Abstract
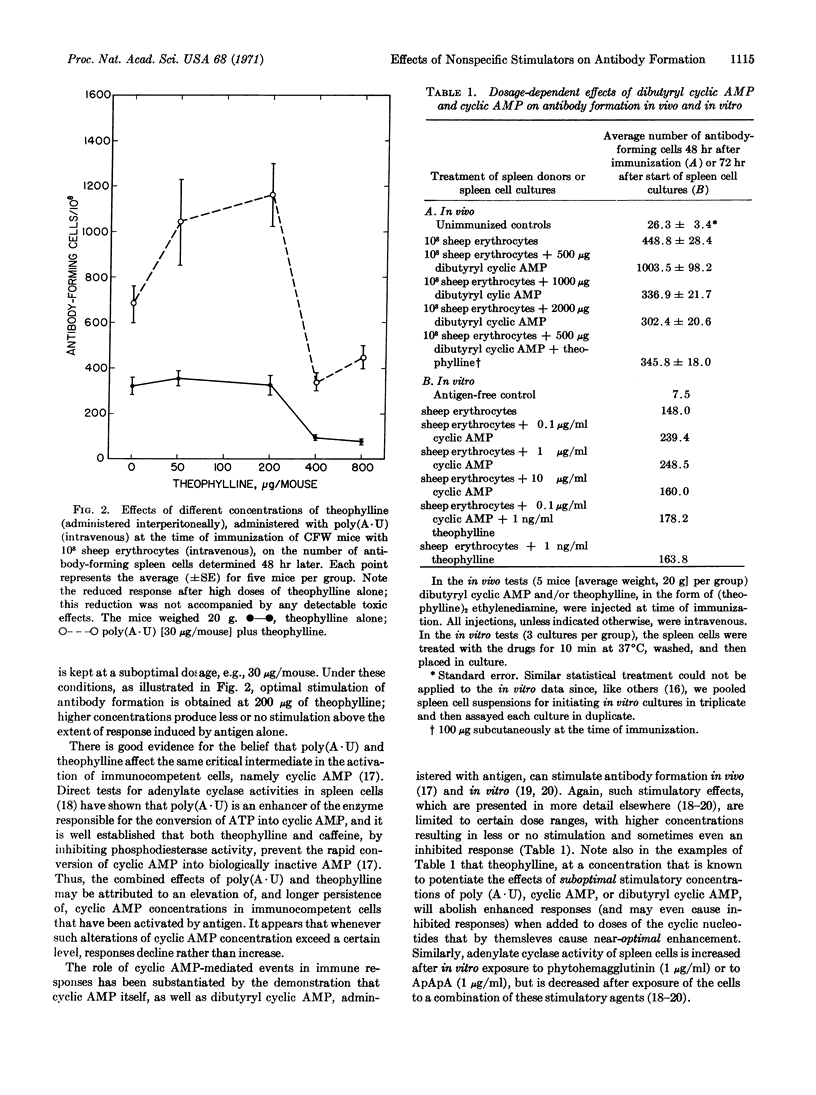
Enhancement of antibody formation in vitro and in vivo by poly(A·U), cyclic AMP, dibutyryl cyclic AMP, or any one of these agents in combination with theophylline or caffeine, is usually limited to certain dose ranges. High doses of these nonspecific stimulators can produce less or no enhancement, or may actually inhibit antibody formation. Such dose-response relationships may be pertinent to an understanding of phenomena of specific immunological nonresponsiveness and certain types of antigenic competition.
Keywords: poly(A·U), theophylline, mouse, in vivo, tissue culture, cyclic AMP
Full text
PDF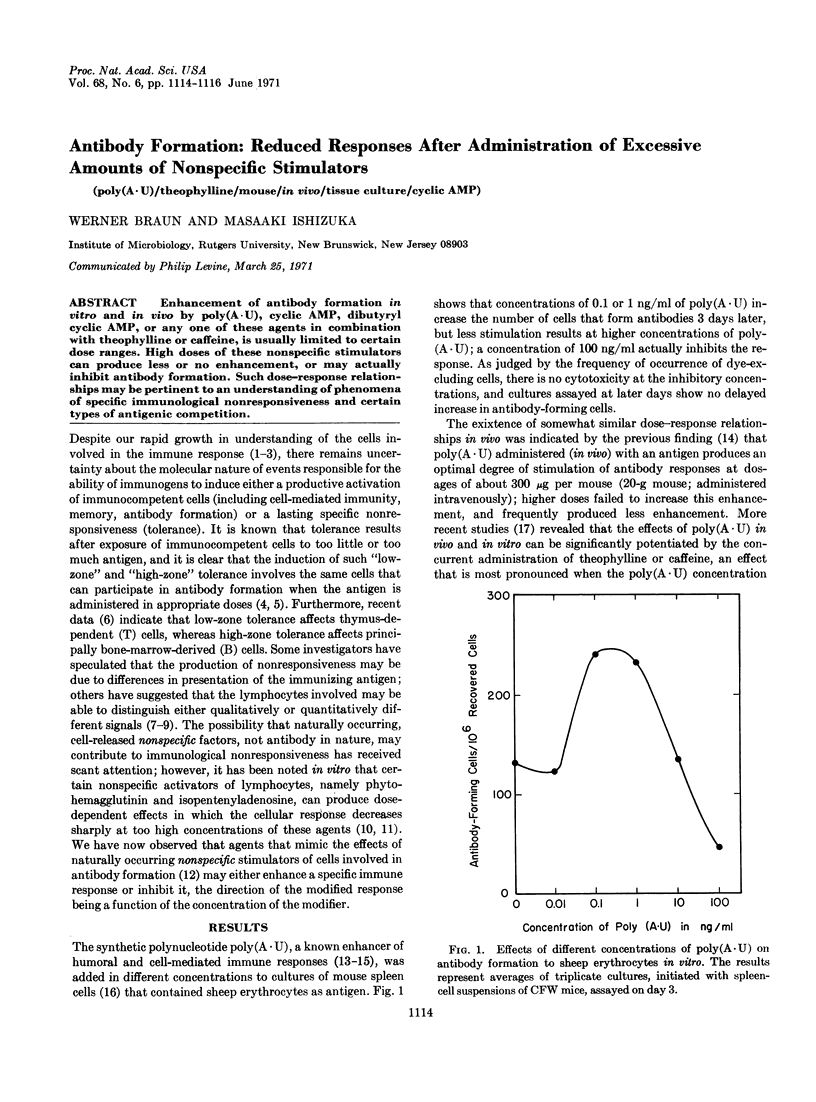

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun W., Nakano M. Antibody formation: stimulation by polyadenylic and polycytidylic acids. Science. 1967 Aug 18;157(3790):819–821. doi: 10.1126/science.157.3790.819. [DOI] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Chan E. L., Mishell R. I., Mitchell G. F. Cell interaction in an immune response in vitro: requirement for theta-carrying cells. Science. 1970 Dec 11;170(3963):1215–1217. doi: 10.1126/science.170.3963.1215. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Diener E., Shortman K., Russell P. Induction of immunity and tolerance in vitro in the absence of phagocytic cells. Nature. 1970 Feb 21;225(5234):731–732. doi: 10.1038/225731a0. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Mitchison N. A. The mechanism of immunological paralysis. Adv Immunol. 1968;8:129–181. doi: 10.1016/s0065-2776(08)60466-6. [DOI] [PubMed] [Google Scholar]
- Fassina G. Antagonistic action of metabolic inhibitors on dibutyrl cyclic 3'5'-adenosine monophosphate-stimulated and caffeine-stimulated lipolysis in vitro. Life Sci. 1967 Apr 15;6(8):825–831. doi: 10.1016/0024-3205(67)90285-8. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Whang-Peng J., Perry S. Isopentenyladenosine stimulates and inhibits mitosis of human lymphocytes treated with phytohemagglutinin. Science. 1969 Jul 25;165(3891):400–402. doi: 10.1126/science.165.3891.400. [DOI] [PubMed] [Google Scholar]
- Hartmann K. U. Induction of a hemolysin response in vitro. Interaction of cells of bone marrow origin and thymic origin. J Exp Med. 1970 Dec 1;132(6):1267–1278. doi: 10.1084/jem.132.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka M., Gafni M., Braun W. Cyclic AMP effects on antibody formation and their similarities to hormone-mediated events. Proc Soc Exp Biol Med. 1970 Sep;134(4):963–967. doi: 10.3181/00379727-134-34921. [DOI] [PubMed] [Google Scholar]
- Liacopoulos P., Perramant F. Inhibition non spécifique de la réponse immunologique au cours de l'induction de la paralysie immunitaire chez la souris adulte. Ann Inst Pasteur (Paris) 1966 Mar;110(3 Suppl):161–170. [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney J. J., Waksman B. H. Activation of normal rabbit macrophage monolayers by supernatants of antigen-stimulated lymphocytes. J Immunol. 1970 Nov;105(5):1138–1145. [PubMed] [Google Scholar]
- Möller G., Sjöberg O. Effect of antigenic competition on antigen-sensitive cells and on adoptively transferred immunocompetent cells. Cell Immunol. 1970 May;1(1):110–121. doi: 10.1016/0008-8749(70)90064-x. [DOI] [PubMed] [Google Scholar]
- Nakano M., Braun W. Cell-released non-specific stimulatiors of antibody-forming spleen cell populations. J Immunol. 1967 Sep;99(3):570–575. [PubMed] [Google Scholar]
- Radovich J., Talmage D. W. Antigenic competition: cellular or humoral. Science. 1967 Oct 27;158(3800):512–514. doi: 10.1126/science.158.3800.512. [DOI] [PubMed] [Google Scholar]
- Trainin N., Burger M., Kaye A. M. Some characteristics of a thymic humoral factor determined by assay in vivo of DNA synthesis in lymph nodes of thymectomized mice. Biochem Pharmacol. 1967 Apr;16(4):711–720. doi: 10.1016/0006-2952(67)90084-6. [DOI] [PubMed] [Google Scholar]
- Waterston R. H. Antigen competition: a paradox. Science. 1970 Dec 4;170(3962):1108–1110. doi: 10.1126/science.170.3962.1108. [DOI] [PubMed] [Google Scholar]


