Abstract
Background
Olfactory assessment is often neglected in clinical practice, although olfactory loss can assist in diagnosis and may lead to significant morbidity. “Sniffin’ Sticks” is a modern test of nasal chemosensory performance that was developed in Germany and validated in many countries. Our aim was to validate the applicability of “Sniffin’ Sticks” in a Turkish population.
Material/Methods
The study included 123 healthy volunteers with a reported normal sense of smell and 51 patients complaining of a reduction in their olfactory function presenting either at rhinology or neurology clinics. The mean age of the subjects tested was 30.2±12.5 years in 126 males and 48 females. The participants were divided into 2 groups according to subjective olfactory function – healthy or abnormal. Each subject’s olfactory function was assessed using the “Sniffin’ Sticks” test.
Results
We found significant differences in “Sniffin’ Sticks” test results between the abnormal and healthy groups. In healthy subjects, the 10th percentiles of odor threshold score, odor discrimination score, odor identification score, and TDI score were 7.25, 12, 11, and 32, respectively. Considering the 2 groups together, apple and turpentine were the least well-recognized odors from the 16 odors presented.
Conclusions
Our study provides an update of normative values for routine clinical use of “Sniffin’ Sticks” in a Turkish population. Also, the present study validates that “Sniffin’ Sticks” olfactory test was applicable for clinical usage in a Turkish population.
Keywords: olfactory function, odor discrimination, odor identification, odor threshold, “Sniffin’ Sticks”
Background
The sense of smell plays important roles in daily human life, and loss of olfaction is mostly expressed in terms of a severe decrease in quality of life. Problems in quality of life were reported primarily in the areas of eating and safety [1–4]. The major causes of olfactory disorders are head injury, infections of upper respiratory tract, sinonasal diseases, head trauma, and toxic exposure.
Approximately 5% of the general population is anosmic (no sense of smell), but about 15% have reduced olfactory function [5–8]. A significant number of patients complain of a reduction in their olfactory function, so it becomes important to use appropriate diagnostic tools (e.g., the University of Pennsylvania UPSIT Smell Identification Test [9], “Sniffin’ Sticks” [10,11], or measurements of event-related potentials [12]). Quantitative smell disorders such as anosmia or hyposmia can be differentiated from normal olfactory function.
The “Sniffin’ Sticks” test was initially developed and validated on large numbers of patients in Germany [10,13]. Currently, the kit is used by many clinicians around the world and has been validated in various countries and populations (e.g., Australia [14], Greece [15,16], Taiwan [17–19], Italy [20], Holland [21], Sri Lanka [22], and Brazil [23]). The “Sniffin’ Sticks” test has been validated and normative data published on a large number of subjects in Northern Europe and smaller studies have proven its usefulness in assessing olfaction in populations around the world, but there is only 1 study evaluating the “Sniffin’ Sticks” olfactory test in Turkey [24]. In that study researchers found quite poor scores in a Turkish population when compared to other worldwide studies. Thus, we aimed to re-investigate the normative data for “Sniffin’ Sticks” olfactory test in healthy Turkish subjects. Another goal of this study was to compare the scores of subjects with and without a complaint of olfactory dysfunction for detecting the clinical applicability of the “Sniffin’ Sticks” olfactory test.
Material and Methods
This study was approved by the Institutional Review Board of Gulhane Military Medicine Academy (GATA) Haydarpasa Training Hospital. The study was conducted in otorhinolaryngology and neurology clinics of GATA Haydarpasa Training Hospital and Istanbul Surgery Hospital and included 51 patients complaining of a reduction in their olfactory function (abnormal group). A group of 123 subjects who stated they have normal olfactory function were also enrolled (healthy group). Informed consent was obtained from all participating subjects. No signs of nasal polyposis, marked septal deviation, or rhinosinusitis were found by endoscopic nasal examination in any of the subjects.
Psychophysical testing of olfactory function was performed with the validated “Sniffin’ Sticks” test. Odorants were presented in commercially available felt-tip pens (“Sniffin’ Sticks” Burghart GmbH, Wedel, Germany) [10,11]. Olfactory testing used 3 tests: tests for odor threshold (testing by means of a single staircase procedure), odor discrimination (3-alternative forced choice), and odor identification (4-alternative forced choice).
For odor presentation, the pen cap was removed by the experimenter for approximately 3 seconds and the tip of the pen was placed approximately 1–2 cm in front of the nostrils. Instead of liquid dye, the tampon of the pens for threshold testing was filled with phenyl ethyl alcohol (PEA, a rose-like odor) diluted in propylene glycol (dilution ratio 1: 2, starting at 4%). Odors were presented in a total of 16 triplets of pens, 1 containing diluted phenyl ethyl alcohol and 2 containing only propylene glycol (negative controls). The interval between presentations of individual pens of a triplet was approximately 3 seconds and presentation of the triplets to a subject occurred every 20 seconds. Employing a 3-alternative, temporal forced choice paradigm, the subjects had to identify the pen that contained the odorant. Subjects were blindfolded to prevent visual identification of the odor-containing pens.
Thresholds (T) were determined using a single-staircase technique. In the present 3-alternative, temporal forced-choice paradigm, 2 successive correct identifications of the pen containing the odor or 1 incorrect identification triggered a reversal of the staircase to the next higher or the next lower dilution step, respectively. Seven reversals had to be obtained [10]. The odor thresholds were determined as the mean of the last 4 staircase reversals.
Assessment of odor threshold was followed by a test of odor discrimination [10]. For odor discrimination (D), 16 triplets of pens were presented, with 2 containing the same odorant and 1 containing the target odorant. The subjects’ task was to identify the sample that had a different smell. To prevent visual detection of the target pen, subjects were blindfolded with a sleeping mask. Subjects were only allowed to sample the odor once. Presentation of triplets was separated by at least 30 seconds. The test result was a sum score of correctly identified pens. The final step a test of odor identification was performed to completely assess the subjects’ objective function [10]. Odor identification (I) was assessed by means of 16 common odors. Using a multiple forced-choice paradigm, identification of individual odors was performed from a list of 4 verbal descriptors each. Each odorant was presented by the experimenter and there was an interval of at least 30 seconds to prevent olfactory desensitization [10]. Subjects were free to sample the odors as often as necessary to make a decision. The test result was a sum score of the correctly identified odors. Results from olfactory testing can be analyzed separately from each other. Overall olfactory function is expressed as the sum of the scores from the 3 individual tests (TDI score) [25,26].
Statistical analysis
Data analysis was performed by SPSS 21.0 (Statistical Package for Social Sciences, SPSS Inc. Chicago, IL). The normal distribution of considered variables was evaluated using the Shapiro-Wilk test. Data are shown as mean ?standard deviation for continuous variables and number of cases was used for categorical variables. Demographic data of the subjects were compared by t test or chi-squared test, as appropriate. To explore general olfactory sensitivity (TDI score) in relation to age and sex, data were submitted to analysis of variance (ANOVA) using the general linear model with between subject factors? age group? and sex?with Bonferroni post hoc tests. The level of significance was set at 0.05.
Results
The study was carried out in 174 subjects between the ages of 18–68 years. The participants were divided into 2 groups according to subjective olfactory function, either healthy or abnormal. Most participants (68%) had a high educational level (high school or university) and there was no difference between the groups in terms of education and age; however, there was significant difference in terms of sex.
Table 1 summarizes the characteristics of each of the variables of interest by disease status. Differences in TDI, T, D, and I were significant between the abnormal and healthy groups.
Table 1.
Comparison of the two groups according to his/her own admission about olfactory dysfunction.
| Characteristics | Abnormal group | Healthy group | p value |
|---|---|---|---|
| Age | 29.9±11.95 | 30.9±13.7 | 0.6 |
| Male gender/N | 43/51 | 83/123 | 0.03 |
| T | 4.96±1.9 | 8.9±1.5 | <0.0001 |
| D | 9.98±3.2 | 14.04±1.3 | <0.0001 |
| I | 8.98±2.7 | 13.3±1.4 | <0.0001 |
| TDI | 23.9±6.1 | 36.4±2.85 | <0.0001 |
TDI – threshold, discrimination and identification.
Descriptive statistics of normative values obtained in healthy subjects are shown in Table 2. When all the age groups were considered together in healthy subjects, the 10th percentiles of odor threshold score, odor discrimination score, odor identification score, and TDI score were 7.25, 12, 11, and 32, respectively.
Table 2.
Descriptive statistics of normative values of “Sniffin’ Sticks” olfactory test obtained in healthy subjects.
| T | D | I | TDI | |
|---|---|---|---|---|
| Age group: 18–35 (n=90) | ||||
| Mean ±SD | 9.04±1.35 | 14.01±1.35 | 13.3±1.28 | 36.4±2.93 |
| Range | 5–11 | 10–16 | 10–16 | 31–42 |
| 10th percentile | 7 | 12 | 11 | 31.1 |
| 25th percentile | 8.25 | 13 | 12.75 | 35 |
| 50th percentile | 9.50 | 14 | 14 | 37 |
| 75th percentile | 10.50 | 15 | 14 | 38.25 |
| 90th percentile | 11 | 16 | 15 | 40 |
| Age group: 36–55 (n=26) | ||||
| Mean ±SD | 8.9±1.87 | 13.7±1.18 | 13.69±1.12 | 36.3±2.48 |
| Range | 2–11 | 12–16 | 10–16 | 31–40 |
| 10th percentile | 6.7 | 12 | 13 | 32 |
| 25th percentile | 8.50 | 13 | 13 | 34.75 |
| 50th percentile | 9.25 | 14 | 14 | 36.5 |
| 75th percentile | 10 | 15 | 14 | 39 |
| 90th percentile | 11 | 15 | 15 | 39 |
| Age group: >55 (n=7) | ||||
| Mean ±SD | 8.6±0.53 | 14.7±1.38 | 12.3±2.3 | 35.5±3.35 |
| Range | 8–9 | 13–16 | 10–15 | 32–39 |
| 10th percentile | 8 | 13 | 10 | 32 |
| 25th percentile | 8.25 | 13 | 10 | 32 |
| 50th percentile | 9.50 | 15 | 11 | 35 |
| 75th percentile | 9.50 | 16 | 15 | 39 |
| 90th percentile | 9.75 | 16 | 15 | 39 |
TDI – threshold, discrimination and identification.
ANOVA testing did not reveal a significant effect of age on T, D, or TDI scores but a significant decrease was detected in I scores in subjects over the age of 55 (p=0.04).
A significant effect of subject sex was found in the I score (p=0.03) and TDI score (p=0.02), with females being more sensitive than males.
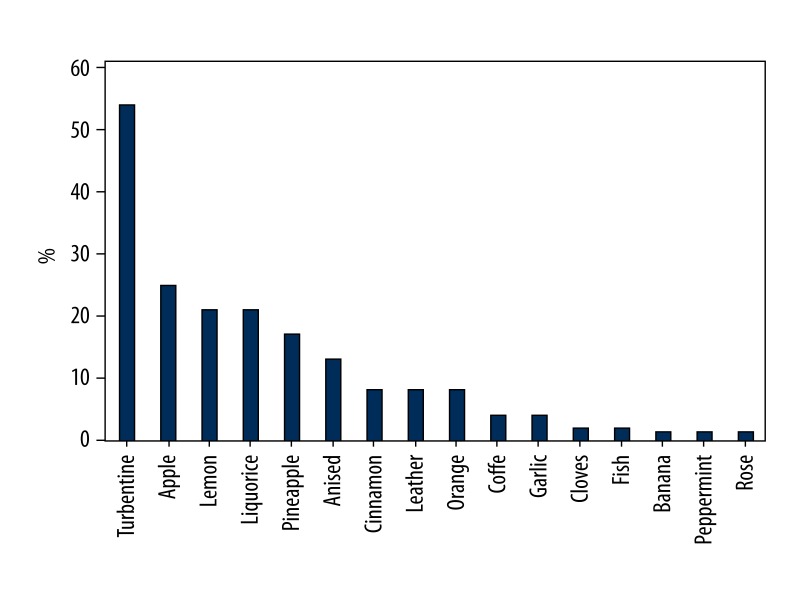
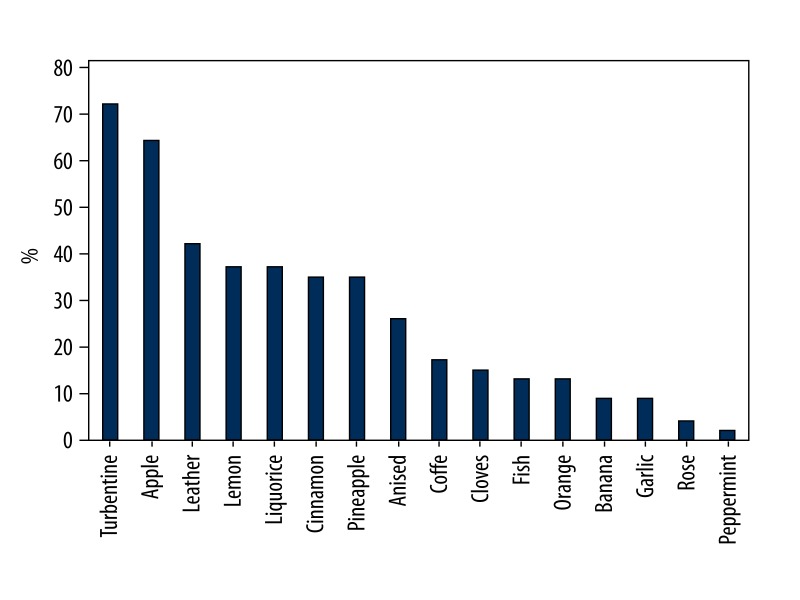
In the healthy group, some odorants were consistently correctly identified but others were not. Peppermint, rose, fish, banana, cloves, garlic, and coffee were the odorants most reliably identified correctly, while turpentine, apple, leather, lemon, and liquorice were most commonly mistaken (Figure 1). In the abnormal group, as expected, a larger proportion of subjects wrongly identified odorants (Figure 2). Considering the 2 groups together, apple and turpentine were the least well-recognized odors from the 16 presented.
Figure 1.
Proportion of wrongly identifying odorants in healthy group.
Figure 2.
Proportion of wrongly identifying odorants in abnormal group.
Discussion
Our study provides an update of normative values for routine clinical use of “Sniffin’ Sticks” in Turkish populations. Also, the present study validated that the “Sniffin’ Sticks” olfactory test is suitable for clinical usage in Turkish populations.
“Sniffin’ Sticks” is a modern olfactory test recommended by the German Olfactology and Gustology Association as a standard for olfactory testing [7]. However, cultural differences make the application of the odor identification tests in different countries difficult, because odor identification is strongly dependent on familiarity with the odors tested. When applied cross-culturally, the “Sniffin’ Sticks” test appears to perform well, but in some populations the test required adaptation using different distracters in the 4 alternative forced multiple-choice identification subtest [16–19].
In a validating study conducted in Taiwan, although the odorants were kept the same, some descriptors were changed from the original version. The authors concluded that the “Sniffin’ Sticks” test appears to be suited for the assessment of olfactory identification in an Asian population after revision of the descriptors [18,19].
Two studies were conducted in Greece on healthy subjects. In 1 of the studies the authors did not encounter any problematic items in the identification list and found the “Sniffin’ Sticks” test well-adapted to testing olfaction in a Greek population [15]. The other, larger, study showed decreased identification (<70%) for 6 of the odorants: aniseed, turpentine, liquorice, apple, lemon, and cinnamon [16]. The modifications in the list of descriptors after linguistic changes significantly increased the identification of problematic items. Translation and cultural adaptation of the descriptor list in the identification subtest was used in studies conducted in Brazil, Sri-Lanka, and the Netherlands, with the test being validated against normative data from Germany [21–23].
In a randomized crossover study on patients with hyposmia and anosmia, the investigators modified the distracters, using more contrasted distracters (contrasted test) [27]. Patients with hyposmia performed better in the contrasted test in odor identification, but patients with anosmia did not. It appears that the use of more contrasted distracters can contribute to better discrimination between patients with hyposmia and anosmia, which is important in a clinical context.
In our Turkish population, the odors most commonly mistaken in the healthy group were turpentine (54%), apple (25%), lemon (21%), liquorice (21%), and pineapple (17%). In the abnormal group, the most commonly mistaken odorants were apple (72%), cinnamon (67%), turpentine (63%), pineapple (55%) and liquorice (55%). A possible explanation for these results could be the similarity of distracters with the odorant, as in the case of apple (distracters are orange, peach, and melon), cinnamon (distracters are honey, vanilla, and chocolate) and lemon (distracters are grapefruit, apple, and peach) or unfamiliarity with an odorant such as turpentine, liquorice, and pineapple. Another possible reason for the difficulties in identification could lie in the odor itself: the currently used apple odor smells like Granny Smith apple and the participants confused this odor with air freshener. In Turkish culture, people are accustomed to more sugary and sweet apple types. Also, some of the odors presented as distracters may not be very familiar to Turkish populations, for example ham, wine, rum, or gummy bears.
According to Hummel et al., a criterion for the selection of odorants was the successful identification of individual odorants from a list of 4 descriptors should be >75% in healthy subjects [28]. Based on this view, we propose replacing turpentine with an odorant more familiar to Turkish culture. Our study had a limitation due to the low number of participants over the age 55. Thus, further studies with a higher number of participants including all age groups are needed for the Turkish population.
Conclusions
These results provide the basis for routine clinical evaluation of patients with olfactory disorders using the “Sniffin’ Sticks” test in Turkish populations.
Acknowledgments
The authors acknowledge the help of Dr. Thomas Hummel in the preparation of this article.
Footnotes
Source of support: Departmental sources
Statement
The authors state that they have no funding, financial relationships, or conflicts of interest.
References
- 1.Jacob S, McClintock MK, Zelano B, Ober C. Paternally inherited HLA alleles are associated with women’s choice of male odor. Nat Genet. 2002;30(2):175–79. doi: 10.1038/ng830. [DOI] [PubMed] [Google Scholar]
- 2.Baron RA. The sweet smell of helping: effects of pleasant ambient fragrance on prosocial behaviour shopping malls. Personal Soc Psychol Bull. 1997;23:489–503. [Google Scholar]
- 3.Vernet-Maury E, Alaoui-Ismaili O, Dittmar A, et al. Basic emotions induced by odorants: a new approach based on autonomic pattern results. J Auton Nerv Syst. 1999;75(2–3):176–83. doi: 10.1016/s0165-1838(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 4.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur Arch Otorhinolaryngol. 2005;262(3):231–35. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 5.Murphy C, Schubert CR, Cruickshanks KJ, et al. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–12. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 6.Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255(8):1121–26. doi: 10.1007/s00415-008-0807-9. [DOI] [PubMed] [Google Scholar]
- 7.Landis BN, Hummel T. New evidence for high occurrence of olfactory dysfunctions within the population. Am J Med. 2006;119(1):91–92. doi: 10.1016/j.amjmed.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 8.Brerson A, Johansson L, Ek L, et al. Prevalence of olfactory dysfunction: The Sk de population-based study. Laryngoscope. 2004;114(4):733–37. doi: 10.1097/00005537-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 9.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function (UPSIT) Physiol Behav. 1984;32(3):489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 10.Hummel T, Sekinger B, Wolf S, et al. “Sniffin’ Sticks” olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold”. Chem Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Kobal G, Klimek L, Wolfensberger M, et al. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 2000;257(4):205–11. doi: 10.1007/s004050050223. [DOI] [PubMed] [Google Scholar]
- 12.Kobal G, Hummel T. Olfactory and intranasal trigeminal event-related potentials in anosmic patients. Laryngoscope. 1998;108(7):1033–35. doi: 10.1097/00005537-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Kobal G, Hummel T, Sekinger B, et al. “Sniffin’ sticks”: screening of olfactory performance”. Rhinology. 1996;34(4):222–226. [PubMed] [Google Scholar]
- 14.Mackay-Sim A, Grant L, Owen C, et al. Australian norms for a quantitative olfactory function test. J Clin Neurosci. 2004;11(8):874–79. doi: 10.1016/j.jocn.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Katotomichelakis M, Balatsouras D, Tripsianis G, et al. Normative values of olfactory function testing using the ‘sniffin’ sticks’. Laryngoscope. 2007;117(1):114–20. doi: 10.1097/01.mlg.0000246518.79894.7e. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinidis I, Printza A, Genetzaki S, et al. Cultural adaptation of an olfactory identification test: the Greek version of Sniffin’ Sticks. Rhinology. 2008;46(4):292–96. [PubMed] [Google Scholar]
- 17.Yuan BC, Lee PL, Lee YL, et al. Investigation of the Sniffin’ Sticks olfactory test in Taiwan and comparison with different continents. J Chin Med Assoc. 2010;73(9):483–86. doi: 10.1016/S1726-4901(10)70103-9. [DOI] [PubMed] [Google Scholar]
- 18.Shu CH, Yuan BC, Lin SH, et al. Cross-cultural application of the “Sniffin’ Sticks” odor identification test. Am J Rhinol. 2007;21(5):570–73. doi: 10.2500/ajr.2007.21.3075. [DOI] [PubMed] [Google Scholar]
- 19.Shu CH, Yuan BC. Assessment of odor identification function in Asia using a modified “Sniffin’ Stick” odor identification test. Eur Arch Otorhinolaryngol. 2008;265(7):787–90. doi: 10.1007/s00405-007-0551-2. [DOI] [PubMed] [Google Scholar]
- 20.Eibenstein A, Fioretti AB, Lena C, et al. Olfactory screening test: experience in 102 Italian subjects. Acta Otorhinolaryngol Ital. 2005;25(1):18–22. [PMC free article] [PubMed] [Google Scholar]
- 21.Boesveldt S, Verbaan D, Knol DL, et al. Odour identification and discrimination in Dutch adults over 45 years. Rhinology. 2008;46(2):131–36. [PubMed] [Google Scholar]
- 22.Silveira-Moriyama L, Sirisena D, Gamage P, et al. Adapting the Sniffin’ Sticks to diagnose Parkinson’s disease in Sri Lanka. Mov Disord. 2009;24(8):1229–33. doi: 10.1002/mds.22545. [DOI] [PubMed] [Google Scholar]
- 23.Silveira-Moriyama L, Carvalho MJ, Katzenschlager R, et al. The use of smell identification tests in the diagnosis of Parkinson’s disease in Brazil. Mov Disord. 2008;23(16):2328–34. doi: 10.1002/mds.22241. [DOI] [PubMed] [Google Scholar]
- 24.Orhan KS, Karabulut B, Keles N, Deger K. Evaluation of Factors Concerning the Olfaction Using the Sniffin’ Sticks Test. Otolaryngol Head Neck Surg. 2011;146(2):240–46. doi: 10.1177/0194599811425019. [DOI] [PubMed] [Google Scholar]
- 25.Wolfensberger M, Schnieper I, Welge-Lussen A. “Sniffin’ Sticks” a new olfactory test battery”. Acta Otolaryngol. 2000;120(2):303–6. doi: 10.1080/000164800750001134. [DOI] [PubMed] [Google Scholar]
- 26.Hummel T, Kobal G, Gudziol H, et al. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264(3):237–43. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- 27.Gudziol V, Hummel T. The influence of distractors on odor identification. Arch. Otolaryngol Head Neck Surg. 2009;135(2):143–45. doi: 10.1001/archotol.135.2.143. [DOI] [PubMed] [Google Scholar]
- 28.Hummel T, Sekinger B, Wolf SR, et al. Sniffin’ sticks : olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]