourse. The device is manufactured and developed by Global Life Technologies and has received press in Urology Times Magazine (http://business.highbeam.com/137412/article-1G1-200116245/external-support-device-alternative-ed-medications). Figure 1 displays the Erektor apparatus. It is composed of two rings attached to an interspaced rigid rod. The penile shaft is placed within the confines of the rings and the rigid rods lie along the ventral aspect of the penis. The penile shaft is stretched when the device is worn and intercourse may then ensue. Each device is individually customized to the patient’s phallic length. However, there has been no documented research or trials to provide the efficacy of the device. Nevertheless, its unique and technologically innovative design deserves mention as an option for patients seeking nonpharmaceutical/noninvasive treatment.
Figure 1.
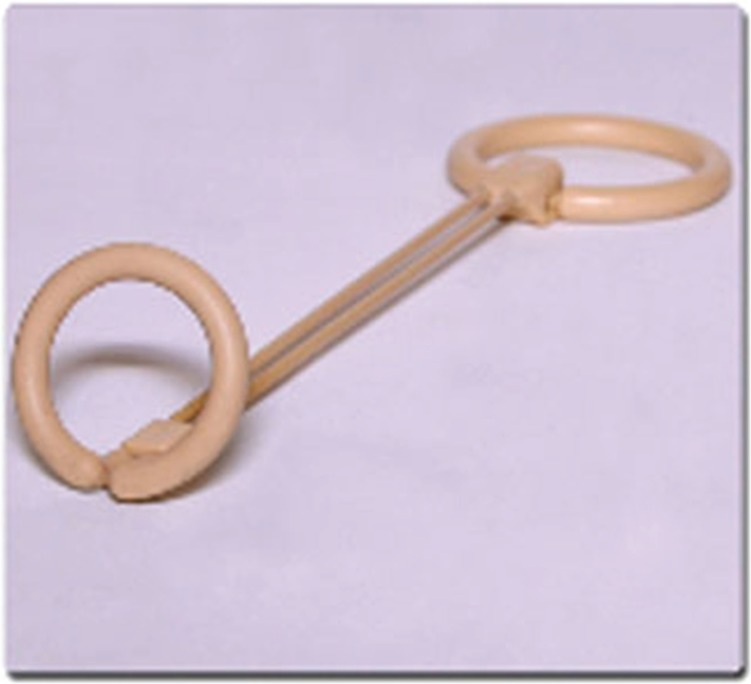
Erektor – composed of two cylindrical rings attached by a rigid rod. The penile shaft is placed within the confines of the rings. The rigid bar sites on the ventral shaft of the penis and provides rigidity for intercourse.
Source: www.erektorforlife.com
Another penile support device consists of a penile cast that is worn externally during intercourse. It is composed of a body and attachments. Three sizes are available: medium, large and extra-large (T. Iwai, personal communication). Two corona glans openings provide sensation during intercourse (Figure 2). There have been no published trials to establish the efficacy of the device at this time. However, it may serve as an option for patients with end-organ failure who may not be candidates for, or unable to afford, penile implants.
Figure 2.
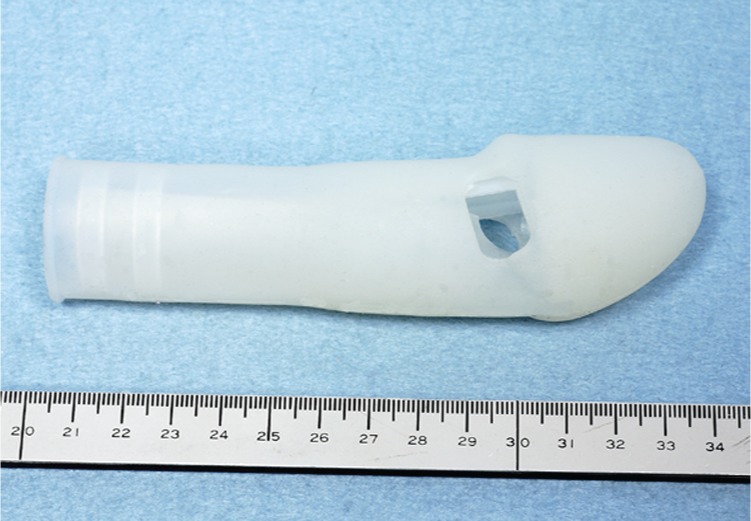
The penile cast worn during intercourse provides rigidity to the penile shaft and sensation is gained through the coronal glans window.
Source: Courtesy of Takehisa Iwai, MD, PhD.
Vibrator
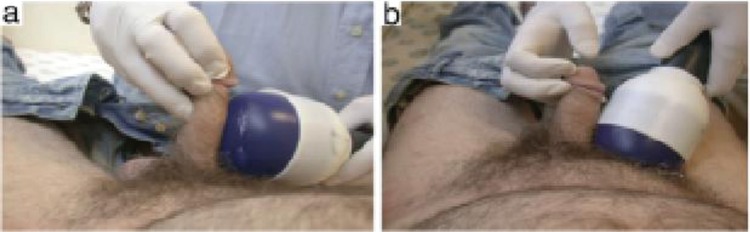
The use of penile vibratory stimulation (PVS) to induce penile erection and ejaculation was first described by Sobrero and colleagues in 1965 in men without spinal cord injury (SCI) [Sobrero et al. 1965; Sonksen and Ohl, 2002]. The first reported use of PVS in a man with SCI was with a hand device in 1970 [Sonksen and Ohl, 2002]. Refinements in technique, advancements in technology and portability led to the first US Food and Drug Administration (FDA) approved penile vibratory stimulator for ED in July 2011. Viberect, developed by Reflexonic, Chambersburg, Pennsylvania, is an FDA approved class II medical device used to provoke penile erection in men with ED and to provoke ejaculation in men with SCI (Figures 3 and 4). The device has received an abundance of online and commercial press. The mechanism of action of the device is through vibratory stimulation to branches of the pudendal nerve along the penile shaft.
Figure 3.

Viberect device. The blue circular pads are placed in contact with the penile shaft and using a touch pad on the dorsal aspect of the device the individual is able to customize activation.
Source: www.reflexonic.com
Figure 4.
Demonstration of the external handheld shockwave device being applied to the penile shaft and crura.
Source: Vardi et al. [2012].
Penile erection requires a complex interplay of the central nervous system, local and endothelial mediators. Sexual stimulation causes a release of neurotransmitters from the cavernous nerve terminals. Specifically, stimulation of the pudendal nerve causes a reflex parasympathetic erection through an activation of the parasympathetic pathway by pelvic nerves and nonadrenergic noncholinergic (NANC) fibers [Everaert et al. 2010]. Nitric oxide (NO) is the neurotransmitter released by NANC fibers. NANC fibers play a pivotal role in achieving tumescence after central nervous system stimulation by way of facilitating the release of NO and vasoactive intestinal polypeptide (VIP) and inhibiting noradrenaline release from the sympathetic fibers. The cumulative and downstream effect is a dilation of cavernosal and spongiosal smooth muscle through the activation of intracellular second messengers such as cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP). The Viberect handheld device utilizes exogenous stimulation of cavernousal nerve fibers by way of vibratory frequencies to release NO from nerve terminal endings [Tajkarimi and Burnett, 2011]. It may serve as an option for penile rehabilitation after nerve sparing radical prostatectomy. However, well designed clinical trials are needed to evaluate its efficacy.
Low intensity extracorporeal shockwave
The use of low intensity extracorporeal shockwave (LI-ESW) for the treatment of ED has its origins in the research performed in the 1990s by Young and Dyson who discovered the use of ultrasound and its effect to increase angiogenesis by increasing expression of vascular endothelial growth factor (VEGF) [Vardi et al. 2012; Young and Dyson, 1990]. Their research involved the use of ultrasound to promote the formation of new blood vessels in excised full thickness skin from adult rats. By exposing skin to 5 days of ultrasound at an intensity of 0.1 W/cm2 and frequency of 0.75 or 3.0 MHz, they demonstrated the formation of new blood vessels as assessed by microfocal X-ray techniques.
In 2010, a pilot study performed by Vardi and colleagues investigated the use of LI-ESW on men with ED who previously responded to oral phosphodiesterase type 5 inhibitor (PDE5-I). Only 20 men were enrolled in the study and the shockwave treatment consisted of 2 sessions per week for 3 weeks. LI-ESW was applied to the penile shaft and crura at five different nonspecific sites. The International Index of Erectile Function - Erectile Function (IIEF-EF) domain scores were used to measure responses at the end of treatment sessions. Results at 1 month follow up revealed a significant increase in IIEF-ED domain scores in all men and remained unchanged at 6 months follow up [Vardi et al. 2010]. Furthermore the study demonstrated significant increases in the duration of erections and penile rigidity [Vardi et al. 2010]. The study was the first to demonstrate the potential application of LI-ESW to the penile shaft to improve cavernosal hemodynamics. In May of 2012, Vardi and colleagues published the first randomized, controlled study to show that LI-ESW has positive short term clinical and physiological effects on ED for men who respond to PDE5-I. A total of 67 men were randomized to receive 12 sessions of LI-ESW or sham therapy. Erectile function and penile hemodynamics were assessed before the first treatment and after the final treatment using questionnaires and a veno-occlusive strain gauge plethysmography [Vardi et al. 2012]. Results revealed an increase in IIEF-EF domain scores and achievement of sufficient penile rigidity in men who were previously unable to perform penetration. None of the participants had any adverse events or effects from treatment [Vardi et al. 2012]. Correlation of results revealed an increase in penile blood flow.
Based on the animal study, the mechanism of LI-ESW for ED is related to the increased expression of VEGF, smooth muscle and endothelial content through recruitment of endogenous mesenchymal stem cells [Qiu et al. 2013]. Recently, Liu and colleagues also published their research on the effects of different doses of low energy shock wave therapy on ED in diabetic induced rats. Rats were divided into five groups (normal control, diabetic control and three different doses of LI-ESW treated groups) [Liu et al. 2013]. The different doses of LI-ESW included 100, 200 and 300 shocks each treatment on the penile shaft three times a week for total of two weeks. Erectile function was evaluated using intracavernous pressure (ICP) 1 week after treatment commenced. Corpora were also harvested and stained histologically for the study to assess changes in smooth muscle content, endothelium, NO positive nerve fibers and expression of VEGF. The study’s results revealed maximal therapeutic effect with the rats treated with 300 LI-ESW, with increased smooth muscle and endothelial contents, upregulation of VEGF, von Willebrand factor (vWF), alpha smooth muscle actin (α-SMA) and neuronal NO synthase (nNOS) in the corpora cavernosum [Liu et al. 2013].
Low intensity shockwave lithotripsy is currently not an approved therapy for ED and studies need still be conducted to optimize outcomes, dosage and frequency of treatments for patients. The technology and its applicability at present are intriguing and may soon provide a minimally invasive procedure to help patients achieve erection in addition to oral pharmacotherapy.
Impulse magnetic field therapy
Similar to the application of LI-ESW for ED, impulse magnetic field therapy has gained interest in the field of sexual medicine. Magnetic pulse fields induce an alternating current within the body’s electrolytes affecting the cells’ water content, mitochondrial function, physical properties of the membranes, nutrient, oxygen and amino acid uptake, energy production, ion membrane permeability, and macrophage migration [Pelka et al. 2002]. Magnetic fields in adequate forms and doses can increase oxygen uptake by the cell, enhance blood circulation, and reverse functional impairment. Shafik and colleagues were the first to use impulse magnetic-field therapy on humans [Shafik et al. 2000]. Their study included 32 patients with neurogenic ED and 20 healthy volunteers. A magnetic coil was placed over the dorsal aspect of the penis in the vicinity of the symphysis pubis. For 10 minutes, magnetic stimulation at 40% intensity and 20 Hz frequency, 50 seconds on and 50 seconds off, led to gradual increases in length and diameter until full erection was achieved; the penis became firm, rigid and pulsatile. Intracorporeal pressure also increased significantly (p < 0.0001) at full erection. The study demonstrated that magnetic stimulation is a simple, noninvasive method that could induce phallic engorgement and indicated this therapy might be suitable for patients with ED. Further research by Rainer and colleagues in a double-blind, placebo-controlled study assessed the efficacy of 3 weeks of impulse magnetic-field therapy for ED [Rainer et al.]. A total of 20 volunteers who suffered from ED or orgasmic disturbances were included. The results suggested that impulse magnetic field therapy improved erectile function at certain forms and doses (10 minutes, magnetic stimulation at 40% intensity and 20 Hz frequency, 50 seconds on and 50 seconds off) [Pelka et al. 2002]. However, more clinical studies are needed to verify the effectiveness of this therapy (including more patients, a longer time frame and side effects). A special design and easy use of impulse magnetic field therapy for ED is also required.
Tissue engineering
Over the past decade, physicians and scientists have been working to engineer a biological substitute to replace injured, diseased or malfunctioning organs. As applied to urology, much research and development has been seen in the arena of tissue engineering for corporal bodies and tunica albuginea replacement and repair. The construction of a biological penile prosthesis has gained much interest in the urologic community. The first known biological reconstruction of the phallus for impotence had its origin in 1936, when use of bone cartilage, the ‘artificial os penis’ was used to create scaffolding for post traumatic penile reconstruction [Bretan, 1989]. However, as it functioned poorly and had aberrant cosmetic results, this methodology of reconstruction fell out of favor [Patel and Atala, 2011; Yoo et al. 1999]. In 1998, Yoo and colleagues were able to demonstrate the ability to grow cartilaginous rods produced by seeding bovine chondrocytes onto a polyglycolic acid polymer infrastructure [Yoo et al. 1998]. Later studies performed by the same group demonstrated successful implantation of the biologically grown cartilage rods into the corporal spaces of rabbits (Figure 5) [Yoo et al. 1999]. The creation of a neo corpora by seeding human corporeal smooth muscle cells on polymer scaffolds was later demonstrated by the research conducted by Kershen and colleagues [Kershen et al. 2002].
Figure 5.
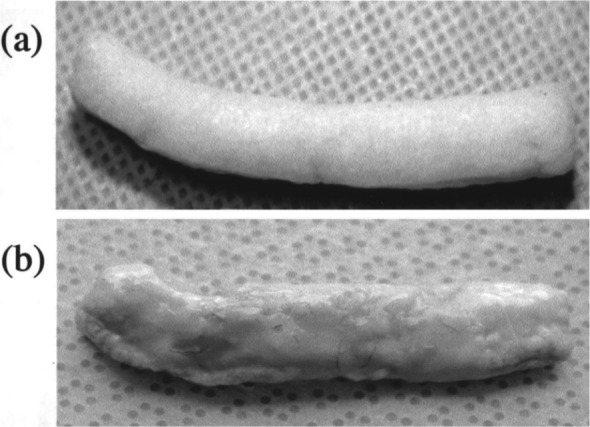
Demonstration of autologous engineered cartilage rods before and after implantation into the corporal spaces of rabbits.
Source: Yoo et al. [1999].
Further studies by Chen and colleagues examined the ability to bioengineer entire pendular penile bodies in a rabbit model. By implanting smooth muscle cells and endothelial cells seeded onto three-dimensional (3D) corporal collagen matrices into excised pendular penile corpora cavities in a rabbit model, they were able to demonstrate the creation of a neo corpora that exhibited good intracorporeal pressures to attain erection, induced relaxation by exposure to NO and carbachol, and mating assessments that revealed an 83% intravaginal ejaculation rate [Chen et al. 2010].
Such research and development has given encouraging hope and direction for individuals with ED due to congenital penile anomalies, penile cancer and penile injury with the possibility of being able to provide a biological substitute. No human tests have been performed, yet the future of such technology is bright and may bring an alternative to implantation of the mechanical penile prosthesis.
Nanotechnology
The application of a topical agent to achieve tumescence has been achieved through the use of nanotechnology. Nanoparticles are packaged molecules that are comparable in size to viruses and can be synthesized to encapsulate biologically active materials, such as PDE5-I. A gel comprised of nanoparticles applied directly to the penile shaft to achieve erection has the added benefit of minimizing the side effect profiles of oral PDE5-I and directed local therapy. In the laboratory, synthesized nanoparticles are created by putting tadalafil, sialorphin and NO into a topical gel.According to the study by Han and colleagues, nanoparticles encapsulating erectogenic agents were applied to the glans and penile shaft of rats, and ICP was recorded [Han et al. 2010]. The control group consisted of nanoparticles, without the encapsulated erectogenic agent, applied to glans and penile shaft in a similar manner. Results revealed an erectile response within an average of 4.5 minutes after the administration of the topic agent comprised of NO nanoparticles, with duration of 1.42 minutes [Han et al. 2010]. Sialorphin nanoparticles achieved a visible erectile response at an average time of 4.5 minutes after application and lasted for 8 minutes. Tadalafil nanoparticles were able to achieve an erectile response at one hour only after stimulation of the cavernosal nerve [Han et al. 2010].
To date, no human trials exist, but the application of nanoparticles to provide localized therapy for ED may soon provide yet another option for patients and the nanoparticle delivery system may revolutionize the localized therapy for ED. Further studies are needed to fully develop these delivery systems with respect to dosage and toxicity.
Endovascular treatment
Vasculogenic disease is one of the most common etiologies of ED. Microsurgical vascular reconstruction for penile artery insufficiency as a treatment for ED was first attempted in 1973. Various vascular conduits were used for reestablishment of arterial inflow, but given the limited studies and wide range of complications, the American Urological Association (AUA) advised against such procedures. Today, our surgical armamentarium has improved with more sophisticated image guided procedures and techniques. The Zen Trial (Zotarolimus-Eluting Peripheral Stent System for the Treatment of ED in Males with Sub-Optimal Response to PDE5 Inhibitors) was launched in 2009 and was the first trial to investigate the use of the drug eluting stent in patients with ED caused by internal pudendal artery stenosis refractory to PDE5-I. Results of the study revealed a four-point improvement in erectile function according to the International Index of Erectile Function (IIEF) in greater than 50% of patients [Goldstein and Koehler, 2012]. No adverse events or complications were encountered. Given the study’s low cohort of only 30 patients and short follow-up period of 30 days, larger scale controlled trials are needed before stents could be introduced as an option for patients with arteriogenic ED.
Other interventional modalities currently being performed include balloon dilation of internal pudendal artery secondary to peripheral arterial disease. A case series published by Babaev and Jhaveri reported significant improvement in erectile function after balloon dilation of the internal pudendal artery after selective angiography demonstrated decreased pudendal arterial patency [Babaev and Jhaveri, 2012]. Various sized drug-eluding coronary stents were additionally deployed within the pudendal artery to maintain patency. Figure 6 demonstrates the findings on selective angiography and postpudendal artery dilation. All patients were discharged the same day and upon follow up reported increased erectile function.
Figure 6.
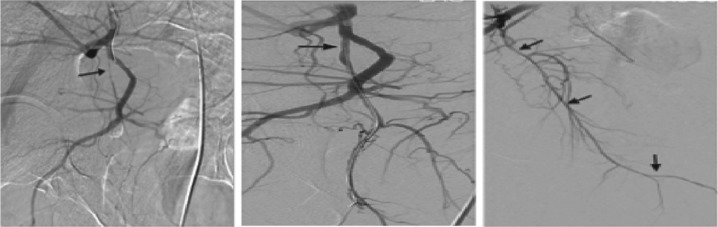
Selective angiographies of the right internal iliac artery show stenosis in the proximal internal pudendal artery, balloon angioplasty and stenting. Final angiographic image demonstrates good distal runoff after revascularization.
Source: Babaev and Jhaveri [2012].
For patients with a veno-occlusive dysfunction, endovascular treatment with selective embolization therapy has shown to be a safe and effective method of treatment for ED. A recent study by Aschenbach and colleagues published data on embolization of the dorsal penile vein with a mixture of N-butyl-2-cyanoacrylate tissue adhesive and LIPIODOL ULTRA. Results of this study revealed an 88% success rate of 24/27 patients recovering from poor tumescence and rigidity [Aschenbach et al. 2013]. The author reported a 0% complication rate. However, given the low cohort of participants and retrospective nature of the larger scale study, prospective data are needed to approve and validate the procedure.
New penile implants
A new innovative penile implant developed in the UK and marketed in Singapore involves the delivering of vasodilators within the corpora cavernosa by implant [Lim, 2003]. Developed by Giles R. Brindley, the device is novel in its approach as the first implanted drug delivery apparatus for the purpose of achieving an erection. The implanted device consists of two parts that are connected together: a cannula inserted into the corpora and a scrotal reservoir (Figure 7). Squeezing the scrotal pump delivers a bolus of the vasoactive drug into the confines of corpora cavernosa. One squeeze of the reservoir delivers 0.16 cc (80 mg) of sodium nitroprusside. The reservoir holds approximately 7 cc. Refilling of the reservoir can performed in an office setting by direct injection through the scrotal skin. The device has been implanted into 31 patients in the UK and Singapore; 11 implanted mechanical failures and two device infections were noted in the series. Successful usage and achievement of erection by the other participants has yet to be reported. The medicated penile implant offers a new take on implantable devices. The device is marketed to be less conspicuous than other inflatable prosthesis on the market, causes no damage to the corpora cavernosa and is cost effective. Although much more research is needed to establish efficacy of the device, there remain several questions as to the device’s safety. Reservoir rupture secondary to intercourse, trauma or infection would cause release of sodium nitroprusside systemically and could be fatal. The medicated implant may provide patients with another option for erection in the near future, but further safety testing and research is required before the device may be introduced to the urologic community and considered a viable treatment alternative for ED.
Figure 7.

The medicated penile implant consisting of a reservoir, device tubing and cavernosal drug delivery cannula.
Source: Lim [2003].
Another new implant is under development. This implant is based on a novel application of a nickel–titanium based shape memory alloy (SMA) that alternates between a flaccid and erect configuration with application of heat (Figure 8). The device can mimic a physiologic erection without the use of pumps or reservoirs. A recent study revealed that, compared with the AMS 700 and the Ambicor prosthesis, an SMA-based prosthesis demonstrates comparable mechanical properties. This new device is currently undergoing further refinements and it may provide urologists with a new option in penile prosthesis technology [Le et al. 2013].
Figure 8.
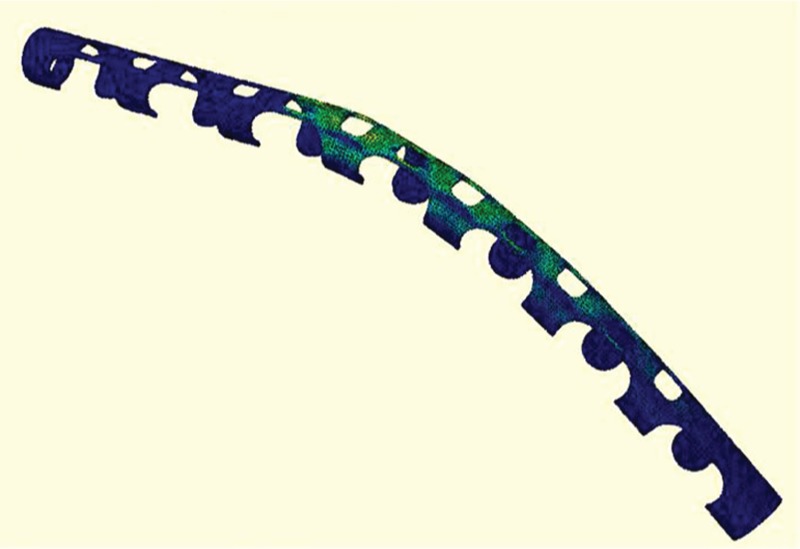
Nickel–titanium based shape memory alloy (SMA) alternates between a flaccid and erect configuration with application of heat.
Source: Le et al. [2013].
Temporal CaSO4 penile cast
Advances in penile prosthesis technology are not only limited to achieving erection, but also in the preservation of the corpora cavernosa after a penile prosthesis infection. It has been estimated that penile prosthesis infection rate is 1–3% and 13.3% for revision cases respectively [Selph and Carson, 2011]. There have been many preventative strategies to reduce the rate of infections such as intraoperative sterile techniques, impregnated penile prosthesis and preoperative systemic antibiotics. When infection does ensue, expedient explantation of the prosthesis is necessary in addition to corporal irrigation with antibiotic solution. However, such removal of the prosthesis can cause corporal fibrosis, loss of phallic length, penile deformity in addition to psychological stress, depression and chronic pain syndrome [Swords et al. 2013]. A new approach to corporal preservation after penile prosthesis explantation secondary to infection has been investigated by Swords and colleagues at the University of South Florida. Their research involves the use of a temporal intracorporeal antibiotic cast composed of synthetic high purity CaSO4 (Figure 9) that is used to provide continuous antibiotic/antifungal medication after penile prosthesis explantation [Swords et al. 2013]. The cast provides the added benefit of reducing corporal fibrosis and maintaining phallic length during healing.
Figure 9.
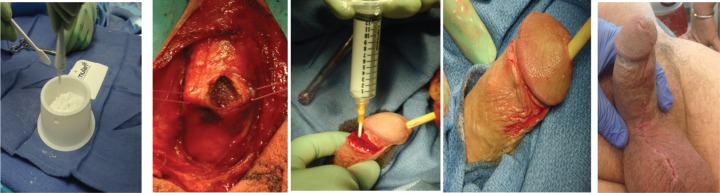
This set of images was taken intraoperatively and displays the steps for injection of the temporal penile cast. The compound of CaSO4 is first mixed in a sterile basin before being filled in a syringe. Injection of the paste into the corpora is then performed. After injection corpora and incision is closed. The postinjection penile shaft demonstrates rigidity.
Source: Swords et al. [2013].
Currently the cast has been implanted in two patients post penile prosthesis removal as a result of infection. The cast self-absorbs after approximately 4–6 weeks. Results of the two test patients revealed successful salvage replacement after the cast had resorbed. Although one patient did have evidence of corporal fibrosis, the other patient was found to have intact penile length and no corporal fibrosis. A more timely approach to re-implantation of the penile prosthesis may be necessary after cast reabsorption to prevent fibrosis in future cases. Both patients were able to be re-implanted with a penile prosthesis and are satisfied with results without recurrence of infection. Further patient research is warranted before such salvage intervention can be considered a treatment option. The following technology is not only innovative, but provides another therapy modality for infected penile implants and a tool for surgeons to provide better outcomes to their patients.
VED: new knowledge
A VED uses negative pressure to distend the corporal sinusoids and to increase blood inflow to the penis. It was first approved by the FDA in 1982 [Lewis and Witherington, 1997]. and recommended as one of the alternatives for organic ED by the AUA in 1996 [Montague et al. 1996]. However, it was not a very popular treatment for ED until the concept of penile rehabilitation was introduced into the urology practice. Recent study showed that it has been the second most commonly used method for penile rehabilitation after radical prostatectomy according to the 2011 AUA survey [Tal et al. 2011]. However, the mechanism of VED therapy for ED after radical prostatectomy was not clear. Recent studies with the use of a unique animal model and a newly designed VED for rats clarified the mechanism of vacuum therapy for penile rehabilitation after radical prostatectomy. By mimicking the clinical use of VED, scientists designed a rat-specific VED based on rat anatomy and the principle of the human VED. The studies demonstrated that VED therapy preserves erectile function through antihypoxic, antiapoptotic and antifibrotic mechanisms by improving the arterial blood flow into the penis [Lin et al. 2013; Yuan et al. 2009, 2010a, 2010b]. This scientific evidence indeed motivated physicians’ recommendation and improved patients’ compliance with the use of VED therapy after prostate cancer treatment.
Conclusion
The current treatment algorithm for ED as outlined by the AUA includes PDE5-I initially followed by intracavernosal injection (ICI), VED, intraurethral suppositories and penile prosthesis implantation (in no particular order). Today’s treatment options are limited. As technological advancements are applied to the treatment of ED, patients will ultimately have a plethora of new management options. For now, the external penile support devices with their unique innovative designs deserve future exploration. The handheld penile vibrator may serve as a good option for penile rehabilitation because it increases the neurotransmitters from the cavernous nerve terminals that are involved in penile erection. LI-ESW appears to improve erectile function through recruitment of endogenous stem cells and it may give hope that ED may one day be curable. Tissue engineering will make biological reconstruction of the phallus possible and nanotechnology will revolutionize local therapies for ED in the very near future. With further improvement of endovascular technology, we may cure ED caused by focal vascular lesions. VED will continue to be a popular penile rehabilitation strategy as a recent animal study has clearly shown that VED increases arterial flow and oxygen to the penis and improves early return of erectile function after radical prostatectomy. Current penile implants will continue to provide the most satisfactory option for patients with severe ED. However, new implants may provide easier patient usage and easier implantation by surgeons. The future of technological advancements in the field of sexual medicine is bright, what technologies gain entrance into the medical field will depend upon their safety, efficacy and patient satisfaction. Hopefully, even using a smart phone app to control the Inflatable penile prosthesis (IPP) may be available in the very near future.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Marshall J. Stein, University of Texas Medical School at Houston, Houston, TX, USA
Haocheng Lin, University of Texas Medical School at Houston, Houston, TX, USA.
Run Wang, Professor of Surgery (Urology), Cecil M. Crigler, MD, Chair in Urology, Director of Sexual Medicine, University of Texas Medical School at Houston and MD Anderson Cancer Center, Houston, TX 77030, USA.
References
- Aschenbach R., Steiner T., Kerl M., Zangos S., Basche S., Vogl T. (2013) Endovascular embolisation therapy in men with erectile impotence due to veno-occlusive dysfunction. Eur J Radiol 82: 504–507 [DOI] [PubMed] [Google Scholar]
- Ayta I., McKinlay J., Krane R. (1999) The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 84: 50–56 [DOI] [PubMed] [Google Scholar]
- Babaev A., Jhaveri R. (2012) Angiography and endovascular revascularization of pudendal artery atherosclerotic disease in patients with medically refractory erectile dysfunction. J Invasive Cardiol 24: 236–240 [PubMed] [Google Scholar]
- Bretan P., Jr. (1989) History of the prosthetic treatment of impotence. Urol Clin North Am 16: 1–5 [PubMed] [Google Scholar]
- Chen K., Eberli D., Yoo J., Atala A. (2010) Bioengineered corporal tissue for structural and functional restoration of the penis. Proc Natl Acad Sci U S A 107: 3346–3350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert K., de Waard W., Van Hoof T., Kiekens C., Mulliez T., D’Herde C. (2010) Neuroanatomy and neurophysiology related to sexual dysfunction in male neurogenic patients with lesions to the spinal cord or peripheral nerves. Spinal Cord 48: 182–191 [DOI] [PubMed] [Google Scholar]
- Goldstein L., Koehler T. (2012) The medtronic zotarolimus-eluting peripheral stent system for the treatment of erectile dysfunction in males with sub-optimal response to PDE5 inhibitors: 6 month results. J Sex Med 9: 111 [Google Scholar]
- Han G., Tar M., Kuppam D., Friedman A., Melman A., Friedman J., et al. (2010) Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J Sex Med 7: 224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershen R., Yoo J., Moreland R., Krane R., Atala A. (2002) Reconstitution of human corpus cavernosum smooth muscle in vitro and in vivo. Tissue Eng 8: 515–524 [DOI] [PubMed] [Google Scholar]
- Le B., Colombo A., Mustoe T., McVary K. (2013) Evaluation of a Ni-Ti shape memory alloy for use in a novel penile prosthesis. J Urol 189(Suppl.): e502 [Google Scholar]
- Lewis R., Witherington R. (1997) External vacuum therapy for erectile dysfunction: use and results. World J Urol 15: 78–82 [DOI] [PubMed] [Google Scholar]
- Lim P. (2003) Recent Advances and Research Updates. [Google Scholar]
- Lin H., Yang W., Zhang J., Dai Y., Wang R. (2013) Penile rehabilitation with a vacuum erectile device in an animal model is related to an antihypoxic mechanism: blood gas evidence. Asian J Androl 15: 387–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhou F., Li G., Wang L., Li H., Bai G., et al. (2013) Evaluation of the Effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci 14: 10661–10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi M., Filippi S., Ledda F., Magini A., Forti G. (2000) Erectile dysfunction: from biochemical pharmacology to advances in medical therapy. Eur J Endocrinol 143: 143–154 [DOI] [PubMed] [Google Scholar]
- Montague D., Barada J., Belker A., Levine L., Nadig P., Roehrborn C., et al. (1996) Clinical guidelines panel on erectile dysfunction: summary report on the treatment of organic erectile dysfunction. The American Urological Association. J Urol 156: 2007–2011 [DOI] [PubMed] [Google Scholar]
- Patel M., Atala A. (2011) Tissue engineering of the penis. Sci World J 11: 2567–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka R., Jaenicke C., Gruenwald J. (2002) Impulse magnetic-field therapy for erectile dysfunction: a double-blind, placebo-controlled study. Adv Ther 19: 53–60 [DOI] [PubMed] [Google Scholar]
- Qiu X., Lin G., Xin Z., Ferretti L., Zhang H., Lue T., et al. (2013) Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 10: 738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selph J., Carson C. (2011) Penile prosthesis infection: approaches to prevention and treatment. Urol Clin North Am 38: 227–235 [DOI] [PubMed] [Google Scholar]
- Shafik A., El-Sibai O., Shafik A. (2000) Magnetic stimulation of the cavernous nerve for the treatment of erectile dysfunction in humans. Int J Impot Res 12: 137–141; discussion 141–132. [DOI] [PubMed] [Google Scholar]
- Sobrero A.J., Stearns H.E., Blair J.H. (1965) Technic for the Induction of Ejaculation in Humans. Fertil Steril 16: 765–767 [DOI] [PubMed] [Google Scholar]
- Sonksen J., Ohl D. (2002) Penile vibratory stimulation and electroejaculation in the treatment of ejaculatory dysfunction. Int J Androl 25: 324–332 [DOI] [PubMed] [Google Scholar]
- Swords K., Martinez D., Lockhart J., Carrion R. (2013) A preliminary report on the usage of an intracorporeal antibiotic cast with synthetic high purity CaSO4 for the treatment of infected penile implant. J Sex Med 10: 1162–1169 [DOI] [PubMed] [Google Scholar]
- Tajkarimi K., Burnett A. (2011) Viberect® device use by men with erectile dysfunction: safety, ease of use, tolerability, and satisfaction survey. J Sex Med 8: 441–441 [Google Scholar]
- Tal R., Teloken P., Mulhall J. (2011) Erectile function rehabilitation after radical prostatectomy: practice patterns among AUA members. J Sex Med 8: 2370–2376 [DOI] [PubMed] [Google Scholar]
- Vardi Y., Appel B., Jacob G., Massarwi O., Gruenwald I. (2010) Can low-intensity extracorporeal shockwave therapy improve erectile function? A 6-month follow-up pilot study in patients with organic erectile dysfunction. Eur Urol 58: 243–248 [DOI] [PubMed] [Google Scholar]
- Vardi Y., Appel B., Kilchevsky A., Gruenwald I. (2012) Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol 187: 1769–1775 [DOI] [PubMed] [Google Scholar]
- Yoo J., Lee I., Atala A. (1998) Cartilage rods as a potential material for penile reconstruction. J Urol 160: 1164–1168; discussion 1178. [DOI] [PubMed] [Google Scholar]
- Yoo J., Park H., Lee I., Atala A. (1999) Autologous engineered cartilage rods for penile reconstruction. J Urol 162: 1119–1121 [DOI] [PubMed] [Google Scholar]
- Young S., Dyson M. (1990) The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol 16: 261–269 [DOI] [PubMed] [Google Scholar]
- Yuan J., Hoang A., Romero C., Lin H., Dai Y., Wang R. (2010a) Vacuum therapy in erectile dysfunction–science and clinical evidence. Int J Impot Res 22: 211–219 [DOI] [PubMed] [Google Scholar]
- Yuan J., Lin H., Li P., Zhang R., Luo A., Berardinelli F., et al. (2010b) Molecular mechanisms of vacuum therapy in penile rehabilitation: a novel animal study. Eur Urol 58: 773–780 [DOI] [PubMed] [Google Scholar]
- Yuan J., Westney O., Wang R. (2009) Design and application of a new rat-specific vacuum erectile device for penile rehabilitation research. J Sex Med 6: 3247–3253 [DOI] [PubMed] [Google Scholar]


