Abstract
Incubation of rat calvaria for short times in the presence of a labeled amino acid revealed the existence of a collagen fraction (procollagen) that functions as a biosynthetic precursor of collagen. Procollagen contains an α1-like chain (pre-α1) that elutes earlier from CM-cellulose than does rat-bone α1 and has a molecular weight, estimated by acrylamide gel electrophoresis, of 120,000. A time-dependent conversion of pre-α1 to α1 was demonstrated by incubation of calvaria for periods varying from 9 to 60 min and by a pulse-chase experiment. Limited cleavage of procollagen with pepsin resulted in a molecule with a chain resembling α1 in chromatographic properties, molecular weight, and relative hydroxyproline and proline contents. Thus, conversion of procollagen to collagen is likely to occur in vivo by a proteolytic mechanism. The additional peptide sequences in procollagen may serve to initiate chain association in triple-helix formation, to facilitate molecular transport, and to inhibit intracellular fibrogenesis.
Keywords: zymogen, pepsin digestion, chromatography, gel electrophoresis, isotopic labeling
Full text
PDF
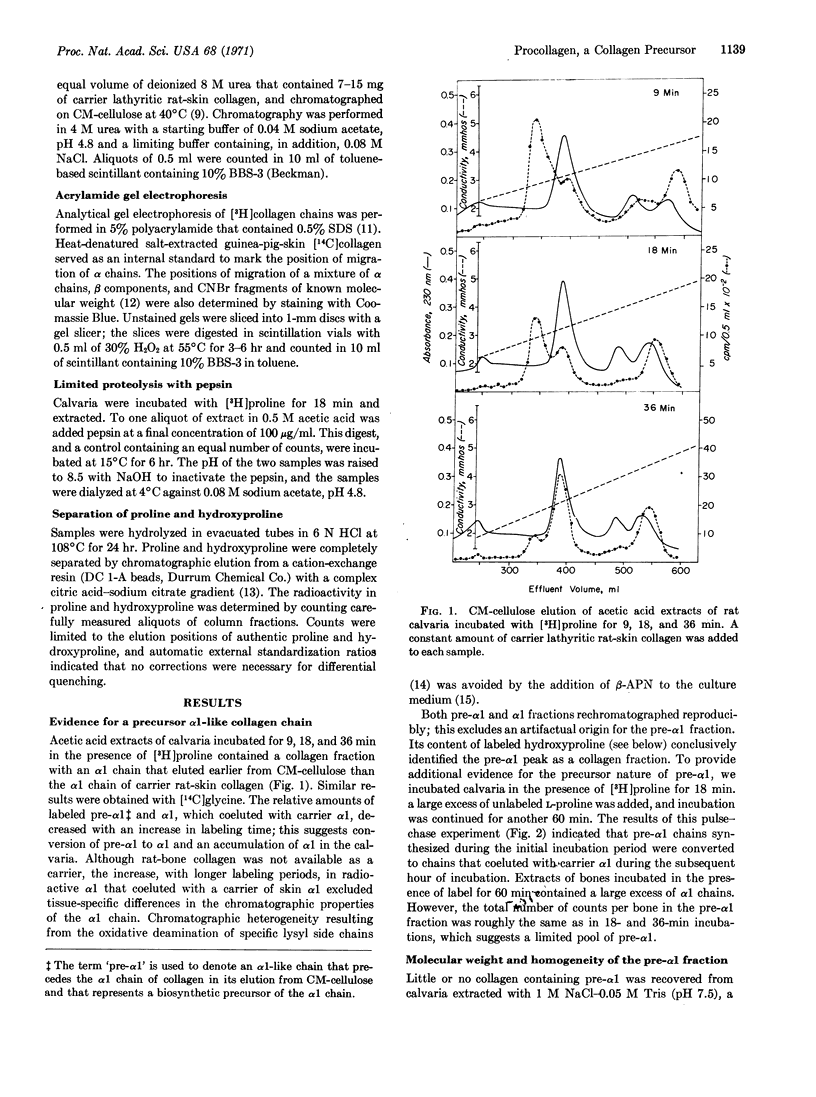
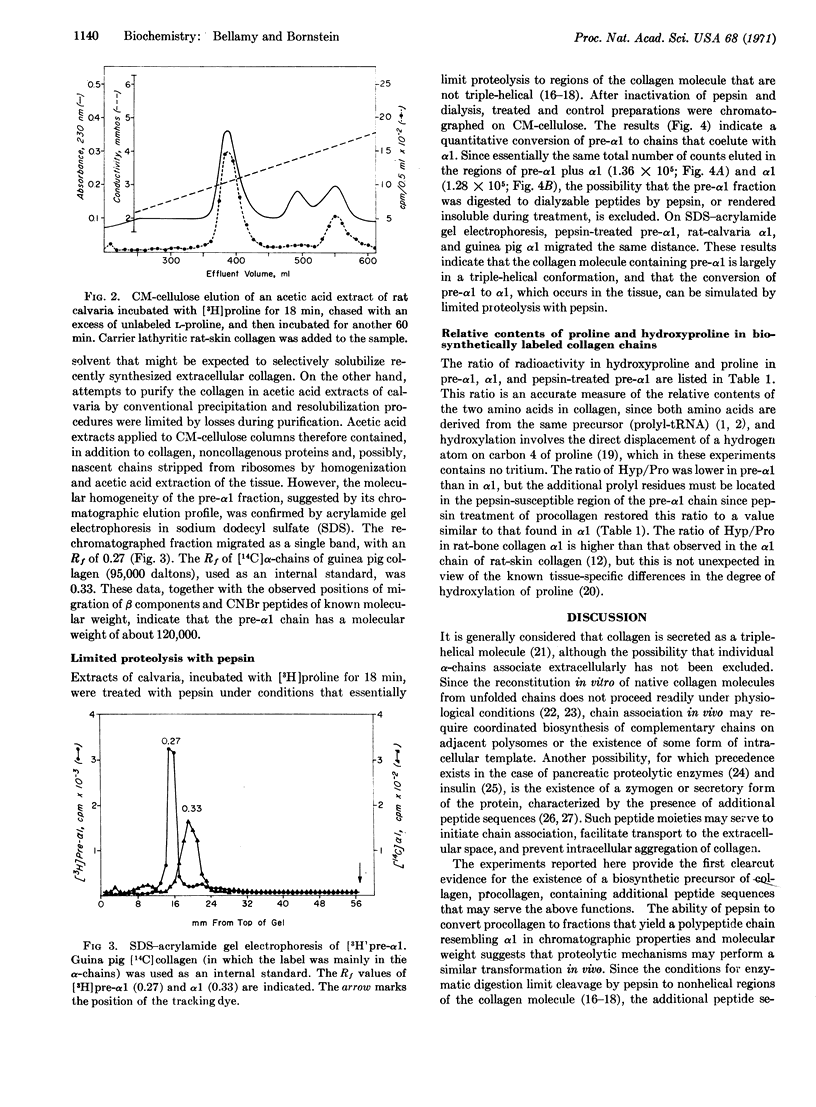
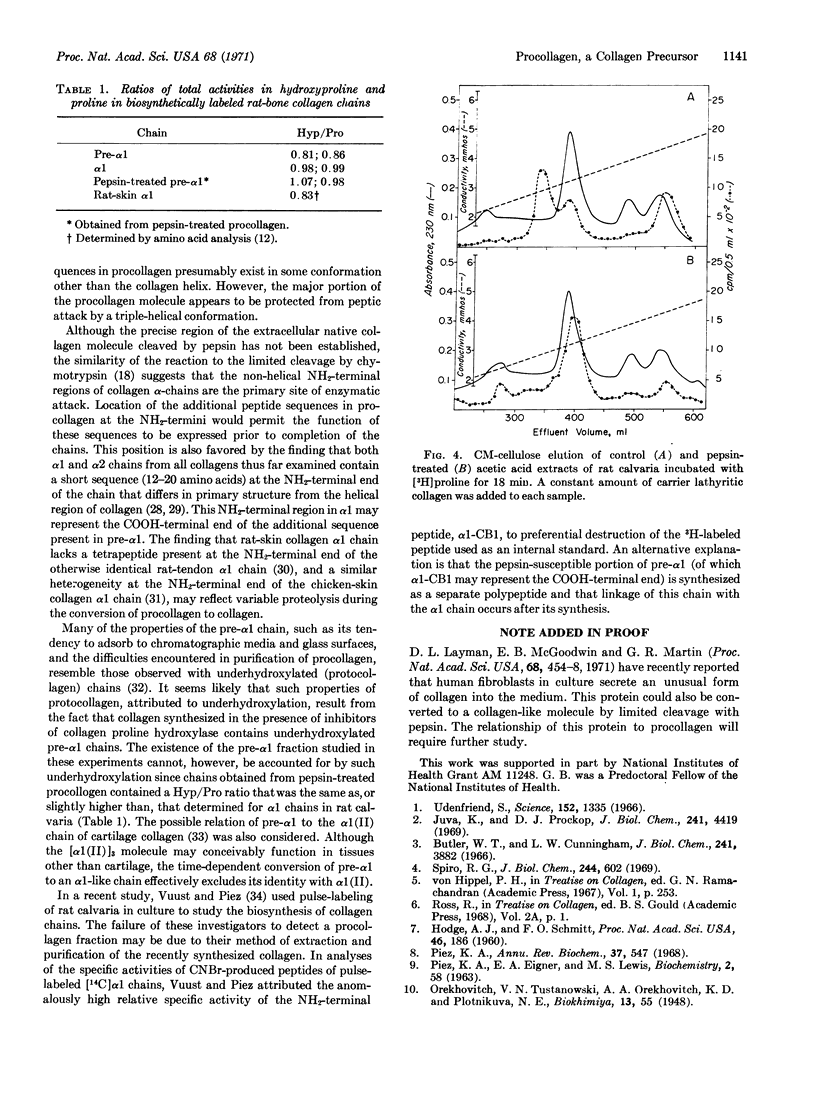

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier G., Engel J. The renaturation of soluble collagen. Products formed at different temperatures. Biochemistry. 1966 Aug;5(8):2744–2755. doi: 10.1021/bi00872a035. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Comparative sequence studies of rat skin and tendon collagen. I. Evidence for incomplete hydroxylation of individual prolyl residues in the normal proteins. Biochemistry. 1967 Oct;6(10):3082–3093. doi: 10.1021/bi00862a015. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Comparative sequence studies of rat skin and tendon collagen. II. The absence of a short sequence at the amino terminus of the skin alpha-1 chain. Biochemistry. 1969 Jan;8(1):63–71. doi: 10.1021/bi00829a010. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Cunningham L. W. Evidence for the linkage of a disaccharide to hydroxylysine in tropocollagen. J Biol Chem. 1966 Sep 10;241(17):3882–3888. [PubMed] [Google Scholar]
- Butler W. T., Piez K. A., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha-1 chain of rat skin collagen. Biochemistry. 1967 Dec;6(12):3771–3780. doi: 10.1021/bi00864a022. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Rao N. V. Collagen structure in solution. I. Kinetics of helix regeneration in single-chain gelatins. Biochemistry. 1970 Sep 15;9(19):3714–3724. doi: 10.1021/bi00821a010. [DOI] [PubMed] [Google Scholar]
- Hodge A. J., Schmitt F. O. THE CHARGE PROFILE OF THE TROPOCOLLAGEN MACROMOLECULE AND THE PACKING ARRANGEMENT IN NATIVE-TYPE COLLAGEN FIBRILS. Proc Natl Acad Sci U S A. 1960 Feb;46(2):186–197. doi: 10.1073/pnas.46.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. An effect of puromycin on the synthesis of collagen by embryonic cartilage in vitro. J Biol Chem. 1966 Oct 10;241(19):4419–4425. [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the alpha-chains of chick skin collagen and the nature of the NH2-terminal cross-link region. Biochemistry. 1969 Sep;8(9):3648–3655. doi: 10.1021/bi00837a023. [DOI] [PubMed] [Google Scholar]
- Layman D. L., McGoodwin E. B., Martin G. R. The nature of the collagen synthesized by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1971 Feb;68(2):454–458. doi: 10.1073/pnas.68.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens L. N. The formation of collagen alpha chains in the absence of proline hydroxylation. J Biol Chem. 1970 Feb 10;245(3):453–461. [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Piez K. A. An accelerated single-column procedure for the automatic analysis of amino acids in collagen and elastin hydrolyzates. Anal Biochem. 1966 Aug;16(2):320–326. doi: 10.1016/0003-2697(66)90161-8. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A., Winter W. P. Evolution of structure and function of proteases. Science. 1967 Dec 29;158(3809):1638–1644. doi: 10.1126/science.158.3809.1638. [DOI] [PubMed] [Google Scholar]
- Piez K. A., Bladen H. A., Lane J. M., Miller E. J., Bornstein P., Butler W. T., Kang A. H. Comparative studies on the chemistry of collagen utilizing cyanogen bromide cleavage. Brookhaven Symp Biol. 1968 Jun;21(2):345–357. [PubMed] [Google Scholar]
- Piez K. A. Cross-linking of collagen and elastin. Annu Rev Biochem. 1968;37:547–570. doi: 10.1146/annurev.bi.37.070168.002555. [DOI] [PubMed] [Google Scholar]
- SCHMITT F. O. Contributions of molecular biology to medicine. Bull N Y Acad Med. 1960 Nov;36:725–749. [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C., Pinnell S. R., Martin G. R. Cross-linking of collagen and elastin. Properties of lysyl oxidase. Biochemistry. 1970 Nov 10;9(23):4486–4492. doi: 10.1021/bi00825a004. [DOI] [PubMed] [Google Scholar]
- Speakman P. T. Proposed mechanism for the biological assembly of collagen triple helix. Nature. 1971 Jan 22;229(5282):241–243. doi: 10.1038/229241a0. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J Biol Chem. 1969 Feb 25;244(4):602–612. [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Udenfriend S. Formation of hydroxyproline in collagen. Science. 1966 Jun 3;152(3727):1335–1340. doi: 10.1126/science.152.3727.1335. [DOI] [PubMed] [Google Scholar]
- Vuust J., Piez K. A. Biosynthesis of the alpha chains of collagen studied by pulse-labeling in culture. J Biol Chem. 1970 Nov 25;245(22):6201–6207. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



