Abstract
Background
Although many treatments for advanced gastric cancer have been developed, only poor treatment results have generally been obtained. We performed a prospective study on the combination chemotherapy of paclitaxel and cisplatin (PC). The primary objectives of the study were elucidating the disease response and evaluating the drug regimen's safety.
Methods
Patients with metastatic or recurrent gastric cancer received intravenous paclitaxel 175 mg/m2, and cisplatin 70 mg/m2 on day 1. This cycle was repeated every 3 weeks.
Results
From January 2000 to March 2004, 37 patients from 3 different hospitals were enrolled in this study. A total of 135 treatment cycles (median: 3 cycles) were administered. The responses were evaluable in 34 patients; 24 patients received this regimen as their first-line treatment for metastatic cancer and the other patients received it as their second-line treatment for recurrent cancer. The objective response rate (RR) was 26.5% (95% CI: 11.7-41.3) with two complete responses, and stable disease was observed in 41.1% of the patients. Importantly, an RR of 33.3% (95% CI: 0.6-66.0) was achieved for the eight patients who received this regimen as a first-line treatment. The median follow up duration was 14 months for all the patients, and the median time to progression was 6 months (95% CI: 1.9-10.2). The overall survival time was 8.9 months (95% CI: 7.0-11.0) with a 1-year survival rate of 18.7% (95% CI: 5.6-31.8). The most common toxicity was neutropenia.
Conclusion
PC exhibited promising activity against gastric cancer for the previously untreated patients as a first-line treatment with an acceptable toxicity profile.
Keywords: Stomach Neoplasms, Paclitaxel
INTRODUCTION
Gastric cancer is the second leading cause of cancer mortality in the world and as such, it remains a major challenge for the global medical community1). 75% of patients with gastric cancer are considered incurable at the time of diagnosis due to their advanced disease. Even among the patients with clinically resectable tumors, the relapse rate is high for 40% to 65% of these patients2). The prognosis for the patients with metastatic disease is very poor, with the 5-year survival being less than 5%3).
Randomized clinical trials have shown a modest improvement in the quantity and quality of life when using chemotherapy over the best supportive care alone for patients with advanced gastric cancer4). 5-Fluorouracil-based chemotherapy is commonly used in this setting, but it is not considered the standard of care because the responses are generally incomplete and brief, with few of the patient's responses lasting longer than four months5). A combined chemotherapy generally provides superior response rates over any single-agent therapy, but this has not been associated with a survival advantage6). It is imperative that new agents and new treatment strategies be evaluated.
Paclitaxel is an antimitotic agent that stabilizes microtubules and induces a mitotic block, and it has shown in vitro activity against a variety of malignancies including gastric cancer7). In addition, the growth inhibitory effect of paclitaxel on the primary cultures of gastric cancer is greater than that noted for cisplatin and adriamycin8). Paclitaxel has been reported to have a modest level of activity against the newly diagnosed or refractory gastric cancers as a first-line chemotherapy in phase I-II studies9). Cisplatin has a well known activity against gastric cancer, and it has an objective response rate of 19% when it was used as a single agent. It is interesting that responses to this agent have been seen in those patients with gastric cancer that was refractory to prior chemotherapy. Paclitaxel is synergistic with cisplatin in vivo and in vitro, and especially when paclitaxel precedes platinum administration10,11). The paclitaxel and cisplatin combination has produced antitumor activity with tolerable safety profiles when they have been given to patients having ovarian cancer, advanced non-small cell lung cancer (NSCLC), head and neck cancer and esophageal carcinomas12-15).
The recent phase II studies that have used a paclitaxel containing combination chemotherapy for the treatment of patients with advanced gastric cancer have now been reported on16). A combination regimen of paclitaxel 160 mg/m2 plus cisplatin 60 mg/m2 every 2 weeks produced a response rate of 44% with a median survival time of 11 months. In spite of giving G-CSF support, depending on the absolute neutrophil counts on the day of chemotherapy administration, one third of the patients experienced grade 3 or 4 neutropenia. A triple drug combination of paclitaxel, cisplatin and 5-FU with or without folinic acid has demonstrated favorable response rates of 48~51%. However, the toxicity was substantial and the median overall survival was just 6~11 months17,18). Given the fact that chemotherapy in those patients with advanced gastric cancer is directed at producing palliative effects because these patients are expected to fare poorly, the anticancer activity and adverse effects of different drug regimens must be weighted carefully. The results of the recently published studies on patients with metastatic cancer have suggested that the paclitaxel and cisplatin combination therapy is relatively well tolerated19). However, the efficacy of paclitaxel and cisplatin combination therapy for the treatment of gastric cancer has not been extensively explored. We conducted this phase II study to evaluate the efficacy and the tolerability of this drug combination for patients with pretreated or chemotherapy-nave advanced gastric cancer.
MATERIALS AND METHODS
Patients
This study enrolled patients with histologically proven gastric cancer; measurable disease was a requirement of the study and the patients had to be ≥18 years of age, have an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 and a life expectancy > 3 months. The laboratory criteria included an absolute granulocyte count ≥1,500 cells/mm3, platelets ≥100,000 cells/mm3, hemoglobin ≥9 g/dL, serum creatinine ≤2 mg/dL, bilirubin ≤1.5 mg/dL and transaminases ≤4 times the upper normal limit. The study's protocol was approved by the institutional review board, and all the patients gave us a written informed consent before their enrolment.
Treatment schedule
Paclitaxel (Taxol, Bristol-Myers Squibb Company, Princeton, NJ, USA) was administered at a dose of 175 mg/m2; this was infused over a 3 hour period and then cisplatin was infused every 3 weeks at a dose of 70 mg/m2 over a 1 hour period along with a standard hydration method. The prophylactic antiemetic medication consisted of dexamethasone and 5-hydroxytryptamine-3 antagonists. Before paclitaxel administration, the patients were premedicated with dexamethasone 20 mg i.v., dephenhydramine 50 mg i.v. and cimetidine 300 mg i.v. 30 minutes prior to therapy to prevent the onset of any hypersensitivity reactions and to reduce and/or delay skin toxicity.
Dose modification for adverse events
The toxicity was evaluated before each treatment cycle according to the National Cancer Institute of Canada Common Toxicity Criteria, version 2.0. The drug doses were reduced by 25% in the case of febrile neutropenia grade 4, if the lowest platelet count dropped to less than 25,000 cells/mm3, in the case of grade 1 nephrotoxicity or grade 2 neuropathy, or if any grade 2 non-haematologic toxicity was observed during the previous cycle. Paclitaxel administration was stopped in any case of grade 4 skin toxicity and/or grade 3 anaphylactic reaction. Both chemotherapeutic drugs were discontinued in the event of transitory ≥grade 2 renal toxicity or if there was any definitive decrease of creatinine clearance (<60 mL/min), ≥a grade 3 neuropathy and if any severe toxicity recurred despite the dose attenuation. If the treatment was delayed for 21 days, then the patient was excluded from the study.
Pretreatment and follow-up evaluation
Pretreatment evaluation included a complete medical history and physical examination, a complete blood count and the biochemistry profiles. EKG, chest X-ray and CT scans were performed to define the extent of disease. Complete blood counts and the differential count were obtained weekly, and the biochemical profiles were assessed before each treatment cycle.
Response evaluation
The primary endpoint of this trial was the objective response rate (RR: complete responses plus partial responses), which was evaluated according to WHO standard criteria. Tumor assessment for all lesions was performed at the end of every three cycles. A complete response (CR) was defined as complete resolution of disease being noted on the physical and radiographic examination, and there were no new lesions and no disease related symptoms. A partial response (PR) was defined as a ≥50% reduction for the sum of the products of the perpendicular measurements of all the sites of measurable disease, and progressive disease (PD) was defined as a ≥25% increase for the sum of the products of the perpendicular diameters of all measurable lesions. Stable disease (SD) was defined as any condition other than an objective response or progressive disease. For those patients who achieved CR or PR, confirmatory tumor assessment was done 4 weeks after the initial response assessment.
The secondary endpoints include the duration of the treatment response (measured from the onset of the best response to the date of disease progression), the time to disease progression (calculated from the start of therapy to the time of progression or relapse) and the overall survival.
Statistics
According to the optimal two-stage phase II design20), the treatment program was designed to reject a response rate less than 20% (P0) and to provide a statistical power of 80% for assessing the activity of the regimen in terms of a 40% response rate (P1) with an error of less than 0.05. If fewer than 4 responses were noted in the first 18 eligible patients, the study would be halted. Because responses were observed, additional patients were enrolled to get a final accrual of 34 patients. For the response rate, 95% confidence intervals were calculated as previously described. Overall survival, the time to progression and the response duration were calculated using the Kaplan-Meier method21).
RESULTS
A total of 37 patients were enrolled on this study from January 2000 to March 2004. Two of the patients who were enrolled did not receive any medication because their performance status decreased to ECOG 3 prior to the first cycle of treatment; one patient was lost to follow-up after the first cycle of treatment. 34 patients were evaluable for their treatment response. Table 1 summarizes the patient characteristics.
Table 1.
Patients characteristics
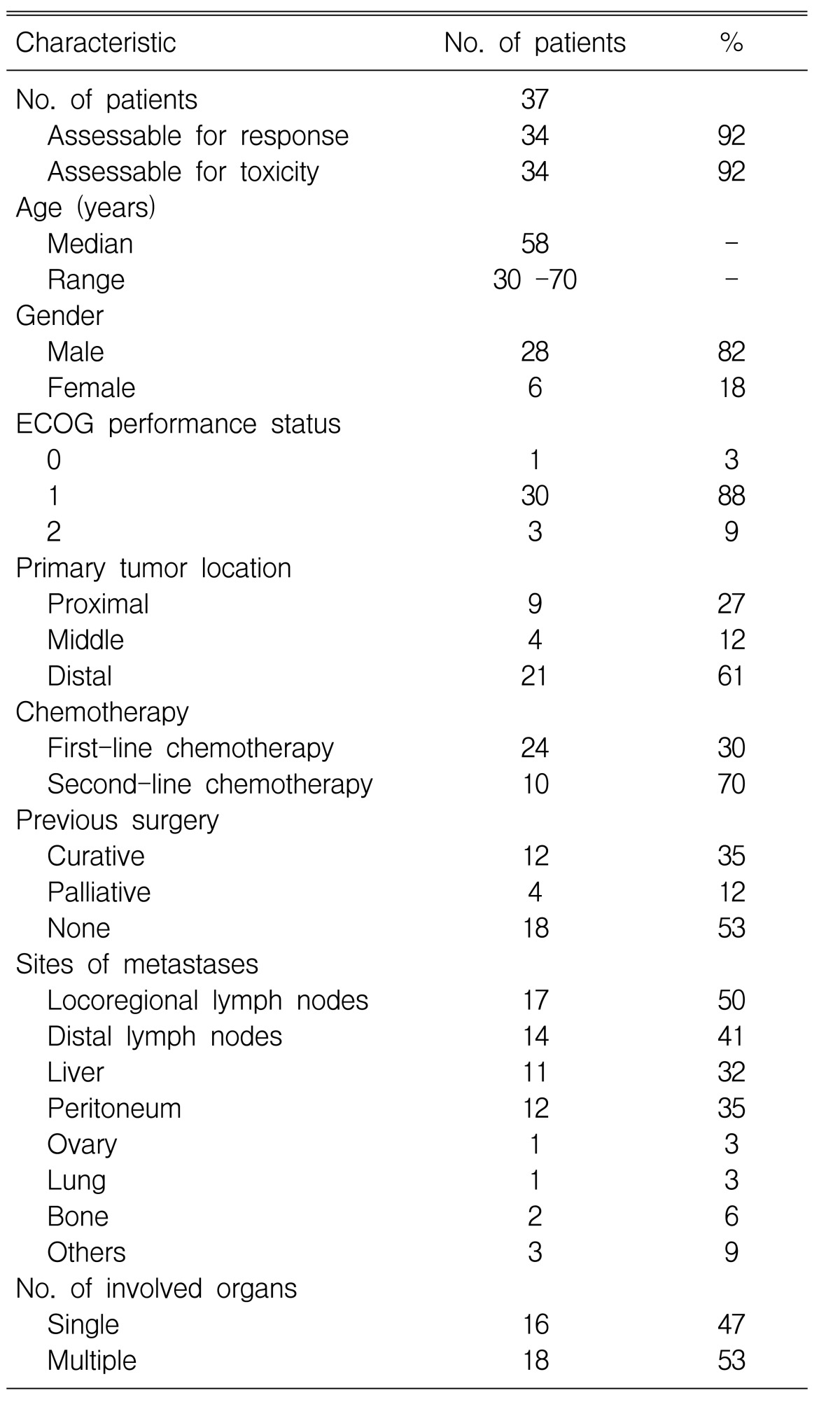
The median age of the 28 (82%) male patients and the six (18%) female patients was 58 years (range: 30 to 70 years), and the median ECOG performance status was 1 (range: 0-2). 18 (53%) patients had multiple metastases involving two or more organ systems. 16 (47%) patients underwent surgery; 12 of them underwent curative resection and the remaining four patients required palliative surgery.
Among the 34 patients, 24 patients (71%) received the test medication as their first-line treatment, and others (29%) received the test medication as their second-line treatment after first failing with 5-FU based chemotherapy (5-FU, epirubicin, mytomycin C/etoposide, doxorubicin and cisplatin).
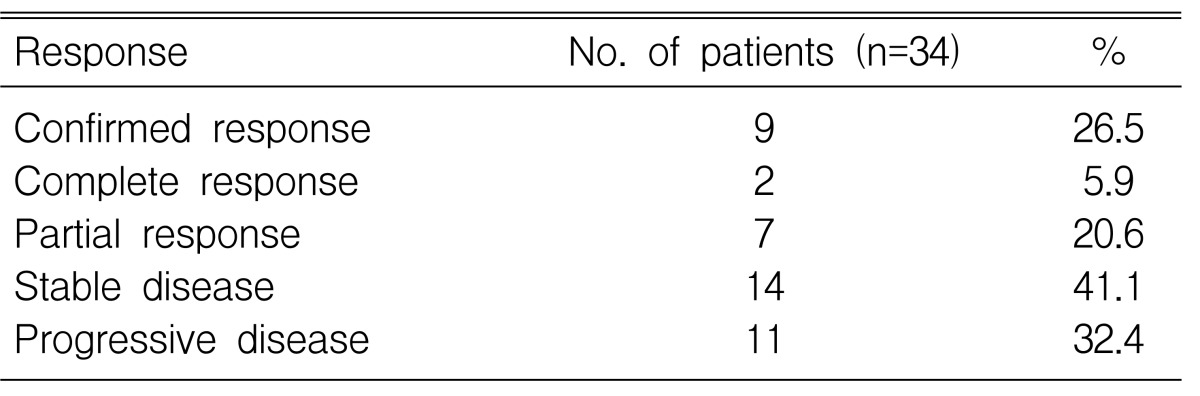
The objective response rate was 26.5% (95% CI: 11.7-41.3), including two confirmed CRs and seven confirmed PRs (Table 2). Importantly, RRs of 33.3% (95% CI: 0.6-66.0) were achieved in 8 patients as a first-line treatment. The median duration of response was 5.1 months (95% CI: 3.6-7.4).
Table 2.
Antitumor activity
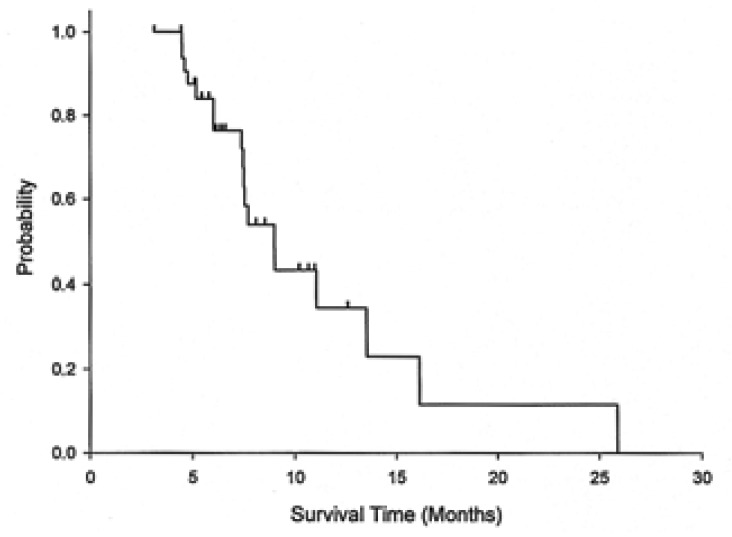
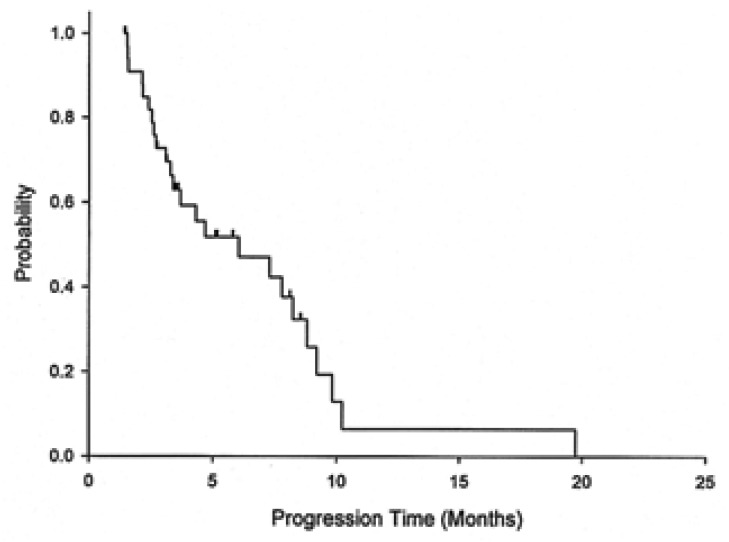
Responses according to the metastasis sites were noted in distal lymph nodes (four of 14, 29%), the liver (two of 11, 18%), and the peritoneum (three of 12, 25%). With a median follow up duration of 14 months, the median overall survival was 8.9 months (95% CI: 7.0-11.0) for all the patients, 9 months (95% CI: 6.3-11.7) for the chemotherapy-nave cases, and 7.5 months (95% CI: 7.2-7.8) for the pre-treated cases. The 1-year survival rate for all the patients was 18.7% (95% CI: 5.6-31.8) (Figure 1). The median time to disease progression (TTP) was 6 months (95% CI: 1.9-10.2) (Figure 2). The median TTP was 7.8 months (95% CI: 2.3-13.3) for the chemotherapy-nave cases and 4.5 months (95% CI: 1.1-7.6) for the pre-treated cases.
Figure 1.
Overall survival for all the patients.
Figure 2.
Time to progression in the treated population.
Toxicities and treatment delays/reduction
A total of 135 treatment cycles (median: 3 cycles; range: 1 to 6) were administered of which 129 cycles were assessable for safety. The most severe toxicities associated with treatment are reported in Table 3. The most common toxicity was neutropenia, with grade 3 and 4 neutropenia being observed in 26% of the 34 patients. Neutropenic fever developed in one patient, and this patient recovered with intravenous antibiotics and G-CSF. Grade 3 and 4 anemia was seen in six patients (18%).
Table 3.
The worst toxicities associated with treatment
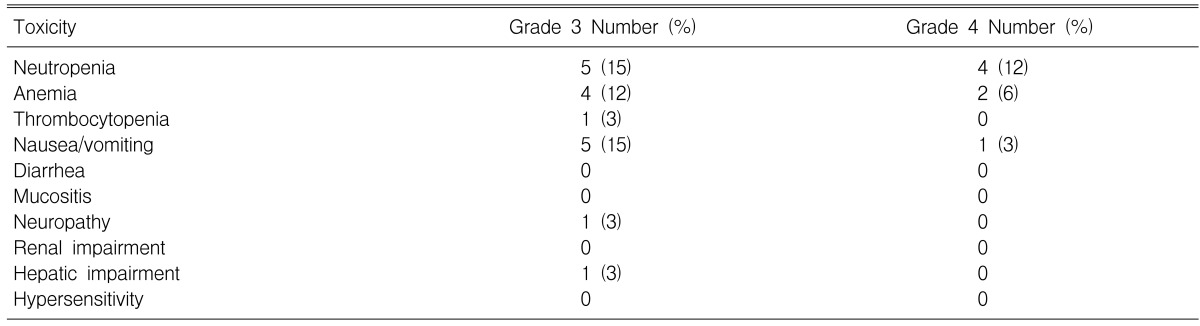
The most common non-hematological toxicities were nausea, peripheral neuropathy and grade 3 hyperbilirubinemia. There were no treatment related deaths during the study. Treatment was delayed in 29 cycles and the drug dose was reduced in three cycles. Treatment delays were for the following reasons: hematological toxicity (20 cycles), and the patients' choice (9 cycles). The median dose intensity for all the treatment cycles was 53.5 mg/m2/week (range: 32.8-58.3) and 19.4 mg/m2/week (range: 13.3-23.3) for paclitaxel and cisplatin, respectively.
DISCUSSION
The prognosis for advanced gastric cancer remains poor at best. Although gastric cancer is considered to be a chemosensitive disease and palliative chemotherapy leads to improving the quality of life and a prolonged survival, chemotherapy in the advanced setting is limited by a low CR rate, a short response duration and the considerable toxicities. In addition, the median survival time that has been achieved by employing chemotherapy only ranges from 6 and 8 months. New drugs and novel therapeutic interventions need to be tested to improve the response rates and the survival time for patients with advanced gastric cancer.
Some studies have reported that the paclitaxel, as a single agent, has a clinical activity against advanced gastric cancer22,23). Cisplatin has shown documented activity against gastric cancer when it was used as a single agent24). Combination chemotherapy of paclitaxel and cisplatin has shown apparent synergistic efficacy and safety for those patients with ovarian cancer, head and neck cancer and NSCLC12-15).
Although several reports on combination chemotherapy of docetaxel and cisplatin have been published, there has been no report on the combination chemotherapy with paclitaxel and cisplatin administered at the usual dose and on the schedule used for NSCLC or ovary cancer.
The results of the present study indicate that the combination of paclitaxel and cisplatin is active and well tolerated for advanced gastric cancer patients. The median TTP of the 34 patients analyzed in this study was 6 months, and the median OS was 8 months. These results are comparable to those results reported for the standard combination regimens such as ECF (epirubicin, cisplatin and 5-FU), CF (cisplatin and 5-FU) and FAMTX (5-FU, doxorubicin and methotrexate) that have overall survival times of 8.7, 7.2 and 6.7 months, respectively, and these three regimens are known to cause substantial toxicities. The response rate for all the patients treated with PC was 26.5%, and this included two CRs (5.9%) and 7 PRs (20.6%). However the response rate for the patients who did not received prior chemotherapy was 33%, which was comparable to the results reported from studies using FAMTX, CF and DC (docetaxel and cisplatin).
In addition to the antitumor efficacy, toxicity is a critically important issue for the choice of treatment in the palliative setting. In the study of Kornek et al. a combination regimen of paclitaxel 160 mg/m2 plus cisplatin 60 mg/m2 every 2 weeks produced a response rate of 44% with a median survival of 11 months16). In spite of giving G-CSF support, depending upon the absolute neutrophile counts on the day of chemotherapy administration, one third of the patients experienced grade 3 or 4 neutropenia. In the studies with DC being given every 3 weeks25,26), over 50% of patients experienced grade 4 neutropenia and the incidence of grade 3 or worse gastrointestinal toxicity was substantial in the recent randomized studies27) with DCF (docetaxel, cisplatin and 5-FU) versus CF. For the patients with advanced gastric cancer, the RR and TTP of the DCF regimen were superior to those of the CF regimen (RR, 38.7% vs. 23. 2%, respectively, and TTP, 5.2 vs. 3.7 months, respectively), while the difference in the OS (10.2 vs. 8.5 months, respectively) did not reach statistical significance. However the toxicities reported in the DCF arm were significant: the grade 3/4 neutropenia was 84% and the grade 3/4 febrile neutropenia was 16%. The current paclitaxel/cisplatin regimen has an advantage for toxicity over the two weeks administration of docetaxel/cisplatin or PC. In our experience, the main grade 3/4 toxicity associated with the paclitaxel/cisplatin regimen was neutropenia, and this was short-lived and easily managed. The incidence of adverse events other than neutropenia was rather low.
In conclusion, our study showed a moderate activity for the PC combination chemotherapy against metastatic and recurrent gastric cancer, especially as a fist-line treatment, along with a favorable toxicity profile. The PC combination may be considered as an active and well-tolerated treatment regimen for patients suffering with advanced gastric cancer.
References
- 1.Wong JE, Ito Y, Correa P, Peeters KC, van de Velde CJ, Sasako M, Macdonald J. Therapeutic strategies in gastric cancer. J Clin Oncol. 2003;21:267s–269s. doi: 10.1200/JCO.2003.08.536. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 3.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin. 1996;46:5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald JS, Schein PS, Woolley PV, Smythe T, Ueno W, Hoth D, Smith F, Boiron M, Gisselbrecht C, Brunet R, Lagarde C. 5-Fluorouracil, doxorubicin and mitomycin (FAM) combination chemotherapy for advanced gastric cancer. Ann Intern Med. 1980;93:533–536. doi: 10.7326/0003-4819-93-4-533. [DOI] [PubMed] [Google Scholar]
- 6.Cullinan SA, Moertel CG, Fleming TR, Rubin JR, Krook JE, Everson LK, Windschitl HE, Twito DI, Marschke RF, Foley JF. A comparison of three chemotherapeutic regimens in the treatment of advanced pancreatic and gastric carcinoma: fluorouracil vs fluorouracil and doxorubicin vs fluorouracil, doxorubicin, and mitomycin. JAMA. 1985;253:2061–2067. [PubMed] [Google Scholar]
- 7.Chang YF, Li LL, Wu CW, Liu TY, Liu WY, Peng FK, Chi CW. Paclitaxel-induced apoptosis in human gastric carcinoma cell lines. Cancer. 1996;77:14–18. doi: 10.1002/(SICI)1097-0142(19960101)77:1<14::AID-CNCR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka H, Yano K, Saito T, Seo Y, Tomoda H. Cytotoxicity of paclitaxel in comparison with other anticancer agents against neoplastic cells obtained from clinical gastrointestinal carcinoma tissue. Anticancer Res. 1995;15:2001–2006. [PubMed] [Google Scholar]
- 9.Cascinu S, Graziano F, Cardarelli N, Marcellini M, Giordani P, Menichetti ET, Catalano G. Phase II study of paclitaxel in pretreated advanced gastric cancer. Anticancer Drugs. 1998;9:307–310. doi: 10.1097/00001813-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Thigpen JT, Blessing JA, Beecham J, Homesley H, Yordan E. Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas. J Clin Oncol. 1991;9:1962–1966. doi: 10.1200/JCO.1991.9.11.1962. [DOI] [PubMed] [Google Scholar]
- 11.Vanhoefer U, Harstrick A, Wilke H, Schleucher N, Walles H, Schroder J, Seeber S. Schedule-dependent antagonism of paclitaxel and cisplatin in human gastric and ovarian carcinoma cell lines in vitro. Eur J Cancer. 1995;31A:92–97. doi: 10.1016/0959-8049(94)00440-g. [DOI] [PubMed] [Google Scholar]
- 12.Ilson DH, Ajani J, Bhalla K, Forastiere A, Huang Y, Patel P, Martin L, Donegan J, Pazdur R, Reed C, Kelsen DP. Phase II trial of paclitaxel, fluorouracil, cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol. 1998;16:1826–1834. doi: 10.1200/JCO.1998.16.5.1826. [DOI] [PubMed] [Google Scholar]
- 13.Khuri FR, Shin DM, Glisson BS, Lippman SM, Hong WK. Treatment of patients with recurrent or metastatic squamous cell carcinoma of the head and neck: current status and future directions. Semin Oncol. 2000;27(4 Suppl 8):25–33. [PubMed] [Google Scholar]
- 14.Gatzemeier U, von Pawel J, Gottfried M, ten Velde GP, Mattson K, DeMarinis F, Harper P, Salvati F, Robinet G, Lucenti A, Bogaerts J, Gallant G. Phase III comparative study of high dose cisplatin versus a combination of paclitaxel and cisplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18:3390–3399. doi: 10.1200/JCO.2000.18.19.3390. [DOI] [PubMed] [Google Scholar]
- 15.Pfisterer J. Optimierung der primären Chemotherapie des fortgeschrittenen Ovarialkarzinoms. Onkologie. 2000;23:31–43. [Google Scholar]
- 16.Kornek GV, Raderer M, Schull B, Fiebiger W, Gedlicka C, Lenauer A, Depisch D, Schneeweiss B, Lang F, Scheithauer W. Effective combination chemotherapy with paclitaxel and cisplatin with or without human granulocyte colony-stimulating factor and/or erythropoietin in patients with advanced gastric cancer. Br J Cancer. 2002;86:1858–1863. doi: 10.1038/sj.bjc.6600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollmannsberger C, Quietzsch D, Haag C, Lingenfelser T, Schroeder M, Hartmann JT, Baronius W, Hempel V, Clemens M, Kanz L, Bokemeyer C. A phase II study of paclitaxel, weekly, 24-hour continous infusion 5-fluorouracil, folinic acid and cisplatin in patients with advanced gastric cancer. Br J Cancer. 2000;83:458–462. doi: 10.1054/bjoc.2000.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honecker F, Kollmannsberger C, Quietzsch D, Haag C, Schroeder M, Spott C, Hartmann JT, Baronius W, Hempel V, Kanz L, Bokemeyer C. Phase II study of weekly paclitaxel plus 24-h continuous infusion 5-fluorouracil, folinic acid and 3-weekly cisplatin for the treatment of patients with advanced gastric cancer. Anticancer Drugs. 2002;13:497–503. doi: 10.1097/00001813-200206000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Park SR, Oh DY, Kim DW, Kim TY, Heo DS, Bang YJ, Kim NK, Kang WK, Kim HT, Im SA, Suh JH, Kim HK, Kim HK. A multi-center, late phase II clinical trial of Genexol (paclitaxel) and cisplatin for patients with advanced gastric cancer. Oncol Rep. 2004;12:1059–1064. [PubMed] [Google Scholar]
- 20.Simon R. How large should a phase II trial of a new drug be? Cancer Treat Rep. 1987;71:1079–1085. [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:458–481. [Google Scholar]
- 22.Ajani JA, Fairweather J, Dumas P, Patt YZ, Pazdur R, Mansfield PF. A phase II study of Taxol in patients with advanced untreated gastric carcinoma [Abstract] Proc Annu Meet Am Soc Clin Oncol. 1997;16:A933. [Google Scholar]
- 23.Ohtsu A, Boku N, Tamura F, Muro K, Shimada Y, Saigenji K, Akazawa S, Kitajima M, Kanamaru R, Taguchi T. An early phase II study of a 3-hour infusion of paclitaxel for advanced gastric cancer. Am J Clin Oncol. 1998;21:416–419. doi: 10.1097/00000421-199808000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Wils J. The treatment of advanced gastric cancer. Semin Oncol. 1996;23:397–406. [PubMed] [Google Scholar]
- 25.Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, Morant R, Borner MM, Herrmann R, Honegger H, Cavalli F, Alberto P, Castiglione M, Goldhirsch A. Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Ann Oncol. 2000;11:301–306. doi: 10.1023/a:1008342013224. [DOI] [PubMed] [Google Scholar]
- 26.Ridwelski K, Gebauer T, Fahlke J, Kroning H, Kettner E, Meyer F, Eichelmann K, Lippert H. Combination chemotherapy with docetaxel and cisplatin for locally advanced and metastatic gastric cancer. Ann Oncol. 2001;12:47–51. doi: 10.1023/a:1008328501128. [DOI] [PubMed] [Google Scholar]
- 27.Ajani JA, van Cutsem E, Moiseyenko V, Tjulandin S, Fodor M, Majlis A, Boni C, Zuber E, Blattmann A. Docetaxel (D), cisplatin, 5-fluorouracil compare to cisplatin (C) and 5-fluorouracil (F) for chemotherapy-naive patients with metastatic or locally recurrent, unresectable gastric carcinoma (MGC): interim results of a randomized phase III trial (V325) Proc Am Soc Clin Oncol. 2003;22:249. [Google Scholar]