Abstract
Purpose
To compare dose-volume histogram (DVH) variables for the internal and external urinary sphincters (IUS/EUS) with urinary quality of life after prostate brachytherapy.
Materials and Methods
Subjects were 42 consecutive men from a prospective study of brachytherapy as monotherapy with 125I for intermediate-risk localized prostate cancer. No patient received hormone therapy. Preplanning constraints included prostate V100 >95%, V150 <60%, and V200 <20% and rectal R100 < 1 cm3. Patients completed the EPIC quality of life questionnaire before and 1, 4, 8, and 12 months after implantation, and urinary domain scores were analyzed. All structures including the IUS and EUS were contoured on T2-weighted MRI at day 30, and doses received were calculated from identification of seeds on CT. Spearman's (nonparametric) rank correlation coefficient (ρ) was used for statistical analyses.
Results
Overall urinary morbidity was worst 1 month after the implant. Urinary function declined when the IUS V285 was 0.4% (ρ =–0.32, p=0.04); bother worsened when the IUS V35 was 99% (ρ=–0.31, p=0.05) or the EUS V240 was 63% (ρ=–0.31, p=0.05); irritation increased when the IUS V35 was 95% (ρ=–0.37, p=0.02) and the EUS V265 was 24% (ρ=–0.32, p=0.04); and urgency worsened when the IUS V35 was 99.5% (ρ=–0.38, p=0.02). Incontinence did not correlate with EUS or IUS dose
Conclusions
Doses to the IUS and EUS on MRI/CT predicted worse urinary function, with greater bother, irritative symptoms, and urgency. Incorporating MRI-based DVH analysis into the treatment planning process may reduce acute urinary morbidity after brachytherapy.
Keywords: Health-related quality of life, Expanded Prostate cancer Index Composite (EPIC) survey, MRI/CT
INTRODUCTION
The American Cancer Society estimates that in 2010, 217,730 men will be diagnosed with prostate cancer in the United States [1]. Men with early-stage, low-grade disease can live for years with or without treatment [2, 3]. Typical treatment options in such cases include active surveillance, radical prostatectomy, and definitive radiation therapy with either external-beam radiation therapy or brachytherapy. Biochemical progression-free survival rates have been shown to be equivalent for patients treated with surgery or radiation [4-6]. However, the side-effect profile for each modality is different and hence the morbidity patterns are different as well [7]. The incidence, types, and severity of acute urinary morbidity after prostate brachytherapy are well documented, but the causes remain a topic of debate [4-13].
Currently, no definitive consensus has been reached about which factors have the greatest influence on urinary morbidity after prostate brachytherapy. Some studies suggest that clinical factors such as larger prostate volume or poorer baseline International Prostate Symptom Score (IPSS) predict worse acute urinary morbidity, whereas others suggest that dosimetric factors such as dose to a urethral segment can predict acute urinary morbidity [7-13]. Magnetic resonance imaging (MRI) provides superior soft tissue delineation of the urinary structures compared with computed tomography (CT), ultrasound, and fluoroscopy (10). Improved visualization of the anatomy and robust evaluation of patient quality-of-life patterns may help identify factors associated with lesser—or greater—urinary morbidity. Here we used MRI-based dose-volume histogram (DVH) analysis to compare DVH variables for the internal urinary sphincter (IUS) and the external urinary sphincter (EUS) with prospectively collected data on urinary quality-of-life outcomes.
METHODS AND MATERIALS
Patients and treatment design and delivery
The study group consisted of 42 consecutive men with intermediate-risk localized prostate cancer treated with prostate brachytherapy as monotherapy. All patients were treated with iodine-125 (125I) seeds at The University of Texas MD Anderson Cancer Center in an institutional review board–approved prospective phase II trial. All patients underwent disease staging and then treatment simulation with rectal ultrasound. The prostate volume and dimensions were calculated and used for treatment planning. The implantations were done by one physician (SJF) to a prescribed dose of 145 Gy to the planning target volume (PTV). The standard dosimetric variables for each implant were prostate V100 >95%, V150 <60%, and V200 <20%, urethral U200=0, and rectal R100 <1 cm3. Pelvic CT and MRI scans were obtained for all patients at day 0 and on day 30 after implantation. Patients returned for follow-up at 1 month, 4 months, 8 months, and 12 months after implantation and completed an Expanded Prostate Index Composite (EPIC) quality-of-life survey at each follow-up visit.
Structure contouring
For optimal visualization of the prostate and surrounding structures, we used axial T2-weighted 3T pelvic MRI. The prostate, penile bulb, bladder, IUS, EUS, and urethra were identified and contoured by using Variseed brachytherapy software version 7.2 (Varian Medical Systems, Inc., Palo Alto, CA). The MRI-based prostate lengths and volumes were compared with the corresponding pre-implant ultrasound contours, and any discrepancies were reviewed and contours modified as appropriate based on the MR images; any remaining differences were assumed to be overestimates on ultrasound given the better visualization by MRI. The penile bulb was identified by the area of increased signal inferior to the prostate and contoured on all slices. The bladder was identified and contoured from the superior aspect through the bladder neck. The bladder neck and IUS were distinguished according to McLaughlin et al. [10]. The IUS was contoured from the superior aspect, set as 1 cm superior to the prostate base, to the inferior aspect, set at the verumontanum. The inferior aspect of the EUS was defined from the first image on which a muscular ring was visible around the urethra, and contours were continued superiorly into the prostate to the verumontanum. When nearing the verumontanum, the diameters of the IUS and EUS closely approximated the diameter of the urethra. The verumontanum was localized on axial, sagittal, and coronal views. The urethra was identified on axial views, and localization improved with the sagittal and coronal views.
MRI-CT fusion process
Thirty days after the implantation procedure, all patients were evaluated by pelvic CT and MRI. To minimize anatomic variations, we obtained images with both modalities on the same day, within 4 hours of each other. No significant differences in bladder and rectal filling were noted between the two modalities. All CT scans were obtained with a helical 16-slice Lightspeed CT scanner, and MR images with a 3-T Signa Excite MRI scanner (both from GE Medical Systems, Milwaukee, WI). Axial CT and MR images were obtained while the patient was supine. The CT and MRI matrix size was 512x512; and the axial pixel size was approximately 1 mm for CT and 0.352 mm for MRI. A torso phased array coil was used for MRI in all cases. Multiplanar T1- and T2-weighted MR images were obtained with and without gadolinium contrast.
CT and axial T2 MR images were electronically fused by using a manual technique with the same Variseed software as that used for contouring the structures of interest. Bony and soft tissue landmarks were used for approximate alignment and then precise seed-to-seed matches were performed. Although extraprostatic seeds can be difficult to visualize on MRI, those within the prostate show up quite clearly as signal voids, which were matched to the readily visible seed images on the CT scan slices. For all patients, the contouring on MR images was done by one physician (SPR) and reviewed by another (SJF). Soft tissue contours were obtained exclusively from the MR images, and the CT scans provided information for seed localization. Coronal and sagittal MR images were used to verify the prostate apex. After doses had been computed, DVHs were exported from Variseed for statistical analysis.
The EPIC survey and follow-up
Health-related quality-of-life (HRQOL) indicators are essential tools for evaluating outcomes after prostate brachytherapy. We used the Expanded Prostate cancer Index Composite (EPIC) survey to assess HRQOL in this study. Of the four summary domains of the EPIC (urinary, bowel, sexual, and hormonal), we focused on the urinary summary domain, which includes subscales of incontinence, irritative/obstructive symptoms, function, and bother. Each subscale comprises 4-7 questions, with some overlap between questions. All of the men in this study were given alpha blockers before the implant, and these drugs were discontinued when the men's urinary function returned to baseline. All men were required to have documented follow-up of at least 4 months after the implant to allow comparison of EPIC scores at three times (baseline and at 1 and 4 months after the implant). Urinary morbidity was evaluated in terms of relative changes in scores instead of absolute scores. The EPIC scores for each of the four urinary subscales, plus urinary urgency, were then correlated with the DVH findings for the IUS and EUS.
Statistical analysis
Data were analyzed by using SAS/STAT software, Version 9 of the SAS System for Windows. Spearman's rank correlation coefficient (rho [ρ]) was used to investigate potential relationships between variables. P values of 0.05 were considered to indicate statistical significance.
RESULTS
Patient characteristics
A total of 42 consecutive patients with localized intermediate-risk prostate cancer (mean age, 66.3 years) were treated with 125I brachytherapy as monotherapy at MD Anderson Cancer Center. More than 90% of patients had clinical T1c disease and 95% had a Gleason score of 7. The mean prostate-specific antigen level was 7.5 ng/mL.
EPIC scores
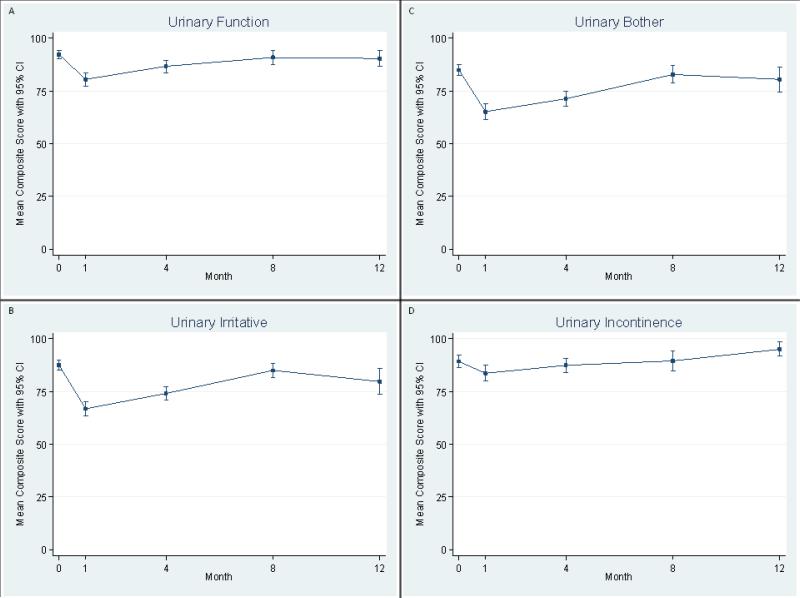
Acute urinary morbidity peaked near 1 month and then improved towards baseline levels over the following months. Scores for all four urinary subscales and urinary urgency followed the same pattern (Figure 1). EPIC scores for bother and irritation showed the largest decrease indicating worsening symptoms (Table 1). Urinary incontinence scores had declined slightly relative to baseline at 1 month but generally remained near baseline throughout the study. Post-implants urinary symptoms correlated with baseline urinary function (p=0.01), urinary incontinence (p=0.02), and urinary urgency (p=0.02), but not urinary bother (0.06) or irritative symptoms (p=0.29).
Fig. 1.
Mean scores on the Expanded Prostate cancer Index Composite (EPIC) survey for urinary morbidity at baseline, 1 month, 4 months, 8 months, and 12 months after an 125I implant for prostate brachytherapy. (A) urinary function, (B) urinary irritative/obstructive symptoms, (C) urinary bother, (D) urinary incontinence. Bars show 95% confidence intervals (CIs).
Table 1.
EPIC Urinary Domain Scores
| Measurement Time (months) | Overall Urinary Morbidity | Urinary Function | Urinary Bother | Urinary Irritation | Urinary Incontinence |
|---|---|---|---|---|---|
| Baseline | 92.4 | 97.5 | 88.8 | 90.6 | 96.6 |
| 1 | 70.0 | 79.3 | 63.4 | 62.2 | 86.4 |
| 4 | 74.7 | 82.9 | 68.7 | 71.1 | 83.7 |
| 8 | 87.4 | 93.6 | 83 | 85.3 | 92.5 |
| 12 | 85.6 | 91.5 | 81.4 | 82.1 | 94.3 |
Spearman coefficients
Relationships between changes in EPIC scores for urinary function, incontinence, bother, irritation, and urgency and the radiation dose to the IUS and EUS are illustrated in Figure 2. For the IUS, we found that urinary urgency, bother, and irritation showed the largest negative correlation at doses <50 Gy. On the other hand, urinary function and incontinence had the largest negative correlation at doses approaching 300 Gy. For the EUS, we found the largest negative correlations for urinary bother, function, and irritation at doses >240 Gy. The largest negative correlation for urgency was <100 Gy, and we found no correlations with incontinence.
Fig. 2.
Spearman correlation coefficients between each urinary morbidity measure and dose to the (A) internal urinary sphincter and (B) external urinary sphincter. The horizontal red line demarcates a coefficient of 0. Points above the line indicate a positive correlation, and points below the line indicate a negative correlation. The graph labeled “urinary” is overall acute urinary morbidity.
Dose–urinary morbidity correlations
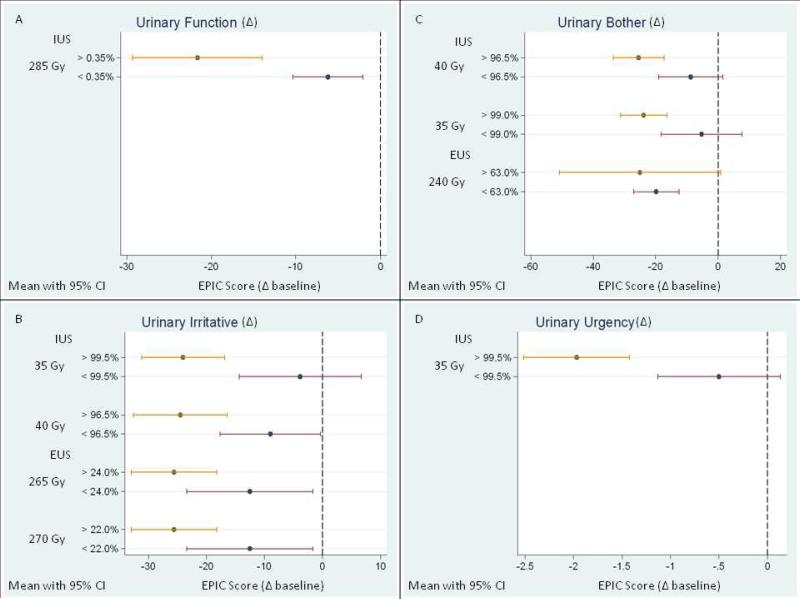
Most subscales showed negative correlations between dose and urinary morbidity except for urinary incontinence and urgency (Figure 3). Urinary function correlated with the IUS V285 (ρ=–0.32, p=0.04). When IUS V285 exceeded 0.4%, the EPIC score for urinary function decreased by more than 20 points; when less than 0.4% of the IUS volume had received that dose, the score decreased by less than 10 points. Urinary bother also correlated both IUS and EUS dose. The IUS V35 and V40 both correlated with bother (ρ=–0.31 for both and p =0.05 and 0.04, respectively). When the V35 was greater than 99%, the bother score decreased by almost 25 points; when less than 99% of the IUS received that dose, the bother score decreased by almost 5 points from baseline. The EUS dose also correlated with EUS V240 (ρ=–0.31, p=0.05). When the EUS V240 was greater than 63%, the bother score decreased by almost 25 points; conversely, when the EUS V240 was less than 63%, the bother score decreased by less than 20 points.
Fig. 3.
Correlations between the dose to the internal and external urinary sphincters and (A) urinary function, (B) urinary irritative/obstructive symptoms, (C) urinary bother, and (D) urinary urgency. Percentages indicate the portion of sphincter volume receiving the specified dose. The baseline EPIC score is referenced to 0 and identified as the vertical dashed line. The change in EPIC score is referenced from baseline. IUS, internal urinary sphincter; EUS, external urinary sphincter; CI, confidence interval.
Urinary incontinence seemed to correlate with the dose to the IUS, but these apparent correlations were not statistically significant (for example, IUS ρ=–0.27, p==0.08). Dose to the EUS did not correlate with incontinence. Dose to the IUS correlated with urinary irritative/obstructive symptoms (IUS V35, ρ=–0.37, p=0.02; IUS V40, ρ=–0.31, p=0.04). When the IUS V35 was greater than 99.5%, the irritative score decreased by almost 25 points; conversely, when the IUS V35 was less than 99.5%, the irritative score decreased by approximately 5 points from baseline. The dose to the EUS also correlated with irritation, with correlation coefficients ranging from –0.30 to –0.32 and p values of 0.05 or 0.04, respectively. When the EUS V265 was greater than 24%, the irritative score decreased by almost 25 points, but when it was less than 24%, the irritative score decreased by slightly more than 10 points. Finally, urinary urgency correlated with dose to the IUS (IUS V35, ρ=–0.38, p=0.02). When at least 99.5% of the IUS received 35 Gy, the symptom score decreased by almost 2 points (Figure 3). However, when less than 99.5% of the IUS received 35 Gy, the symptom score decreased by approximately 0.5 points. An MRI/CT 3-D reconstruction of representative contours and the aspects of urinary morbidity significantly associated with each sphincter are shown in Figure 4.
Fig. 4.
A 3-dimensional reconstruction from fused CT/MRI illustrating a lateral view of the prostate and other organs at risk, including both urinary sphincters. Inferior is to the right and superior to the left. The urinary morbidity measures significantly associated with the dose to each sphincter are listed below the sphincter label. SV, seminal vesicles.
DISCUSSION
To our knowledge, this is the first study to identify an MRI-based dose response between the internal and external sphincters and urinary morbidity, estimated with a validated patient-reported quality-of-life questionnaire, after prostate brachytherapy. Our results suggest that heterogeneous doses to both the IUS and EUS that deviate from preplanning dosimetric constraints predict worse urinary function and increased urinary bother, irritative symptoms, and urgency.
The incidence and types of urinary morbidity associated with prostate brachytherapy have been well documented [14, 15], but the clinical and dosimetric factors influencing urinary morbidity remain debatable [7-13]. Our findings are consistent with several studies that suggest urinary morbidity is influenced by dosimetric factors such as the radiation dose to various segments of the urethra and bladder neck [7, 9, 10, 13]. Crook et al. identified the bladder neck, prostatic urethra, and membranous urethra as critical organs of concern with regard to urinary morbidity [9]. Their recommendations included evaluating the urethral D5, D30, and V150 for adequate dosimetric review, but specific goals for these constraints have not been clearly defined. Parsons et al. studied the patterns of urinary morbidity among men who underwent standard treatment options for localized prostate cancer [7]. They found patients treated with radiotherapy to have a notably lower rate of urinary incontinence, but a higher incidence of urinary bother and irritative symptoms. They also commented that urinary bother and irritative symptoms tend to improve with time, but urinary incontinence associated with radiotherapy, if it develops, tends to worsen. Overall, that group found that poor long-term outcomes were uncommon after radiotherapy, but acute urinary morbidity was relatively common. Pinkawa et al. studied HRQOL in men after prostate brachytherapy and recommended keeping the D10 to the base of seminal vesicles at <190 Gy to achieve satisfactory urinary function [13], having found that doses higher than this threshold led to higher rates of pad usage (28% vs. 8%), and perhaps worse urinary bother score (58 vs. 71) and urinary function score (72 vs. 82) during the acute period. Overall, both urinary function and urinary bother scores dropped precipitously during the acute period compared with baseline values (function 92 vs. 77; bother 82 vs. 65). McLaughlin et al. studied visualization of the prostate using MRI and the potential implications for treatment planning [10]. They concluded that optimal visualization of the urinary structures is necessary to improve the study of dose and toxicity relationships, to limit dose to critical structures appropriately, and to improve quality of life after therapy [10].
Contrary to the studies cited above, several others have suggested that clinical factors have more influence on urinary morbidity than do urethral dosimetric factors and therefore that regional urethral dosimetry yields little clinical benefit [8, 11, 12]. One study by Allen et al. looked at the ability of regional urethral dosimetry to predict urinary morbidity [8]. They found in multivariate analyses that the maximum dose received by the prostate apex and genitourinary diaphragm predicted the greatest increase in IPSS [8]. Overall, they concluded that with tight adherence to urethral-sparing approaches, detailed urethral dosimetry was of no clinical benefit [8]. In another study, Merrick et al. analyzed both dosimetric and clinical factors with regard to urinary morbidity [11] and found that the IPSS before the implant and the prostate D90 were the only factors that predicted the worst dysuria score. However, they also found a significant difference in the percentage of patients with resolution of IPSS according to minimum peripheral dose to the urethra (66% for <150% and 42.6% for >150%) [11]. Neill et al. evaluated doses to 3 segments of the prostate in terms of urinary morbidity [12] and found no relationship between peak or average dose of each urethral segment with irritative or obstructive symptoms, total IPSS, or time to IPSS resolution [12]. However, they did identify several clinical factors, including edema, baseline IPSS, and pretreatment prostate volume, that did correlate with some of these urinary morbidity measures [12]. The current study differs from these three studies in distinct ways that may explain the differences in our results. First, we identified and contoured all of the critical urinary structures on MR images, which allowed better visualization than standard CT images. Second, we focused specifically on the urinary sphincters. Finally, we used the EPIC survey, a more robust quality of life assessment tool than the IPSS, to measure urinary morbidity.
Our study did have several limitations. The MRI/CT fusion process has inherent errors due to anatomic variations of the pelvic anatomy between studies. Moreover, the MRI/CT seed-to-seed match process is limited by the close approximation of seed locations, especially on MR images, where the seed is identified as a signal void. We have begun investigating how to improve MRI techniques for seed localization [16, 17]. An additional limitation is the limited number of patients in this study with significant patient-to-patient variability in the post-implant symptomatology. However, our results provide a meaningful framework to advance the understanding of the relationship of sphincter dose to quality of life, and a multivariate analysis at the conclusion of this 300 patient prospective study will provide a more comprehensive understanding of urinary symptoms by implanted isotopes (i.e. Iodine-125, Paladium-103, and Cesium-131).
A potential strength of our study is that incorporating MRI for better visualization during the planning and evaluation process could improve dosimetric evaluation of an implant, and over time this could help physicians to make necessary modifications to their technique or approach for individual patients to decrease the risk of urinary morbidity. One avenue that should also be explored further is modifications of implant technique to achieve new dosimetric parameters through a combination of different needle insertion approaches and radioactive seed placement distributions.
In conclusion, control of dose heterogeneity through appropriate placement of radioactive seeds during the implant is important to limit the dose to the EUS to 240 Gy. Controlling heterogeneity within the implant may alleviate declines in quality of life associated with urinary bother, irritative symptoms, and function. As for the IUS, doses as low as 35 Gy were found to result in increased urinary bother and urgency. Therefore, in addition to providing optimal coverage of the base of the prostate gland, clinicians should be prepared to appropriately manage common urinary symptoms from radiation exposure to the IUS during the acute period of at baseline and at each follow-up visit after prostate brachytherapy
Acknowledgments
Christine Wogan for substantive editing of the manuscript.
Financial support: This work was supported in part by NIH Cancer Center Support Grant CA16672.
Glossary
- IUS
Internal urinary sphincter
- EUS
External urinary sphincter
- MRI
Magnetic resonance imaging
- DVH
Dose volume histogram
- EPIC
Expanded Prostate Cancer Index Composite
- CT
Computed tomography
- PTV
Planning target volume
- HRQOL
Health related quality of life
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification:
No conflicts to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong YN, Mitra N, Hudes G, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA. 2006;296:2683–2693. doi: 10.1001/jama.296.22.2683. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 5.Eade TN, Horwitz EM, Ruth K, et al. A comparison of acute and chronic toxicity for men with low-risk prostate cancer treated with intensity-modulated radiation therapy or (125)I permanent implant. Int J Radiat Oncol Biol Phys. 2008;71:338–345. doi: 10.1016/j.ijrobp.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupelian PA, Potters L, Khuntia D, et al. Radical prostatectomy, external beam radiotherapy <72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int J Radiat Oncol Biol Phys. 2004;58:25–33. doi: 10.1016/s0360-3016(03)00784-3. [DOI] [PubMed] [Google Scholar]
- 7.Parsons BA, Evans S, Wright MP. Prostate cancer and urinary incontinence. Maturitas. 2009;63:323–328. doi: 10.1016/j.maturitas.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Allen ZA, Merrick GS, Butler WM, et al. Detailed urethral dosimetry in the evaluation of prostate brachytherapy-related urinary morbidity. Int J Radiat Oncol Biol Phys. 2005;62:981–987. doi: 10.1016/j.ijrobp.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 9.Crook JM, Potters L, Stock RG, Zelefsky MJ. Critical organ dosimetry in permanent seed prostate brachytherapy: defining the organs at risk. Brachytherapy. 2005;4:186–194. doi: 10.1016/j.brachy.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin PW, Troyer S, Berri S, et al. Functional anatomy of the prostate: implications for treatment planning. Int J Radiat Oncol Biol Phys. 2005;63:479–491. doi: 10.1016/j.ijrobp.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 11.Merrick GS, Butler WM, Wallner KE, et al. The impact of radiation dose to the urethra on brachytherapy-related dysuria. Brachytherapy. 2005;4:45–50. doi: 10.1016/j.brachy.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Neill M, Studer G, Le L, et al. The nature and extent of urinary morbidity in relation to prostate brachytherapy urethral dosimetry. Brachytherapy. 2007;6:173–179. doi: 10.1016/j.brachy.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Pinkawa M, Fischedick K, Piroth MD, et al. Health-related quality of life after permanent interstitial brachytherapy for prostate cancer: correlation with postimplant CT scan parameters. Strahlenther Onkol. 2006;182:660–665. doi: 10.1007/s00066-006-1530-z. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JF, Swanson DA, Levy LB, et al. Urinary side effects and complications after permanent prostate brachytherapy: the MD Anderson Cancer Center experience. Urology. 2009;74:601–605. doi: 10.1016/j.urology.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 15.Pardo Y, Guedea F, Aguilo F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 28:4687–4696. doi: 10.1200/JCO.2009.25.3245. [DOI] [PubMed] [Google Scholar]
- 16.Frank SJ, Stafford RJ, Bankson JA, et al. A novel MRI marker for prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2008;71:5–8. doi: 10.1016/j.ijrobp.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Frank SJ, Tailor RC, Kudchadker RJ, et al. Anisotropy characterization of I-125 seed with attached encapsulated cobalt chloride complex contrast agent markers for MRI-based prostate brachytherapy. Med Dosim. 2011;36(2):200–205. doi: 10.1016/j.meddos.2010.03.004. [DOI] [PubMed] [Google Scholar]