Abstract
Background
We sought to test whether patients with apical hypertrophic cardiomyopathy (APH) have different clinical features compared to those with typical asymmetric septal hypertrophy (ASH).
Methods
Among 32,534 patients who underwent routine echocardiography at Asan Medical Center from January 2000 to December 2001, 305 patients (0.9%), who were finally diagnosed with hypertrophic cardiomyopathy (HCMP), were evaluated. The type of HCMP was classified according to the echocardiographic findings.
Results
ASH was the most frequent type (n=160, 53%, group I), and APH was the second most frequent (n=91, 30%, group II). Mean age (60.8±10 vs. 48.2±14 years, p<0.001) and prevalence of hypertension (32% vs. 19%, p=0.022) were significantly higher in group II than in group I. Family history of HCMP (4.4% vs. 0% p=0.043) and sudden cardiac death (8.8% vs. 1.1% p=0.014) was more prevalent in group I. During the follow-up period of 32.0± 37.2 months, cardiac events occurred at a significantly higher rate in group I (25.5% vs. 8.8%, p=0.003).
Conclusion
APH comprises a significant proportion of HCMP in Korea and patients with APH show different clinical features compared to those with ASH.
Keywords: Hypertrophic cardiomyopathy, Prognosis, Echocardiography
INTRODUCTION
Hypertrophic cardiomyopathy (HCMP) is a complex, primary, and inherited cardiac muscle disorder characterized by a wide spectrum of morphological manifestations, natural history, and prognosis1-3). Apical hypertrophic cardiomyopathy (APH) is a relatively rare form of HCMP, which was initially described by Japanese investigators in 1976 as a form of non-obstructive hypertrophy predominantly involving the apex of the left ventricle, associated with giant precordial negative T-waves and a typical ace-of-spades configuration of the left ventriculogram4,5). APH is reported to be relatively common in the Asian population6-8) and racial difference in morphological and clinical features of HCMP remains an interesting subject. This study was undertaken to investigate the clinical profiles of HCM in a relatively large Korean population and to test whether patients with APH have different clinical features compared to those with typical asymmetric septal hypertrophy (ASH).
MATERIALS AND METHODS
Patients
Among 32,534 patients who underwent routine echocardiography at Asan Medical Center from January 2000 to December 2001, 305 patients (0.9%) were found to have final diagnoses of HCMP. Medical records, including the echocardiography reports of these 305 patients, were retrospectively reviewed; we included not only newly diagnosed patients, but also those who had already been diagnosed.
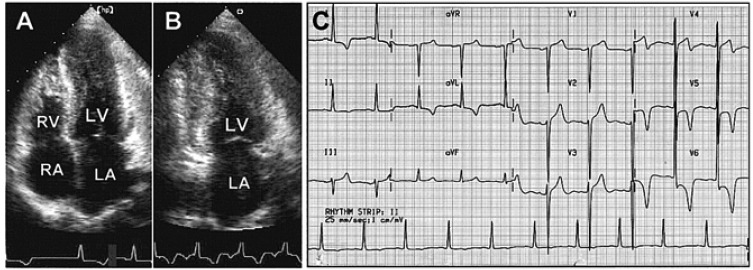
Two-dimensional echocardiograms were obtained using commercially available machines (HP SONOS 2500 & 5500, Andover, United States). Besides routine measurements of the left atrial and ventricular cavity, left ventricular wall thickness at the end of diastole was obtained. According to the site of maximum wall thickness, we classified HCMP into five types: (1) ASH (interventricular septal thickness ≥15 mm and a ratio of interventricular septum to left ventricular posterior wall ≥1.3); (2) APH (apical wall thickness ≥15 mm and a ratio of apical to left ventricular posterior wall ≥1.3, Figure 1A and 1B); (3) mixed type of ASH and APH; (4) mid-ventricular type (hypertrophy confined to the mid-ventricular level with flow acceleration); and (5) diffuse type (hypertrophy involving the entire left ventricle). The initial electrocardiogram at the diagnosis was analyzed for the presence of left ventricular hypertrophy using the Sokolow-Lyon criteria. It was also analyzed for the presence of T-wave inversion. 'Giant' T-wave negativity was defined as a voltage of a negative T-wave ≥1 mV in any of the leads (Figure 1C)9).
Figure 1.
Two-dimensional echocardiograms of apical 4 (A) and 2 (B) chamber view, and 12-lead electrocardiogram (C) in a patient with apical hypertrophic cardiomyopathy and giant negative T-wave.
Clinical follow-up and cardiac events
Late follow-up was obtained by direct telephone contact with patients or by clinic chart review of all patients. Cardiac death was defined as death of cardiovascular origin, such as myocardial infarction, intractable arrhythmia, congestive heart failure, or stroke. Cardiac events included: 1) new onset atrial fibrillation or flutter; 2) sustained ventricular tachycardia or ventricular fibrillation; 3) hospital admission due to chest pain or congestive heart failure; 4) mitral valve replacement due to severe mitral regurgitation; 5) stroke or transient ischemic attack.
Statistics
Data were expressed as mean±SD for continuous variables, and as frequencies for the categorical variables. Differences between APH and ASH were analyzed by the unpaired Student t-test for continuous variables and by the chi-square test for categorical variables. Cardiovascular event-free survival was calculated and compared using the Kaplan-Meier method and log-rank test. A p-value of <0.05 was considered statistically significant.
RESULTS
Types of hypertrophic cardiomyopathy: ASH vs. APH
According to the echocardiographic findings, typical ASH was present in 160 patients (52.5%), APH in 91 patients (29.8%), a mixed type of ASH and APH in 40 patients (13.1%), and mid-ventricular type in 2 patients (0.7%). The other 12 patients showed diffuse type hypertrophy. Comparison of the clinical features between the two major groups (ASH versus APH) is summarized in Table 1. Mean age at diagnosis (60.8±10 vs. 48.2±14 years, p<0.001) and prevalence of past medical history of hypertension (29/91 vs. 31/160, p=0.022) and stroke (11/91 vs. 2/160, p<0.001) were significantly higher in the APH group than in the ASH group. Family history of HCMP (4.4% vs. 0% p=0.043) and sudden cardiac death (8.8% vs. 1.1% p=0.014) were more prevalent in the ASH group. Chest pain and dyspnea were the two most common symptoms at initial presentation in both groups; however, patients with functional class ≥3 at the presentation were more common in the ASH group (17.1% vs.7.9%, p=0.043).
Table 1.
Comparison of baseline characteristics between ASH and APH
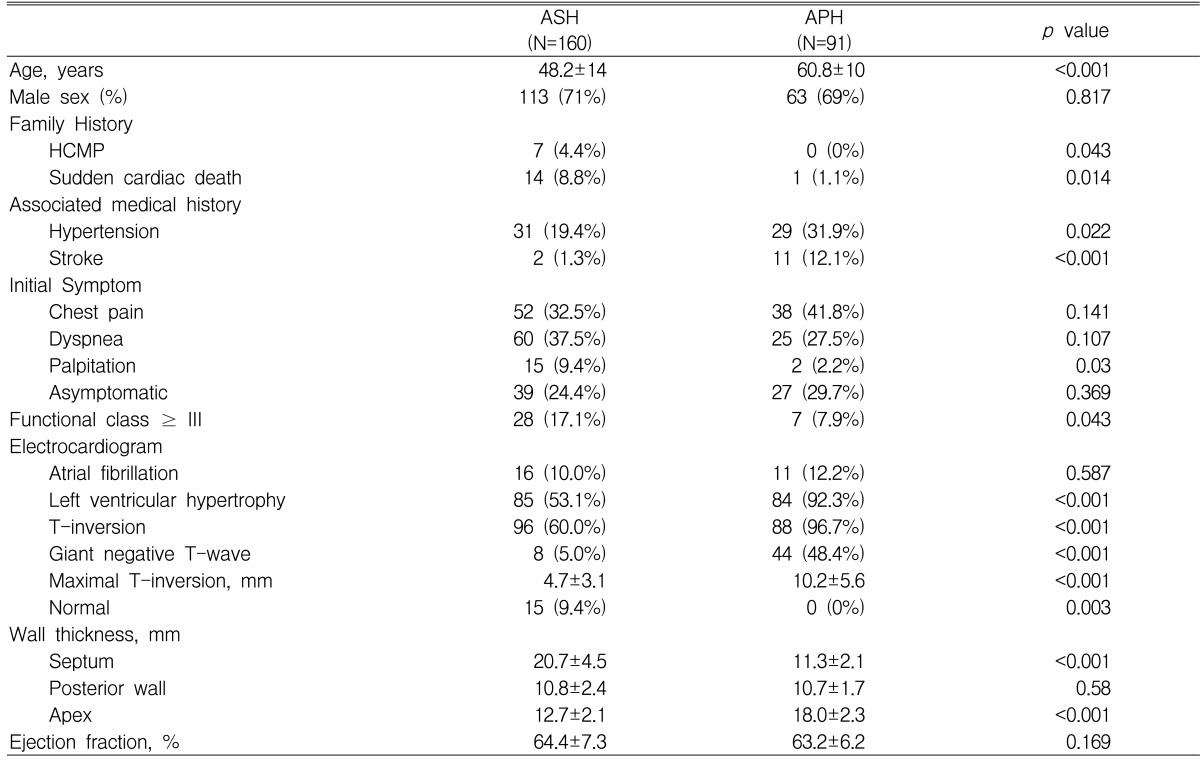
Although the prevalence of atrial fibrillation was the same at presentation (10.0% vs. 12.2%, p=NS), the frequency of electrocardiographic signs of left ventricular hypertrophy (92.3% vs.53.1%, p<0.001), T-wave inversion (96.7% vs.60.0%, p<0.001), and giant negative T-waves (48.4% vs.5.0%, p<0.001) were significantly higher in the APH group than in the ASH group. In the ASH group, 15 patients (9.4%) showed normal electrocardiographic findings.
The mean ratio of septal/posterior wall thickness was 1.96± 0.43 (range 1.30-3.44) in the ASH group and the ratio of apical/posterior wall thickness was 1.72±0.31 (range 1.30-2.75) in the APH group. Systolic anterior motion of the anterior mitral valve with resting left ventricular outflow tract obstruction was noted in 54 of 160 ASH, but none in APH.
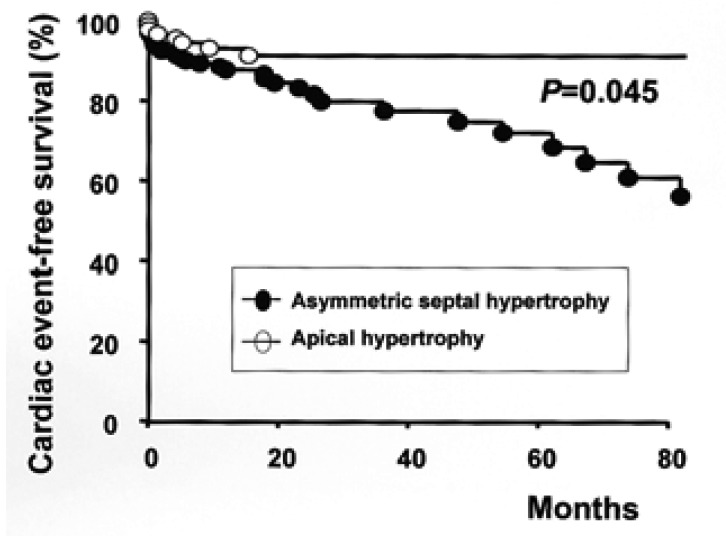
These 251 patients were followed for 32.0±37.2 months. Nonsurgical septal ablation or antiarrhythmic therapy using implantable cardiac defibrillator was exclusively applied in the ASH group and beta-blockers were also more frequently prescribed in the ASH group (63.1% vs. 45.1%, p=0.016). During follow-up, the number of patients with cardiovascular events was higher in the ASH group than in the APH group (25.5% vs. 8.8%, p=0.003, Table 2). The five-year event-free survival rate was 72.0±5.6% and 91.3%±3.2% in the ASH and the APH group, respectively (p=0.045) (Figure 2).
Table 2.
Comparison of cardiovascular-events between ASH and APH
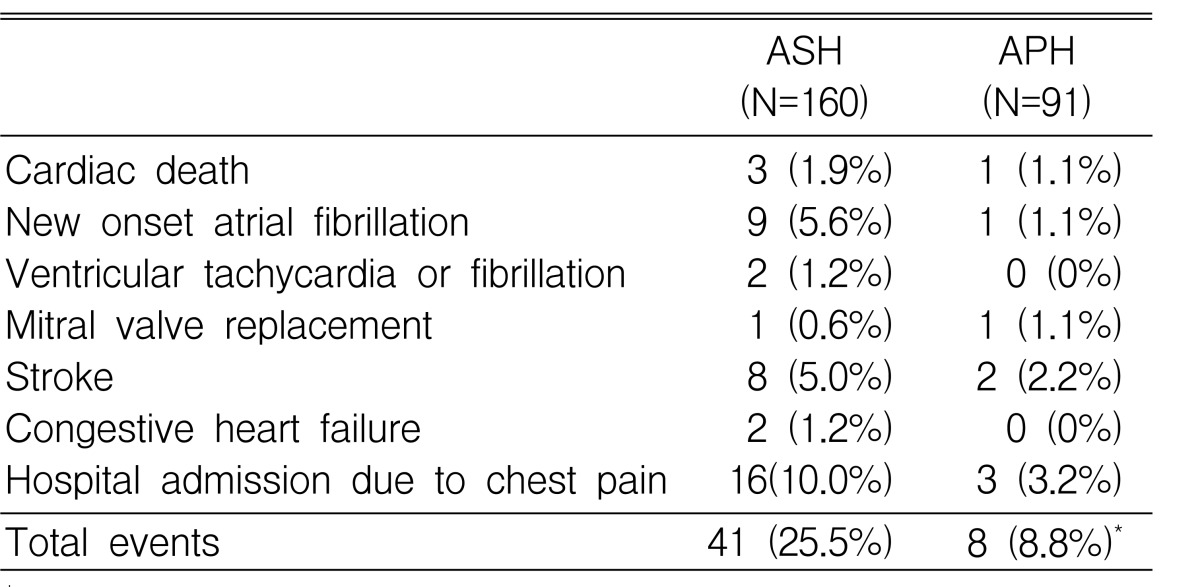
*p<0.05
Figure 2.
Kaplan-Meier cumulative cardiac event-free survival rates of patients with asymmetrical septal hypertrophy and apical hypertrophy
DISCUSSION
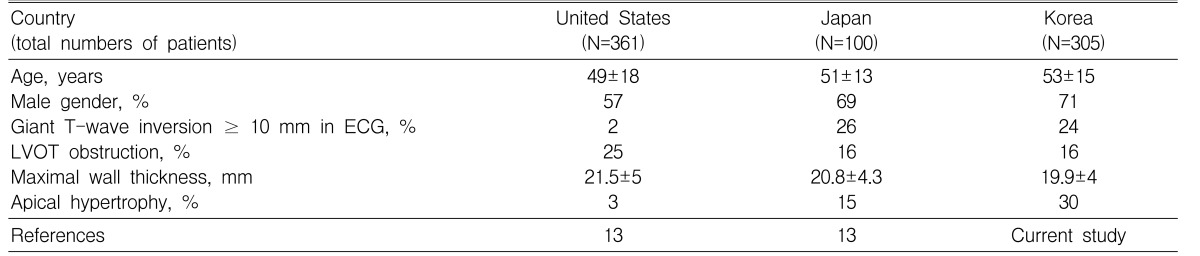
In the present study, we have shown that patients with APH comprise a significant proportion of HCMP in Korea and show different clinical features compared to those with ASH; patients with APH had higher mean age, less frequent family history of sudden cardiac death, higher prevalence of hypertension, and better event-free survival rates than those with typical ASH. In the literature, there are many reports showing marked racial differences in the prevalence of APH; APH is considered to be common in Japan with a prevalence of up to 30%, whereas the prevalence of APH outside of Japan is reported to be about 2% of HCMP in a large series of studies10-12). Recently, Kitaoka et al.13) directly compared cohorts of HCMP from Japan (n=100) and those from the United States (n=361); the mean age of patients with HCMP was not significantly different between the groups (51±13 vs. 49±18 years), but the prevalence of APH was significantly higher in the Japanese cohort than in the American cohort (15% vs 3%, p=0.01). The baseline characteristics of Korean patients with HCMP were quite similar to those of Japanese patients (Table 3); they showed similar mean ages and similar prevalence of male gender, giant T-wave inversion, and resting left ventricular outflow tract obstruction. Further study is necessary to determine whether the higher prevalence of APH is universal in all Asian countries.
Table 3.
Comparison of Baseline Characteristics of Patients with Hypertrophic Cardiomyopathy in Different Ethnic Groups
To date, with the exception of some reports describing the characteristics of APH as a unique subtype of HCMP12,15-18), there has been no direct comparison of APH with typical ASH in such a large scale. The mean age and the prevalence of medical history of hypertension were significantly higher in the APH group, whereas family history of HCMP or sudden cardiac death, and dyspnea of the New York Heart Association functional class ≥3 at presentation were more prevalent in the ASH group. The prevalence of giant negative T-waves was one of the marked features of APH (48% vs. 5% p<0.001); localized apical hypertrophy may lead to a recovery process of lengthened duration between the apex and the other areas, resulting in the genesis of a giant T-wave inversion19). Three independent factors associated with the giant negative T-wave were thickness of apex (OR 1.33, 95% confidence interval of 1.214-1.463), age at diagnosis (OR 1.04, 95% CI 1.012-10.70), and male sex (OR 2.64, 95% CI 1.237-5.652).
The natural history of HCMP has been described in several large studies; reported annual mortality is 2 to 6% in tertiary care centers20,21) or 0.7% in a relatively unselected population base study14,22). In this study, the total annual mortality of patients with HCMP was < 1%. Total cardiovascular events occurred more commonly in patients with ASH than in those with APH, and the cardiac event-free survival rate was better in the APH group. Recently, the long-term outcome of North American patients with APH was investigated and it was concluded that they have a benign prognosis in terms of cardiovascular mortality23). Despite important risk factors of coronary artery disease in our patients with APH, such as old age and high prevalence of hypertension, only a few cardiovascular events occurred, emphasizing the benign course of APH. This finding is likely to influence medical fields to focus on counseling and management of patients with APH.
This study is a retrospective analysis of the database of a single, tertiary referral center with relatively short follow-up duration. Thus, the real prevalence of APH should be reassessed in a large population study. However, considering that patients in a tertiary referral center tend to be characterized by severe symptoms or a malignant family history, we believe that our data support a relatively higher prevalence of APH in Korea compared to other countries. Because of the short follow-up duration, we evaluated cardiovascular events rather than cardiac mortality, and demonstrated better cardiac event-free survival rate in patients with APH. Although most of the cardiac events in the APH group occurred within 18 months of diagnosis, longer follow-up is necessary to confirm the relatively benign clinical course of the APH group.
In conclusion, APH comprises 30% of HCMP in Korea, similar to Japan, and patients with APH show higher mean age, less frequent family history of sudden cardiac death, higher prevalence of hypertension, and better event-free survival rate than those patients with typical ASH. Although we have shown better prognoses for patients with APH compared to those with ASH, the incidence of severe clinical events, such as cardiac death or development of heart failure or stroke, was similar between groups. Moreover, we have failed to demonstrate identification of clinical variables, including electrocardiographic changes, which are useful for predicting the prognosis of these patients. A multi-center longitudinal clinical study is necessary to determine the prognostic factors in patients with APH.
References
- 1.Roberts R, Sigwart U. New concepts in hypertrophic cardiomyopathies, part I. Circulation. 2001;104:2113–2116. doi: 10.1161/hc4201.097429. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ. Hypertrophic cardiomyopathy. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 3.McKenna WJ, Behr ER. Hypertrophic cardiomyopathy: management, risk stratification, and prevention of sudden death. Heart. 2002;87:169–176. doi: 10.1136/heart.87.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto T, Tei C, Murayama M, Ichiyasu H, Hada Y. Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle: echocardiographic and ultrasono-cardiotomographic study. Jpn Heart J. 1976;17:611–629. doi: 10.1536/ihj.17.611. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi H, Ishimura T, Nishiyama S, Nagasaki F, Nakanishi S, Takatsu F, Nishijo T, Umeda T, Machii K. Hypertrophic nonobstructive cardiomyopathy with giant negative T waves (apical hypertrophy): ventriculographic and echocardiographic features in 30 patients. Am J Cardiol. 1979;44:401–412. doi: 10.1016/0002-9149(79)90388-6. [DOI] [PubMed] [Google Scholar]
- 6.Koga Y, Itaya K, Toshima H. Prognosis in hypertrophic cardiomyopathy. Am Heart J. 1984;108:351–359. doi: 10.1016/0002-8703(84)90624-0. [DOI] [PubMed] [Google Scholar]
- 7.Park YB, Lee WS, Kim DK, Choi YS, Seo JD, Lee YW. Clinical and morphological features of hypertrophic cardiomyopathy in Korean patients. J Korean Med Sci. 1989;4:163–169. doi: 10.3346/jkms.1989.4.4.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumori A, Furukawa Y, Hagesawa K, Sato Y, Nakgawa H, Morikawa Y, Miura K, Ohno Y, Tamakoshi A, Inaba Y, Sasayama S. Epidemiologic and clinical characteristics of cardiomyopathies in Japan: results from nationwide surveys. Circ J. 2002;66:323–336. doi: 10.1253/circj.66.323. [DOI] [PubMed] [Google Scholar]
- 9.Alfonso F, Nihoyannopoulos P, Stewart J, Dickie S, Lemery R, Mckenna WJ. Clinical significance of giant negative T waves in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1990;15:965–971. doi: 10.1016/0735-1097(90)90225-e. [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Schiffers A, Klues HG. Comparison of phenotypic expression of hypertrophic cardiomyopathy in patients from the United States and Germany. Am J Cardiol. 1999;83:626–627. doi: 10.1016/s0002-9149(98)00932-1. [DOI] [PubMed] [Google Scholar]
- 11.Chikamori T, Doi YL, Akizawa M, Yonezawa Y, Ozawa T, McKenna WJ. Comparison of clinical, morphological, and prognostic features in hypertrophic cardiomyopathy between Japanese and western patients. Clin Cardiol. 1992;15:833–837. doi: 10.1002/clc.4960151108. [DOI] [PubMed] [Google Scholar]
- 12.Louie EK, Maron BJ. Apical hypertrophic cardiomyopathy: clinical and two-dimensional echocardiographic assessment. Ann Intern Med. 1987;106:663–670. doi: 10.7326/0003-4819-106-5-663. [DOI] [PubMed] [Google Scholar]
- 13.Kitaoka H, Doi Y, Casey SA, Hitomi N, Furuno T, Maron BJ. Comparison of the prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am J Cardiol. 2003;92:1183–1186. doi: 10.1016/j.amjcard.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Cannan CR, Reeder GS, Bailey KR, Melton LJ, 3rd, Gersh BJ. Natural history of hypertrophic cardiomyopathy: a population-based study, 1976 through 1990. Circulation. 1995;92:2488–2495. doi: 10.1161/01.cir.92.9.2488. [DOI] [PubMed] [Google Scholar]
- 15.Keren G, Belhassen B, Sherez J, Miller HI, Megidish R, Berenfeld D, Laniado S. Apical hypertrophic cardiomyopathy: evaluation by noninvasive and invasive techniques in 23 patients. Circulation. 1985;71:45–56. doi: 10.1161/01.cir.71.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Rovelli EG, Parenti F, Devizzi S. Apical hypertrophic cardiomyopathy of "Japanese type" in a Western European person. Am J Cardiol. 1986;57:358–359. doi: 10.1016/0002-9149(86)90927-6. [DOI] [PubMed] [Google Scholar]
- 17.Vacek JL, Davis WR, Bellinger RL, McKiernan TL. Apical hypertrophic cardiomyopathy in American patients. Am Heart J. 1984;108:1501–1506. doi: 10.1016/0002-8703(84)90698-7. [DOI] [PubMed] [Google Scholar]
- 18.Webb JG, Sasson Z, Rakowski H, Liu P, Wigle ED. Apical hypertrophic cardiomyopathy: clinical follow-up and diagnostic correlates. J Am Coll Cardiol. 1990;15:83–90. doi: 10.1016/0735-1097(90)90180-w. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto T. Apical hypertrophic cardiomyopathy (apical hypertrophy): an overview. J Cardiol. 2001;37(Suppl 1):161–178. [PubMed] [Google Scholar]
- 20.Hardarson T, de la Calzada CS, Curiel R, Goodwin JF. Prognosis and mortality of hypertrophic obstructive cardiomyopathy. Lancet. 1973;2:1462–1467. doi: 10.1016/s0140-6736(73)92730-x. [DOI] [PubMed] [Google Scholar]
- 21.McKenna W, Deanfield J, Faruqui A, England D, Oakley C, Goodwin J. Prognosis in hypertrophic cardiomyopathy: role of age and clinical, electrocardiographic and hemodynamic features. Am J Cardiol. 1981;47:532–538. doi: 10.1016/0002-9149(81)90535-x. [DOI] [PubMed] [Google Scholar]
- 22.Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patients population. Circulation. 2000;102:858–864. doi: 10.1161/01.cir.102.8.858. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson MJ, Sonnenberg B, Woo A, Rakowski P, Parker TG, Wigle ED, Rakowski H. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39:638–645. doi: 10.1016/s0735-1097(01)01778-8. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki T, Azuma A, Asada S, Hadase M, Kamitani T, Kawasaki S, Kuribayashi T, Sugihara H. Heart rate turbulence and clinical prognosis in hypertrophic cardiomyopathy and myocardial infarction. Circ J. 2003;67:601–604. doi: 10.1253/circj.67.601. [DOI] [PubMed] [Google Scholar]