Abstract
Background and aims
Hepatocellular carcinoma (HCC) frequently results from synergism between chemical and infectious liver carcinogens. Worldwide, the highest incidence of HCC is in regions endemic for the foodborne contaminant aflatoxin B1 (AFB1) and hepatitis B virus (HBV) infection. Recently, gut microbes have been implicated in multisystemic diseases including obesity and diabetes. Here, the hypothesis that specific intestinal bacteria promote liver cancer was tested in chemical and viral transgenic mouse models.
Methods
Helicobacter-free C3H/HeN mice were inoculated with AFB1 and/or Helicobacter hepaticus. The incidence, multiplicity and surface area of liver tumours were quantitated at 40 weeks. Molecular pathways involved in tumourigenesis were analysed by microarray, quantitative real-time PCR, liquid chromatography/mass spectrometry, ELISA, western blot and immunohistochemistry. In a separate experiment, C57BL/6 FL-N/35 mice harbouring a full-length hepatitis C virus (HCV) transgene were crossed with C3H/HeN mice and cancer rates compared between offspring with and without H hepaticus.
Results
Intestinal colonisation by H hepaticus was sufficient to promote aflatoxin- and HCV transgene-induced HCC. Neither bacterial translocation to the liver nor induction of hepatitis was necessary. From its preferred niche in the intestinal mucus layer, H hepaticus activated nuclear factor-κB (NF-κB)-regulated networks associated with innate and T helper 1 (Th1)-type adaptive immunity both in the lower bowel and liver. Biomarkers indicative of tumour progression included hepatocyte turnover, Wnt/β-catenin activation and oxidative injury with decreased phagocytic clearance of damaged cells.
Conclusions
Enteric microbiota define HCC risk in mice exposed to carcinogenic chemicals or hepatitis virus transgenes. These results have implications for human liver cancer risk assessment and prevention.
Liver cancer, the third leading cause of cancer mortality worldwide, frequently arises in a setting of combined chemical and infectious carcinogen exposures.1 A major risk factor for hepatocellular carcinoma (HCC) is aflatoxin ingestion from foods contaminated by the moulds Aspergillus flavus and A parasiticus.2Worldwide, the incidence of HCC is highest in regions endemic for aflatoxin B1 (AFB1) and hepatitis B virus (HBV).3 However, there is also significant geographic variation in the incidence of AFB1-associated liver cancer in the absence of viral hepatitis, and vice versa.4 Similarly, the distribution of liver cancer in patients chronically infected with hepatitis C virus (HCV) is not uniform.5 This suggests that additional, unrecognised environmental agents influence HCC risk. Because intestinal bacteria have been implicated in multisystemic diseases including obesity and diabetes, 6,7,8 we used mouse models to test the hypothesis that gut microbes influence the outcome of liver cancer associated with chemical and viral transgenic hepatocarcinogens.
Materials and methods
Mice
Sixteen timed-pregnant Helicobacter-free C3H/HeN female mice were purchased from the National Cancer Institute (Frederick, Maryland, USA). Absence of Helicobacter spp. infection was confirmed upon arrival by PCR as described below. Offspring (n = 85) were randomised into four groups: (1) sham, (2) AFB1, (3) H hepaticus or (4) AFB1 + H hepaticus (fig 1a). Pups at 10–12 days of age were inoculated intraperitoneally with a single injection of 7 µg/g body weight AFB1 (Sigma, St Louis, Missouri, USA) dissolved in 100 µl of corn oil, or vehicle only. Others have shown that a single injection of AFB1 to infant mice is necessary and sufficient to induce HCC, whereas adults are more resistant.9 Beginning at 3 weeks, animals were gavaged with 2×107 colony-forming units of H hepaticus strain 3B1 (ATCC 51449) or broth only every 48 h for three doses as previously described10. Mice were euthanatised at 40 weeks by CO2 inhalation, and blood and tissues were evaluated as described below. In a separate experiment, Helicobacter-free FL-N/35 mice bearing a full-length HCV transgene (minus certain regulatory elements) on a C57BL/6 background were crossed with C3H/HeN mice. B6×C3H F1 offspring were genotyped by DNA PCR for the presence or absence of the transgene according to protocol.11 A total of 163 offspring (65 females, 98 males) were entered into four groups characterised by the presence or absence of the HCV transgene and H hepaticus infection as described above. Livers were collected at 3, 9 or 15 month necropsy and submitted for histopathology and quantitative real-time PCR (qRT-PCR). All animal procedures were compliant with the US Public Health Service Policy on Humane Care and Use of Laboratory Animals and approved by the Massachusetts Institute of Technology Committee on Animal Care.
Figure 1.
Helicobacter hepaticus persistently colonises the lower bowel without inducing clinical enteric disease. (a) Aflatoxin study design. (b) Helicobacter hepaticus fluoresence in situ hybridisation. (c) Serum concentration of lipopolysaccharide (LPS) and high mobility group box-1 (HMGB1) in control (Ctrl), AFB1-, H hepaticus (Hh)- and AFB1 + Hh-treated groups.
Histopathology and special stains
H&E-stained sections of formalin-fixed liver were scored by a board-certified veterinary pathologist blinded to sample identity on a 0–4 scale for inflammation and neoplasia as described elsewhere.12 Statistical comparisons for all groups were performed by Kruskal–Wallis one-way analysis of variance (ANOVA), and between groups by Mann–Whitney U test using Prism 4 software (GraphPad, San Diego, California, USA). Tumour incidence, multiplicity and total surface area were calculated as described elsewhere13 and compared statistically by Fisher exact test (incidence) or one-way ANOVA and unpaired t test (multiplicity and surface area). Bacteria were visualised in the lower bowel by fluorescence in situ hybridisation (FISH) following a basic protocol described elsewhere.14 The H hepaticus-specific oligoprobe (5′-Cy3/CCCACACTCCAGAGTAACAGT-3′; Integrated DNA Technologies, Coralville, Iowa. USA) was designed with MacVector 7 software (MacVector, Cary, North Carolina, USA). All chromogenic and fluorescence immunohistochemistry (IHC) procedures were performed as described.15
PCR and microarray
DNA and RNA from liver and lower bowel were isolated as described elsewhere.16 Helicobacter hepaticus was detected using a nested DNA PCR protocol with near single-copy sensitivity.17 Microarray of liver and lower bowel from two female mice in each experimental group was performed using the GeneChip Mouse Genome 430 2.0 Array (Affymetrix, Santa Clara, California, USA). Primary microarray data were deposited with the NCBI Gene Expression Omnibus (#GSE11382). In addition to visual comparison in Excel (Microsoft, Redmond, Washington, USA), microarray data were analysed with Spotfire (TIBCO Software, Palo Alto, California, USA), Partek Genomics Suite (Partek, St Louis, Missouri, USA) and Ingenuity Pathways Analysis (Ingenuity Systems, Redwood City, California, USA). qRT-PCR for liver genes was performed using a previously described SYBR Green-based system.12 Immune-associated lower bowel genes were quantiated with the RT2 Profiler PCR Array (PAMM-011; SuperArray Bioscience, Frederick, Maryland, USA). Parametric statistics were performed as described above. Mice from the HCV study were evaluated for a battery of genes shown previously to be associated with hepatitis and/or cancer by SYBR Green qRT-PCR as described.12, 16.
Serum immunoassays
Serum lipopolysaccharide (LPS) was quantitated with the QCL-1000 Endpoint Chromogenic LAL Assay (Lonza, Basel, Switzerland). High mobility group box-1 (HMGB1) was measured by commercial ELISA (Shino-Test, Tokyo, Japan). Semi-quantitative assessment of circulating protein adducts of 9,12-dioxo-10(E)-dodecenoic acid (DODE) was performed by chemiluminescent western blot using a rabbit polyclonal antibody produced in the laboratory of SRT. Serum cytokines were measured using the Bio-Plex Pro Mouse Cytokine 23-plex Assay (#M60-009RDPD, Bio-Rad Laboratories, Hercules, California, USA) according to the manufacturer’s instructions.
DNA adducts and oxidative damage
To measure aflatoxin–DNA adducts, 18 pups (9 of each sex) at 10 days of age were inoculated with [3H]AFB1 (Moravec Biochemicals, Brea, California, USA) as described above, and liver DNA tritium incorporation was quantitated by scintigraphy according to the method of Groopman et al.18 Etheno adducts of DNA and RNA in terminal liver samples were determined by liquid chromatography/mass spectrometry (LC/MS) according to a method described elsewhere.19Markers of oxidative cellular damage detected by IHC in liver included DODE and 4-hydroxynonenal (Calbiochem, San Diego, California, USA).
Results
Helicobacter hepaticus colonised the lower bowel without clinical signs
Our model organism was H hepaticus, a ubiquitous murine bacterium known to alter the intestinal microbial census without producing clinical signs in wild-type (WT) mice.20. The presence or absence of H hepaticus in appropriate groups was confirmed by bacterial DNA PCR on frozen caecal tissue. There was no evidence of invasion of H hepaticus or other bacteria beneath the colonic surface epithelium by FISH (fig 1b) or by standard histopathology, nor were there lesions consistent with typhlocolitis (inflammation of the caecum and colon) or endotoxaemia (vascular congestion, submucosal oedema). Weight gain in all groups was equivalent, and no mice developed diarrhoea or rectal prolapse indicative of enterocolitis. Additionally, there was no difference between groups for circulating LPS or the tissue distress marker HMGB1(fig 1c). Therefore, by all measured outcomes H hepaticus produced no clinical enteric disease in this group of mice.
Helicobacter hepaticus promoted AFB1-induced liver cancer in both males and females
Next, we determined the impact of AFB1 and H hepaticus, alone and together, on liver disease. No significant lesions in sham-treated mice were identified in tissue sections scored by a board-certified veterinary pathologist blinded to sample identity (fig 2a).12 In contrast, all male mice inoculated with AFB1 developed lesions ranging from preneoplastic intralobular and translobular foci of cellular alteration, to adenomas and malignant HCC. The co-administration of H hepaticus with AFB1 resulted in significantly greater tumour multiplicity, total surface area and ratio of malignant carcinomas relative to benign adenomas (all p<0.05, unpaired t test; fig2b). In females, neither aflatoxin nor H hepaticus alone produced histological alterations, whereas 86% of those exposed to both agents developed dysplastic and/or neoplastic liver lesions (p = 0.02, Fisher exact test; fig 2c). Thus, H hepaticus promoted liver carcinogenesis in both male and female mice.
Figure 2.
Helicobacter hepaticus promotes aflatoxin B1 (AFB1)-induced liver cancer in male and female mice. (a) Liver histopathology of male (top row) and female (bottom row) mice in the four treatment groups; *adenoma, †hepatocellular carcinoma. (b) Adenoma vs carcinoma multiplicity and total tumour surface area comparison in AFB1-exposed males with and without H hepaticus (Hh) (c) Tumour incidence comparison in male and female mice from each treatment group. (d) Acute (4 h), mixed (24 h) and late (48 h) AFB1–DNA adducts in infant male and female mice.
Gender disparity in cancer risk was due to promotion
Our results confirmed data from both humans and rodents that males develop HCC with much higher frequency than females.21,22 In order to determine whether this gender disparity was due to differences in cancer initiation or promotion, we quantitated early (4 h), mixed (24 h) and late (48 h) [3H]AFB1–DNA liver adducts in male and female pups.18 There were no significant differences between the sexes for AFB1–DNA adducts at any time point (fig 2d). Therefore, the influence of sex on HCC risk in this model occurred at the level of promotion and not initiation.
Helicobacter hepaticus increased cell proliferation and activated Wnt/β-catenin signalling
In order to correlate tumour promotion by H hepaticus with tissue markers of liver cancer, we performed a battery of immunohistochemical stains. As expected, tumours contained rapidly proliferating hepatocytes with a high Ki-67 labelling index (fig 3a). In H hepaticus-infected but not Helicobacter-free animals, there also was a significant increase in proliferation in non-cancerous regions. Although focally variable, there also were increased cleaved caspase-3+ apoptotic hepatocytes in areas of high cell proliferation.23 Thus, H hepaticus stimulated hepatocyte turnover that may have contributed to neoplastic transformation through induction of proinflammatory responses, as has been documented in other mouse models.24 One signalling pathway frequently deregulated in aggressive hepatic malignancies in humans and rodents is Wnt/β-catenin.25 In order to determine whether this pathway was targeted by H hepaticus, we performed β-catenin IHC.15 Tumours from AFB1-exposed males infected with H hepaticus, but not females or Helicobacter-free male mice, exhibited malignant nodules with β-catenin+ nuclei, indicative of constitutive Wnt activation (fig.3b). This suggests that pathways activated by H hepaticus colonisation promote the conversion of aflatoxin-induced liver cancer from a β-catenin-negative to a β-catenin-positive phenotype. In humans and in other rodent models, β-catenin+ HCCs are associated with poor clinical outcomes.26,27,28
Figure 3.
Helicobacter hepaticus increases hepatocyte proliferation and dysregulates Wnt/β-catenin. (a) Ki-67 labelling index comparison in non-cancerous liver with and without H hepaticus (Hh) (b) Surface localisation of β-catenin in normal liver and adenoma (left two panels); constitutive Wnt activation with nuclear β-catenin translocation in a hepatocellular carcinoma (HCC) nodule (arrowheads, right panel). (c) Elevated serum interleukin-12 (IL-12) p40 and keratinocyte-derived chemokine (KC; murine analogue of human IL-8) in male mice with histologically overt liver lesions (hepatitis (Hep)and/or tumours (Tmr)); in females, only KC correlated with tumours. AFB1, aflatoxin B1; F, female; M, male, Norm, normal; Fl-B, fluorescence minus background.
Serum keratinocyte-derived chemokine (KC) and interleukin-12 (IL-12) p40 correlated with liver lesions
To determine whether enteric H hepaticus colonisation promoted liver cancer through induction of secreted cytokines, we analysed 23 serum cytokines in male and female mice. Due in part to wide individual variation, we observed trends but no statistically significant differences between groups stratified by gender, AFB1 inoculation or H hepaticus infection (Supplementary table 1 online). In males, two serum cytokines were significantly associated with histological liver lesions (hepatitis and/or tumours): IL-12 p40 and the murine analogue of human IL-8, keratinocyte-derived chemokine (KC; fig 3c). KC but not p40 also correlated with liver tumours in females. Interestingly, both of these cytokines were also significantly elevated in lesion-free males versus females. In mice with hepatitis and/or tumours, the likely source of IL-12 p40 and KC was liver since only those with overt hepatic lesions showed increased circulating levels versus controls of the same sex. It is possible that serum KC and IL-12 p40 may have use as biomarkers for liver disease progression in male mice. Unfortunately, the only blood available for analysis in our study was taken from the heart. It is possible that portal cytokine levels were different from those in the systemic circulation. A follow-up study will be required to test this possibility.
Gender-specific liver genes were preferentially altered in tumourigenesis
We previously reported that liver carcinogenesis in male mice is associated with the loss of a sex-specific hepatic molecular signature, a process termed “liver-gender disruption”.12 In order to determine whether this process also occurs in females, we performed microarray analysis of livers from female mice in each of the four treatment groups. Using a significance cut-off of ≥2-fold difference and p<0.05, we identified 586 upregulated and 78 downregulated genes associated with adenoma formation in female mice exposed to AFB1 and infected with H hepaticus (Supplementary table 2). In agreement with previous results in male mice, we found a significant over-representation of sex-dependent versus gender-neutral liver gene alterations associated with tumourigenesis. Whereas we previously demonstrated that ~13% of liver genes are sex dependent in the adult mouse liver, 12 in the present study we found that 36% of the top 25 upregulated and downregulated genes in females with adenomas were significantly altered (p = 0.009; table1). Also in agreement with prior data, male- and female-predominant genes were altered in both directions. Thus, targeted alteration of gender-specific genes is a feature of hepatocarcinogenesis in both sexes. Because we previously demonstrated that liver gender disruption in males is driven by proinflammatory cytokines, 12 we performed ingenuity pathways analysis of microarray data from female mice. Nuclear factor-κB (NF-κB) emerged at the hub of the most prominent disease-associated signalling networks both in the lower bowel and in the liver (fig 4).
Table 1.
Top 25 altered genes in liver of aflatoxin B1-exposed, H hepaticus-infected female mice with tumours vs uninfected controls
| Upregulated | FC | Gnd | Downregulated | FC | Gnd |
|---|---|---|---|---|---|
| Ubiquitin D | 26.08 | F | ATPase, H+ transporting, lysosomal V0 subunit A1 | −5.93 | |
| Vacuolar protein sorting 13A (yeast) | 17.03 | Protein C | −5.85 | ||
| RIKEN cDNA 4732454E20 gene | 12.66 | Phytoceramidase, alkaline | −4.58 | ||
| UDP-glucose:ceramide glucosyltransferase | 12.25 | IQ motif and WD repeats 1 | −4.46 | ||
| Serum amyloid A 3 | 11.88 | F | Sulfotransferase family 5A, member 1 | −4.00 | M |
| Neuregulin 3 | 9.90 | RIKEN cDNA A930011E06 gene | −3.98 | ||
| H19 fetal liver mRNA | 8.30 | Nuclear factor, erythroid derived 2 | −3.77 | F | |
| Similar to Ig heavy chain V region 23 | 7.69 | F | Solute carrier family 1, member 2 | −3.65 | |
| RIKEN cDNA 3830431G21 gene | 6.84 | D site albumin promoter-binding protein | −3.41 | F | |
| Chemokine (C-X-C motif) ligand 9 | 6.69 | F | Excision rodent repair deficiency, complementation group 4 | −3.27 | |
| Alpha-fetoprotein | 6.30 | M | ATP citrate lyase | −3.23 | F |
| RIKEN cDNA 4930408O21 gene | 6.13 | F | RIKEN cDNA 4833422M21 gene | −3.05 | M |
| CDK inhibitor 1A (P21) | 5.72 | RIKEN cDNA 4933426I21 gene | −2.97 | ||
| PPAR gamma | 5.60 | Serine (or cysteine) peptidase inhibitor, clade B, member 6c | −2.96 | ||
| Killer cell lectin-like receptor B, 1D | 5.35 | Storkhead box 2 | −2.95 | ||
| Adenylate cyclase 1 | 5.29 | d-Amino acid oxidase 1 | −2.94 | ||
| Interferon-activated gene 203 | 5.06 | Major urinary protein 1 | −2.90 | M | |
| Carboxylesterase 3 | 5.04 | Hypothetical LOC574418 | −2.88 | ||
| RIKEN cDNA 3110047M12 gene | 4.96 | F | Somatostatin receptor 3 | −2.85 | |
| RIKEN cDNA 2610305J24 gene | 4.78 | F | Interleukin-1 receptor-associated kinase 1-binding protein 1 | −2.76 | |
| Vang-like 2 (van gogh, Drosophila) | 4.69 | AF4/FMR2 family, member 2 | −2.76 | ||
| Striatin, calmodulin binding protein 3 | 4.68 | Inhibitor of growth family, member 5 | −2.76 | ||
| Scm-like with four mbt domains 1 | 4.68 | Tripartite motif protein 27 | −2.68 | M | |
| cDNA sequence AB182283 | 4.51 | Neurofibromatosis 2 | −2.66 | ||
| Maternal embryonic message 1 | 4.47 | M | Solute carrier family 25, member 19 | −2.61 |
CDK, cyclin-dependent kinase; FC, fold change; Gnd, gender-dimorphic transcript (F, feminine; or M, or masculine); Ig, immunoglobulin; PPAR, peroxisome proliferatoractivated receptor.
Figure 4.
Helicobacter hepaticus dysregulates hepatic gene networks converging on nuclear factor-κB (NF-κB) in both liver and lower bowel. Ingenuity pathways analysis demonstrates the central role of NF-κB in transcriptional disruption of female aflatoxin B1-exposed H hepaticus-infected mice with liver tumours.
Helicobacter hepaticus promoted liver cancer without leaving the lower bowel
The endogenous niche of H hepaticus is the large intestine. In some strains of mice, bacteria may also translocate to the liver and induce chronic hepatitis. 22 We and others have shown that H hepaticus alone can produce chronic hepatitis and HCC in certain strains of male mice, although lifelong infection is required and tumours typically do not arise until after 18 months of age. 12,29 To determine whether H hepaticus translocation to the liver was necessary to promote aflatoxin-induced HCC by 40 weeks in the present study, we performed highly sensitive nested bacterial DNA PCR. 18 In agreement with previous observations, we found a positive correlation between PCR detection of intrahepatic bacteria and histologically overt hepatitis in a subset of males. 12,30 However, neither hepatitis nor detection of intrahepatic bacteria by PCR correlated with tumour incidence, grade or multiplicity in any group of mice, male or female (all p>0.8, data not shown). In males, canonical inflammatory genes derived primarily from leucocytes correlated with overt hepatitis, but not with tumours (Supplementary fig1). We did not detect H hepaticus in mesenteric lymph nodes (MLNs) of any animal by Warthin–Starry stain or DNA PCR (not shown). Thus, there was neither a requirement for bacterial translocation nor induction of hepatitis to promote aflatoxin-induced HCC. From this we concluded that H hepaticus promoted liver cancer through histologically silent mechanisms originating in the lower bowel.
Helicobacter hepaticus triggered innate and T helper 1 (Th1)-type adaptive immunity
To begin to understand the mechanisms by which H hepaticus from its remote intestinal location promoted liver cancer, we performed microarray analysis of the lower bowel. We identified an even greater number of transcriptional alterations in the lower bowel than in the liver from AFB1-exposed, H hepaticus-infected female mice: 2152 upregulated and 49 downregulated genes (Supplementary table 3). As was the case in the hepatic compartment, altered genes represented a broad variety of pathways including innate immunity, Th1-type adaptive immunity, acute-phase responses and DNA-dependent transcriptional regulation. Also as was the case for the liver, NF-κB anchored disrupted cellular networks in the lower bowel fig 4). To confirm and extend lower bowel microarray results, we performed multiplex qRT-PCR for a battery of genes associated with innate and adaptive immunity. Out of 83 colonic genes expressed at detectable levels, 44 were altered by H hepaticus in female mice (38 up, 6 down), and 23 (19 up, 4 down) in males (Supplementary table 4). There were nine genes upregulated by H hepaticus in both female and male mice; those for: C-reactive protein (an acute-phase reactant), CC chemokine ligands 1 and 12, and receptors 7 and 8 (inflammatory signalling molecules induced by various upstream activators including IL-13),31 interferon γ (a prototype Th1 cytokine), osteopontin (Spp1; a pleiotropic cytokine recently proposed as a prognostic biomarker for HCC), 32 IL-1 family member 6 (Fil1ε; a newly discovered cytokine that may augment NF-κB signalling) 33 and IL-10 (an immunoregulatory cytokine that negatively feeds back on Th1-type responses, especially those invoked by infections). 34 The pleiotropic cytokine IL-16 (associated with allergy and autoimmune diseases) was downregulated in both sexes. 35 Viewed as a whole, genomic and cytokine analyses implicated NF-κB-mediated innate and Th1-type adaptive immune activation as the mechanism of greatest importance in liver tumour promotion by H hepaticus. Innate immunity was recently associated with activation of type I diabetes by gut microbes in an unrelated mouse model. 8
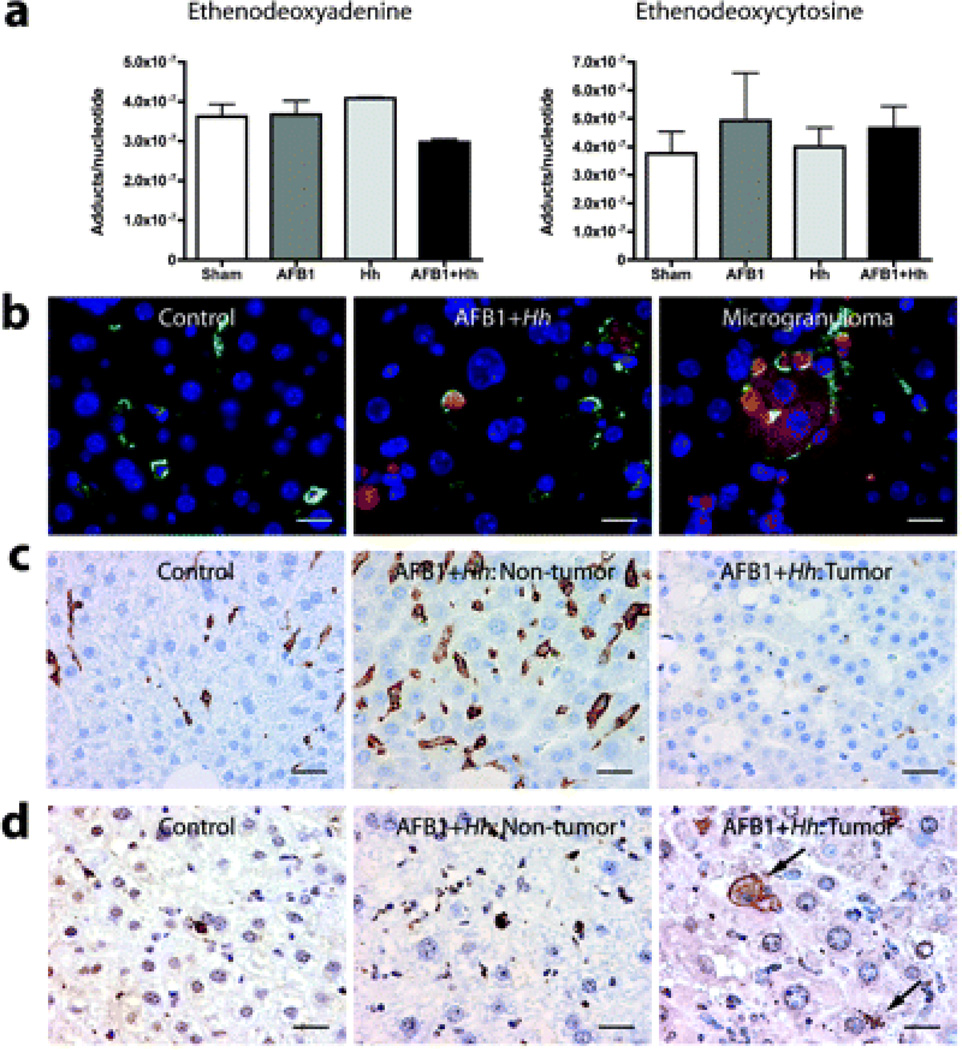
Liver cancer was associated with oxidative damage and depressed intratumoural immunity
Electrophilic attack in a setting of chronic inflammation, including oxidation/nitration and alkylation reactions, has been linked to carcinogenesis. 35 By LC/MS we did not find increased etheno RNA or DNA nucleotide adducts in terminal liver samples(fig 5a). However, by IHC we did observe oxidative damage, including adducts of the highly reactive lipid peroxide DODE, within the cytoplasm of professional phagocytes including Küppfer cells and macrophages. Another cell damage biomarker, 4-hydroxynonenal, was localised to the same cell populations (not shown). DODE+ hepatocytes were rarely encountered in non-cancerous liver, but where present invariably were surrounded by DODE-rich F4/80+ macrophages forming microgranulomas (fig 5b). Within tumours, Küppfer cell and macrophage density was significantly decreased compared with adjacent non-neoplastic tissue (fig 5c). In contrast, intratumoural hepatocytes with DODE+ adducts were much more frequent (fig 5d). Taken together, these observations pointed to decreased phagocyte activity with accumulation of oxidatively damaged hepatocytes inside tumours. Decreased clearance of oxidatively damaged hepatocytes may have contributed to the expansion Wnt/β-catenin-activated malignant nodules described above. We also performed Foxp3 IHC to determine whether natural T regulatory (Treg) cells were altered by H hepaticus infection and/or the presence of liver lesions. Foxp3+ cells were exceedingly rare in uninflamed liver parenchyma regardless of dysplasia/neoplasia grade (not shown). In contrast, we found increased Foxp3+ cells in areas of histologically overt inflammation, presumably recruited to regulate the inflammatory response as we have seen in other models.37 Thus, Foxp3+ Treg cells were associated with hepatitis but not tumours in our model. Taken together, our documentation of proinflammatory cytokine induction, rapid hepatocellular turnover and expansion of oxidatively damaged cells with impaired innate immune surveillance agrees with tumourigenic mechanisms in other mouse models of liver cancer. 38
Figure 5.
Helicobacter hepaticus increases oxidative but not alkylating damage in the liver, and tumours show suppression of immune surveillance with retention and accumulation of oxidatively damaged hepatocytes. (a) Comparison of etheno nucleotide adducts between groups as determined by liquid chromatography/mass spectrometry. (b) Double fluorescence immunohistochemistry (IHC) for Küppfer cells and macrophages (F4/80, fluorescein, green) and 9,12-dioxo-10(E)-dodecenoic acid (DODE; Cy3, red); nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue). (c) Chromogenic IHC for F4/80+ Küppfer cells and macrophages in non-cancerous and cancerous liver (diaminobenzidine (DAB), brown; haematoxylin counterstain). (d) Chromogenic IHC for DODE in non-cancerous and cancerous liver. AFLB1, aflatoxin B1, Hh, H hepaticus.
Helicobacter hepaticus increases oxidative but not alkylating damage in the liver, and tumours show suppression of immune surveillance with retention and accumulation of oxidatively damaged hepatocytes. (a) Comparison of etheno nucleotide adducts between groups as determined by liquid chromatography/mass spectrometry. (b) Double fluorescence immunohistochemistry (IHC) for Küppfer cells and macrophages (F4/80, fluorescein, green) and 9,12-dioxo-10(E)-dodecenoic acid (DODE; Cy3, red); nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI, blue). (c) Chromogenic IHC for F4/80+ Küppfer cells and macrophages in non-cancerous and cancerous liver (diaminobenzidine (DAB), brown; haematoxylin counterstain). (d) Chromogenic IHC for DODE in non-cancerous and cancerous liver. AFLB1, aflatoxin B1, Hh, H hepaticus.
Helicobacter hepaticus promoted liver cancer in HCV-transgenic (Tg) mice
In order to determine whether gut microbes can promote liver cancer induced by a non-chemical carcinogen, we performed a follow-up experiment using transgenic mice expressing a full-length hepatitis C transgene (minus certain regulatory elements). HCV FL-N/35 mice on a C57BL/6 background were bred with WT C3H mice to produce F1 offspring with and without the transgene as determined by DNA genotyping. 11 Unlike the H hepaticus-infected WT C3H males in the aflatoxin study, some of which developed overt hepatitis, no B6×C3 mice in the follow-up experiment developed significant liver inflammation (data not shown). There were no significant lesions in any mice at 3 or 9 months of age, except for sporadic mild to moderate hepatic steatosis that is recognised as a background lesion in B6×C3 F1 males (D Malarkey, NIEHS National Toxicology Program, personal communication). At 18 months, neither HCV transgene expression nor H hepaticus infection alone was sufficient to increase preneoplastic or neoplastic liver lesions over background levels (fig 6a). However, the combination of H hepaticus infection and HCV transgene expression resulted in a significantly greater incidence and multiplicity of preneoplastic and neoplastic liver foci in males (fig 6b). Moreover, qRT-PCR demonstrated that innate immunity and lipid processing genes dysregulated in the liver of other mouse models of cancer, including those encoding C-X-C chemokine ligand 9, cytochrome P450 17a1, stearoyl coA desaturase-2 and trefoil factor 3 (intestinal), were significantly upregulated in male HCV-Tg mice infected with H hepaticus (fig 6c). 12,16,39 Other gene products associated with murine liver cancer including serum amyloid A3, sulfotransferase 5a1 and ubiquitin D also were altered in the H hepaticus-infected HCV-Tg mice but failed to achieve statistical significance (data not shown). We did not observe β-catenin+ HCC in any HCV-Tg males, probably due to weaker tumour-initiating activity of the transgene when compared with AFB1. Nevertheless, our combined studies demonstrate that H hepaticus, from its lower bowel niche and in the absence of overt hepatitis, can promote tumours induced by both chemical and viral transgenic liver
Figure 6.
Helicobacter hepaticus promotes liver cancer in heptatitis C virus (HCV)-transgenic mice and disrupts canonical genes involved in murine hepatocarcinogenesis. (a) Neither H hepaticus (Hh) nor HCV transgene expression alone increased liver cancer risk in B6×C3 mice, whereas the combination of both resulted in a significant increase in burden. (b) Liver genes shown by us and others to be associated with hepatocellular carcinoma (HCC) in mice,12, 16, 29 including C-X-C chemokine ligand 9 (Cxcl9), cytochrome P450 17a1 (Cyt17a1), stearoyl coA desaturase-2 (Scd2) and trefoil factor 3 (Tff3; intestinal), were transcriptionally upregulated in H hepaticus-infected FL-N/35 mice compared with those bearing the HCV transgene alone at 18 months. (c) Histopathology demonstrating mild hepatic steatosis in all B6×C3 males, but HCC only in those expressing the HCV transgene and infected with H hepaticus.
Helicobacter hepaticus promotes liver cancer in heptatitis C virus (HCV)-transgenic mice and disrupts canonical genes involved in murine hepatocarcinogenesis. (a) Neither H hepaticus (Hh) nor HCV transgene expression alone increased liver cancer risk in B6×C3 mice, whereas the combination of both resulted in a significant increase in burden. (b) Liver genes shown by us and others to be associated with hepatocellular carcinoma (HCC) in mice, 12,16,39 including C-X-C chemokine ligand 9 (Cxcl9), cytochrome P450 17a1 (Cyt17a1), stearoyl coA desaturase-2 (Scd2) and trefoil factor 3 (Tff3; intestinal), were transcriptionally upregulated in H hepaticus-infected FL-N/35 mice compared with those bearing the HCV transgene alone at 18 months. (c) Histopathology demonstrating mild hepatic steatosis in all B6×C3 males, but HCC only in those expressing the HCV transgene and infected with H hepaticus.
Discussion
Here we have shown that gut microbes define the incidence and malignancy of liver cancer in mice exposed to the widespread environmental carcinogen AFLB1 or HCV transgenes. We found in both experiments that mice colonised by the enteric bacterium H hepaticus had a greater incidence and burden of HCC than did mice maintained free of infection. Importantly, there was a requirement for neither bacterial colonisation of the liver nor induction of hepatitis for tumour promotion. From its preferred niche in the colonic mucus layer, H hepaticus incited transcriptional responses in both the lower bowel and liver that converged on NF-κB and were consistent with activation of innate and Th1-type adaptive immunity. Oxidative damage from DODE and other electrophilic lipid adducts invoked phagocytic removal of hepatocytes in non-cancerous liver, whereas decreased innate immune surveillance resulted in accumulation of damaged hepatocytes within tumours. Additional mechanisms of liver tumour promotion by H hepaticus included increased hepatocellular turnover and dysregulated Wnt/β-catenin signalling. The conversion of a subset of tumours in H hepaticus-infected mice to a β-catenin+ phenotype was especially significant. This is known to augment specific tumour promoters that accelerate malignancy, including c-myc, cyclin D1 and AP-1/c-jun.28
Our findings reveal intimate cross-talk between gut microbes, the lower bowel and liver in the evolution of HCC, and suggest a novel approach for disease prevention in humans. Enterohepatic Helicobacter spp. including H cinaedi, H pullorum and others have been recovered from humans with and without clinical disease. 40,41,42 Detection of Helicobacter spp. within the liver of humans infected with HCV is associated with increased cancer risk. 43,44 However, our results demonstrate that gut microbes can promote HCC even without leaving the bowel. There are several potential mechanisms that could be involved. First, bacteria may alter colonic mucosal integrity and/or receptor activation, permitting passive or facilitated entry of harmful bacteria or their products into the circulation. We found no evidence of this in the present study. However, we did not sample portal blood and it is possible that microbial products were cleared from the general circulation by the liver. Secondly, microbial colonisation of the lower bowel may invoke release of secreted factors such as cytokines from the intestine and/or MLNs that act upon the liver. We and others have shown that H hepaticus upregulates transcription of numerous cytokines and receptors associated with innate and Th1-type adaptive immunity in the lower bowel. 45,46 IL-6 has been identified as a key regulator of male-predominant liver carcinogenesis. 21 However, we did not observe increased message levels of this cytokine in the lower bowel of H hepaticus-infected mice. In the liver, IL-6 transcription was markedly upregulated in male mice with histologically overt hepatitis, but not in mice with tumours in the absence of hepatitis. Additionally, there were no significant differences between groups in serum IL-6 levels. Nevertheless, IL-6 may have contributed in an autocrine/paracrine fashion to liver tumour promotion in our model. Thirdly, intestinal bacteria may disrupt enterohepatic feedback loops such as those associated with bile acid recirculation (eg, fibroblast growth factors and their receptors). 47 Finally, it is possible that transmission of microbial signals to the liver from the lower bowel and/or MLNs involves direct cell–cell interactions (eg, via migratory dendritic cells). None of these possibilities is mutually exclusive, and we consider it likely that extraintestinal phenotypes influenced by intestinal microbes are polyfactorial in nature. Nevertheless, our results prove in principle that addition of a single agent to the gut microbiome is sufficient to disrupt enterohepatic homeostasis and promote liver cancer. Future discoveries, including those from the Human Microbiome Project, may unmask intestinal bacteria associated with increased risk of HCC in humans. Such microbes would represent attractive therapeutic targets.
Supplementary Material
Acknowledgments
The authors thank Nancy Taylor for laboratory support, Kathleen Cormier, Chakib Boussahmain and Laura Crankshaw for histology, and Kimberly Dufour and Ellen Buckley for diagnostic results. We wish to dedicate this work to the memory of our colleague and dear friend, David Schauer (1961–2009).
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Wogan GN. Aflatoxin as a human carcinogen. Hepatology. 1999;30:573–575. doi: 10.1002/hep.510300231. [DOI] [PubMed] [Google Scholar]
- 3.Wogan GN, Groopman JD, Johnson D, Kensler TW. Aflatoxin and hepatitis B virus biomarkers: a paradigm for complex environmental exposures and cancer risk. Cancer Biomark. 2005;1:5–14. doi: 10.3233/cbm-2005-1103. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn KA, Hunter K, LeVoyer T, et al. Susceptibility to aflatoxin B1-related primary hepatocellular carcinoma in mice and humans. Cancer Res. 2003;63:4594–4601. [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 8.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesselinovitch SD, Mihailovich N, Wogan GN, et al. Aflatoxin B 1, a hepatocarcinogen in the infant mouse. Cancer Res. 1972;32:2289–2291. [PubMed] [Google Scholar]
- 10.Whary MT, Morgan TJ, Dangler CA, et al. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerat H, Honda M, Beard MR, et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352–365. doi: 10.1053/gast.2002.31001. [DOI] [PubMed] [Google Scholar]
- 12.Rogers AB, Theve EJ, Feng Y, et al. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res. 2007;67:11536–11546. doi: 10.1158/0008-5472.CAN-07-1479. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Dinse GE, Foley JF, et al. Comparative prevalence, multiplicity, and progression of spontaneous and vinyl carbamate-induced liver lesions in five strains of male mice. Toxicol Pathol. 2002;30:599–605. doi: 10.1080/01926230290105776. [DOI] [PubMed] [Google Scholar]
- 14.Fox JG, Wang TC, Rogers AB, et al. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 15.Rogers AB, Cormier KS, Fox JG. Thiol-reactive compounds prevent nonspecific antibody binding in immunohistochemistry. Lab Invest. 2006;86:526–533. doi: 10.1038/labinvest.3700407. [DOI] [PubMed] [Google Scholar]
- 16.Boutin SR, Rogers AB, Shen Z, et al. Hepatic temporal gene expression profiling in Helicobacter hepaticus-infected A/JCr mice. Toxicol Pathol. 2004;32:678–693. doi: 10.1080/01926230490524058. [DOI] [PubMed] [Google Scholar]
- 17.Shames B, Fox JG, Dewhirst F, et al. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol. 1995;33:2968–2972. doi: 10.1128/jcm.33.11.2968-2972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groopman JD, Croy RG, Wogan GN. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci USA. 1981;78:5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang B, Zhou X, Yu H, et al. Lipid peroxidation dominates the chemistry of DNA adduct formation in a mouse model of inflammation. Carcinogenesis. 2007;28:1807–1813. doi: 10.1093/carcin/bgm037. [DOI] [PubMed] [Google Scholar]
- 20.Ge Z, Feng Y, Taylor NS, et al. Colonization dynamics of altered Schaedler flora is influenced by gender, aging, and Helicobacter hepaticus infection in the intestines of Swiss Webster mice. Appl Environ Microbiol. 2006;72:5100–5103. doi: 10.1128/AEM.01934-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 22.Rogers AB, Fox JG. Inflammation and Cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am J Physiol Gastrointest Liver Physiol. 2004;286:G361–G366. doi: 10.1152/ajpgi.00499.2003. [DOI] [PubMed] [Google Scholar]
- 23.Rogers AB, Taylor NS, Whary MT, et al. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 24.Sakurai T, He G, Matsuzawa A, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvisi DF, Conner EA, Ladu S, et al. Activation of the canonical Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer. J Hepatol. 2005;42:842–849. doi: 10.1016/j.jhep.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Cieply B, Zeng G, Proverbs-Singh T, et al. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49:821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901–1915. doi: 10.2741/1933. [DOI] [PubMed] [Google Scholar]
- 28.Calvisi DF, Factor VM, Loi R, et al. Activation of beta-catenin during hepatocarcinogenesis in transgenic mouse models: relationship to phenotype and tumor grade. Cancer Res. 2001;61:2085–2091. [PubMed] [Google Scholar]
- 29.Ward JM, Fox JG, Anver MR, et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86:1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 30.Rogers AB, Boutin SR, Whary MT, et al. Progression of chronic hepatitis and preneoplasia in Helicobacter hepaticus-infected A/JCr mice. Toxicol Pathol. 2004;32:668–677. doi: 10.1080/01926230490524247. [DOI] [PubMed] [Google Scholar]
- 31.Cho SJ, Kang MJ, Homer RJ, et al. Role of early growth response-1 (Egr-1) in interleukin-13-induced inflammation and remodeling. J Biol Chem. 2006;281:8161–8168. doi: 10.1074/jbc.M506770200. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Xu HM, Zhou HJ, et al. The prognostic significance of preoperative plasma levels of osteopontin in patients with TNM stage-I of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009 doi: 10.1007/s00432-009-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debets R, Timans JC, Homey B, et al. Two novel IL-1 family members, IL-1 delta and IL-1 epsilon, function as an antagonist and agonist of NF-kappa B activation through the orphan IL-1 receptor-related protein 2. J Immunol. 2001;167:1440–1446. doi: 10.4049/jimmunol.167.3.1440. [DOI] [PubMed] [Google Scholar]
- 34.Lee CW, Rao VP, Rogers AB, et al. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag2−/− mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect Immun. 2007;75:2699–2707. doi: 10.1128/IAI.01788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cruikshank W, Little F. lnterleukin-16: the ins and outs of regulating T-cell activation. Crit Rev Immunol. 2008;28:467–483. doi: 10.1615/critrevimmunol.v28.i6.10. [DOI] [PubMed] [Google Scholar]
- 36.Dedon PC, DeMott MS, Elmquist CE, et al. Challenges in developing DNA and RNA biomarkers of inflammation. Biomarkers Med. 2007;1:293–312. doi: 10.2217/17520363.1.2.293. [DOI] [PubMed] [Google Scholar]
- 37.McBee ME, Zheng PZ, Rogers AB, et al. Modulation of acute diarrheal illness by persistent bacterial infection. Infect Immun. 2008;76:4851–4858. doi: 10.1128/IAI.00745-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakurai T, Maeda S, Chang L, et al. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Theve EJ, Feng Y, Taghizadeh K, et al. Sex hormone influence on hepatitis in young male A/JCr mice infected with Helicobacter hepaticus. Infect Immun. 2008;76:4071–4078. doi: 10.1128/IAI.00401-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiehlbauch JA, Tauxe RV, Baker CN, et al. Helicobacter cinaedi-associated bacteremia and cellulitis in immunocompromised patients. Ann Intern Med. 1994;121:90–93. doi: 10.7326/0003-4819-121-2-199407150-00002. [DOI] [PubMed] [Google Scholar]
- 41.Orlicek SL, Welch DF, Kuhls TL. Septicemia and meningitis caused by Helicobacter cinaedi in a neonate. J Clin Microbiol. 1993;31:569–571. doi: 10.1128/jcm.31.3.569-571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanley J, Linton D, Burnens AP, et al. Helicobacter pullorum sp. nov.-genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology. 1994;140:3441–3449. doi: 10.1099/13500872-140-12-3441. [DOI] [PubMed] [Google Scholar]
- 43.Ponzetto A, Pellicano R, Leone N, et al. Helicobacter infection and cirrhosis in hepatitis C virus carriage: is it an innocent bystander or a troublemaker? Med Hypotheses. 2000;54:275–277. doi: 10.1054/mehy.1999.0987. [DOI] [PubMed] [Google Scholar]
- 44.Dore MP, Realdi G, Mura D, et al. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47:1638–1643. doi: 10.1023/a:1015848009444. [DOI] [PubMed] [Google Scholar]
- 45.Myles MH, Dieckgraefe BK, Criley JM, et al. Characterization of cecal gene expression in a differentially susceptible mouse model of bacterial-induced inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:822–836. doi: 10.1002/ibd.20138. [DOI] [PubMed] [Google Scholar]
- 46.Livingston RS, Myles MH, Livingston BA, et al. Sex influence on chronic intestinal inflammation in Helicobacter hepaticus-infected A/JCr mice. Comp Med. 2004;54:301–308. [PubMed] [Google Scholar]
- 47.Jones S. Mini-review: endocrine actions of fibroblast growth factor 19. Mol Pharm. 2008;5:42–48. doi: 10.1021/mp700105z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.