Abstract
Fertilization triggers activation of a series of pre-programmed signal transduction pathways in the oocyte that establish a block to polyspermy, induce meiosis resumption, and initiate zygotic development. The fusion between sperm and oocyte results in rapid changes in oocyte intracellular free calcium levels which in turn activate multiple protein kinase cascades in the ooplasm. The present study has examined the possibility that sperm-oocyte interaction involves localized activation of oocyte protein tyrosine kinases which could provide an alternative signaling mechanism triggered by the fertilizing sperm. Confocal immunofluorescence analysis with antibodies to phosphotyrosine and phosphorylated protein tyrosine kinases allowed detection of minute signaling events localized to the site of sperm-oocyte interaction that were not amenable to biochemical analysis. The results provide evidence for localized accumulation of phosphotyrosine at the site of sperm contact, binding, or fusion, which suggests active protein tyrosine kinase signaling prior to and during sperm incorporation. The PYK2 kinase was found to be concentrated and activated at the site of sperm-oocyte interaction and likely participates in this response. Widespread activation of PYK2 and FAK kinases was subsequently observed within the oocyte cortex indicating that sperm incorporation is followed by more global signaling by these kinases during meiosis resumption. The results demonstrate an alternating signaling pathway triggered in mammalian oocytes by sperm contact, binding, or fusion with the oocyte.
Keywords: Focal Adhesion Kinase, PYK2 kinase, Fertilization, Egg Activation
Introduction
Fertilization involves the specific interaction between two highly differentiated gametes resulting in combination of the maternal and paternal genomes as well as oocyte activation. In animal species, these interactions include an initial binding event that occurs between the sperm and the oocyte extracellular matrix (jelly coat and chorion in externally fertilizing species or zona pellucida in mammals), a second binding event between the sperm and the oocyte plasma membrane and finally, fusion of the sperm plasma membrane with that of the oocyte. In some cases, interactions between sperm and oocyte trigger signal transduction events that play a significant role in fertilization. For example, binding of sperm to the jelly coat of marine invertebrate eggs or the cumulus cells and zona pellucida in mammals induces the acrosome reaction in the sperm (Watanabe et al. 2009; Naruse et al. 2011; Jin et al. 2011; Gupta et al. 2012). Binding of sperm to the oocyte plasma membrane triggers membrane depolarization and a fast polyspermy block in externally fertilizing species (Cross & Elinson 1980; Longo et al. 1986; Glahn & Nuccitelli 2003) but apparently not in mammals (Okamoto et al. 1977; Gadella & Evans 2011). Finally, the sperm-oocyte fusion event in mammals results in delivery of PLCζ to the ooplasm which initiates the fertilization-induced calcium transient and subsequent oscillations (Swann et al. 2004). In addition to the above well-defined signaling events, other fertilization-induced signaling pathways have not yet been convincingly linked to a specific sperm-oocyte interaction such as gamete binding or fusion. The methods used to differentiate sperm-oocyte binding from fusion include voltage clamp measurement of membrane capacitance (McCulloh & Chambers 1992), electron microscopy (Longo et al. 1986; Longo et al. 1994) or dye transfer (Lawrence et al. 1997; Conover & Gwatkin 2008; Miyado et al. 2008; Barraud-Lange et al. 2012) which have rarely been combined with biochemical studies. This ambiguity applies to the potential role of protein kinases in regulation of ion channel permeability in the oocyte plasma membrane, heterotrimeric G protein activation in sea urchin oocytes (Voronina & Wessel 2006), as well as the fertilization-induced changes in Protein Tyrosine Kinase (PTK) signaling that occur in many marine invertebrate, fish, and amphibian oocytes(Giusti et al. 1999; Tokmakov et al. 2005; Giusti et al. 2000).
Fertilization is known to trigger activation of calcium-calmodulin kinase, protein kinase C, mitogen-activated kinase, and other as yet unidentified protein kinase signaling pathways (Stricker 2009; Kalive et al. 2010; Kim et al. 2013; Krauchunas et al. 2012) including PTK-signaling by Src-family and possibly other tyrosine kinases (McGinnis et al. 2011a). Src-family kinase (SFK) signaling is evident early during the response to sperm-oocyte interaction and is critical for the fertilization-induced calcium transient in oocytes from species that fertilize externally (Kinsey 2012). For example, in sea urchin oocytes, the SFK1 and SFK7 kinases were found to be concentrated in the oocyte cortex, co- localized with filamentous actin and PLCγ and were particularly concentrated at the site of sperm-oocyte interaction (Townley et al. 2009). In zebrafish oocytes, Fyn kinase was detected in the cortical cytoplasm and an antibody to activated (dephosphorylated) SFKs was used to demonstrate that SFKs were activated in the cortex underlying the micropyle within the first minutes of sperm-oocyte contact and then progressed from the site of sperm-oocyte interaction to include the entire oocyte cortex (Sharma & Kinsey 2008). The subsequent finding that the calcium sensitive PYK2 kinase was activated with a similar timing and pattern in zebrafish oocytes (Sharma & Kinsey 2013) raised the possibility that a ‘wave’ of protein tyrosine kinase signaling events triggered at the site of sperm-oocyte interaction and propagated through the cortical cytoplasm might be an important aspect of fertilization in oocytes of other species as well. Analysis of fertilized mouse oocytes demonstrated that phosphotyrosine-containing proteins accumulated within the plasma membrane and/or cortical cytoplasm of fertilized mouse oocytes during anaphase (McGinnis et al. 2007), however activation of SFKs was not concentrated in the oocyte cortex in contrast to the situation in marine invertebrates and fish. The conflicting results obtained from the above localization studies, together with the fact that SFK activity is not required for calcium signaling in mammalian oocytes (Mehlmann & Jaffe 2005; Kurokawa et al. 2004) raised the possibility that mammalian oocytes do not use PTK signaling as part of the early response to fertilization, or that PTKs other than the Src-family are involved. The objective of the present study was to determine whether PTK signaling occurred at the site of sperm-oocyte interaction in the mouse fertilization system and to establish which PTKs might be involved in this response.
Results
Localized PTK signaling events during sperm-egg interaction
In order to determine whether the oocyte exhibited evidence of PTK signaling in response to contact or fusion with the sperm, we tested for accumulation of phosphotyrosine (P-Tyr) at the site of sperm-oocyte interaction using confocal immunofluorescence microscopy. We performed in vitro fertilization (IVF) using cumulus and zona pellucida-free oocytes which undergo fertilization more synchronously than zona-intact oocytes and provide an opportunity to obtain a high frequency of oocytes involved in cell surface interactions with sperm. Oocytes were fertilized by adding capacitated sperm to groups of 20 to 30 oocytes as described in ‘Materials and Methods’. The concentration of capacitated sperm used for IVF was adjusted to produce a majority of monospermic fertilization events with a reasonable level of synchrony (Gardner et al. 2007). Samples were collected during the first 120 minutes post insemination (m.p.i.) and prepared for confocal immunofluorescence using the 4G10 anti-phosphotyrosine antibody to detect any accumulation of P-Tyr-containing proteins in the ooplasm. The different patterns of P-Tyr immunofluorescence observed in multiple in vitro fertilization experiments are presented in Figure 1 and classified in Table 1.
Figure 1. P-Tyr accumulation at sites of sperm-oocyte contact and sperm incorporation.
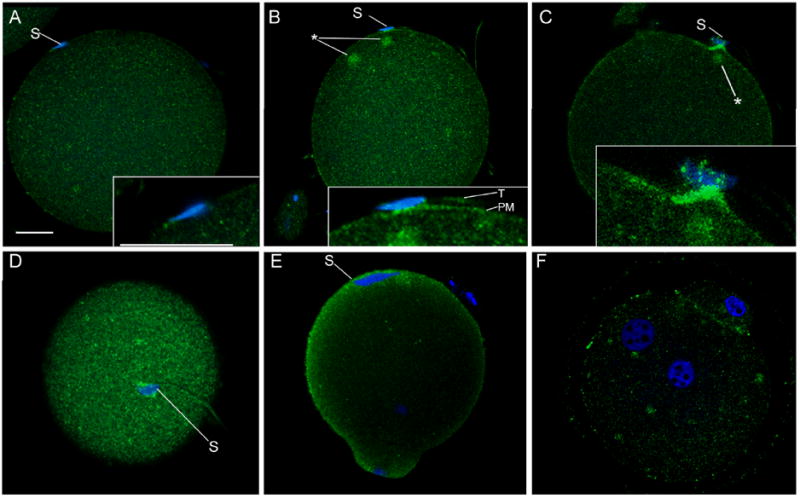
Zona-free oocytes were fertilized in vitro and samples were fixed at different times post-insemination (m.p.i.) to capture different stages of sperm binding, fusion, and incorporation. Oocytes were labeled with the 4G10 anti-p-Tyr antibody and bound antibody was detected with alexa 488-conjugated goat anti-mouse IgG (green) and the nuclear stain ethidium homodimer (blue), then examined by confocal fluorescence as described in ‘Materials and Methods’. Samples presented were fixed at 30 m.p.i. (A,B), 45 m.p.i. (C,D), 90 m.p.i (E), and 120 m.p.i (F). Magnification is indicated by the bar which represents 10 μm. ‘S’ indicates sperm, ‘T’ indicates sperm tail, and ‘PM’ indicates the oocyte plasma membrane. Cytoplasmic foci of P-Tyr-containing protein accumulation are indicated by (*).
Table 1.
Frequency of Oocyte Labeling Patterns Following In Vitro Fertilization
| Probe | n | Under, attached, bound or fused sperm (%) | Over, incorporated sperm (%) | Over MII spindle (%) |
|---|---|---|---|---|
| anti-P-Tyr | 64 | 31 | 82 | 65 |
| anti-FAK PY861 | 54 | 0 | 17 | 15 |
| anti-PYK2 PY579 | 70 | 14 | 75 | 87 |
Oocytes subjected to in vitro fertilization with and without zona removal were fixed at time points where sperm were in contact with, bound, or fused with the oocyte plasma membrane. Samples were labeled with anti-P-Tyr, anti-FAK PY861, or anti-PYK2 PY579 as described in Materials and Methods. Confocal immunofluorescence microscopy was used to image the antibody labeling patterns. Data were collected from 16 experiments. (n) represents the number of oocytes examined within each antibody group.
A fifteen minute exposure to capacitated sperm resulted in no significant changes in P-Tyr distribution in the oocyte even when sperm were present in close proximity to the oocyte plasma membrane (not shown) and only the P-Tyr ‘cap’ that frequently forms in the cortex over the MII spindle (McGinnis et al. 2007) was detected at this early time point. The resolution limitations of the confocal fluorescence technique did not allow us to differentiate between sperm that were in contact with the oocyte plasma membrane, bound to it through functionally significant protein-protein interactions, or in the early stages of gamete fusion. We will therefore use the terms ‘contact, bound, or fused’ to describe the full range of possibilities for sperm that appeared to be in direct contact with the oocyte plasma membrane but showed no evidence of cytoplasmic continuity with the oocyte. Most of the oocytes fixed between 30 to 60 minutes post- insemination exhibited no evidence of P-Tyr labeling near sperm that were in contact or fused with the oocyte plasma membrane (Figure 1, panel A). However, a significant fraction exhibited localized concentrations of P-Tyr-containing proteins in the oocyte cortex at regions where sperm were in contact or fused with the oocyte plasma membrane (Figure 1 panels B-D). These concentrations typically included a region of fluorescence underlying the sperm nucleus and extending laterally in the oocyte plasma membrane or sub-cortical cytoplasm. The plasma membrane of the opposite side of the sperm head that was not in contact with the oocyte exhibited no P-Tyr accumulation. Due to limited resolution of the light microscope when imaging wet, highly processed cells, it was not possible to determine whether the P-Tyr accumulations between the sperm head and the oocyte plasma membrane represented proteins in the sperm plasma membrane, oocyte plasma membrane, or both. However, the fluorescence extending laterally from the region in contact with, bound, or fused with the sperm head was clearly distinguished from the sperm tail (Figure 1B, inset) demonstrating that the oocyte plasma membrane or sub-cortical cytoplasm was the site of active PTK signaling intrinsic to the oocyte. This pattern was detected in 31% of oocytes collected between 30 and 60 minutes post-insemination (Table 1), however only a small fraction (<5%) of the sperm associated with the oocyte plasma membrane exhibited cortical P-Tyr accumulations in the oocyte, suggesting that only a few sperm could illicit this response by the oocyte. Among oocytes that exhibited accumulation of cortical P-Tyr labeling associated with sperm, one or more foci of P-Tyr accumulation was usually observed deeper in the cortical cytoplasm as shown in Figure 1 (panel B, C) where these foci are indicated by an (*).
While most instances of PTK signaling at sites of sperm-egg contact or fusion involved regions of oocyte surface that were smooth in appearance, several cases were found which included cell surface projections from the oocyte that appeared to be in the early stages of encircling the sperm head (Figure 1, panel C and D). These cell processes exhibited more intense P-Tyr labeling than did the smooth regions of oocyte surface. Once the sperm head was incorporated into the oocyte, the cortex overlying the sperm head exhibited further concentration of P-Tyr labeling that formed a cap over the expanding sperm nucleus (Figure 1, panel E) as reported earlier (McGinnis et al. 2007). Oocytes fixed during anaphase or telophase also exhibited widespread cortical P-Tyr accumulation over the entire oocyte cortex (Fig 1, panel E) which almost completely disappeared at the pronuclear stage (Figure 1, panel F).
Together, these data demonstrate that sperm-oocyte contact, binding, or fusion is accompanied by localized accumulation of P-Tyr-containing proteins in the oocyte plasma membrane and/or underlying cortical cytoplasm. Subsequently, oocyte cell surface processes that engulf the sperm also exhibit intense staining with the anti P-Tyr antibody suggesting that dynamic PTK-mediated signaling was occurring in the oocyte. Once the sperm head was incorporated under the oocyte plasma membrane, a ‘cap’ of tyrosine phosphorylated proteins formed in the cortical cytoplasm overlying the sperm head. The entry of the oocyte into anaphase of the second meiotic division was accompanied by widespread accumulation of tyrosine phosphorylated proteins in the cortex, which disappeared at the pronuclear stage.
Identification of PTKs involved in oocyte cortical signaling
As an initial step in identification of the PTKs involved in the above responses to fertilization, we attempted to determine which PTKs exhibited a sub-cellular localization similar to the pattern of P-Tyr accumulation described above. Src-family PTKs have been shown to co-purify with oocyte plasma membranes in several species (Wu & Kinsey 2000; Mahbub Hasan et al. 2005; Tokmakov et al. 2010) and have been localized to the oocyte cortex (Levi et al. 2011). However, in the mouse oocyte, their distribution did not closely match the pattern of P-Tyr accumulation described in Figure 1. Instead, the pool of activated Src-family kinases detected with phosphorylation site-specific antibodies was found to be concentrated near the spindle microtubules and the pronuclear membrane, but not at the oocyte cortex of the mouse oocyte (McGinnis et al. 2007). Finally, suppression of Src-family and Abl- family kinases with chemical inhibitors such as PP2 and SKI-606 had only a limited effect on the accumulation of P-Tyr in the oocyte cortex (not shown). FGR and FES kinases seemed unlikely candidates since FGR was localized primarily at the spindle microtubules and within the germinal vesicle while Fes was expressed at undetectable levels in the mouse oocyte (McGinnis et al. 2011b). We therefore examined the distribution of the focal adhesion kinase family members FAK and PYK2 in the mouse oocyte by western blot and confocal immunofluorescence (Figure 2). FAK was detected in oocytes as a 125 KDa band typical of this protein as well as a 45 KDa band which likely represents the truncated FRNK isoform that results from alternative splicing (Nolan et al. 1999). The 125KDa band was also detected with the phosphorylation site-specific anti-FAK PY861 antibody demonstrating that FAK was activated to some extent in MII oocytes. A 35KDa band was also detected with this antibody and likely represents the phosphorylated FRNK polypeptide migrating differently as a result of phosphorylation. Blots probed with anti-PYK2 revealed the presence of the expected 116KDa band as well as a 25 KDa band similar in size to the truncated PRNK isoform described in somatic cells (Xiong et al. 1998). A higher Mr form migrating at an apparent MW of 200 KDa was also detected with multiple different anti-PYK2 antibodies yet has not been reported in somatic cells. Only the 116 and 200 KDa bands were detected with the phosphorylation-specific anti-PYK2 PY579 antibody indicating that these forms were phosphorylated on tyrosine in vivo.
Figure 2. Detection and sub-cellular distribution of PYK2 and FAK kinases.
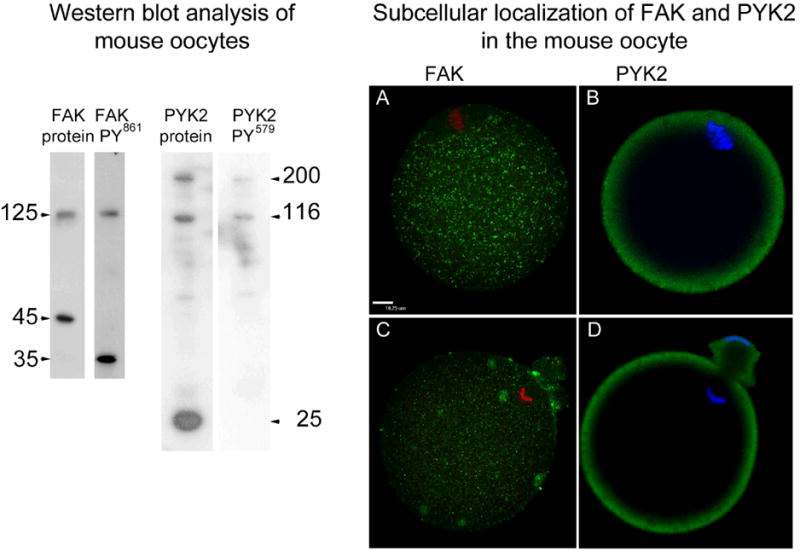
Western blot analysis of MII oocytes (left) was performed on groups of 60 oocytes/ lane probed with antibodies to FAK protein (FAK protein), antibodies to phosphorylated FAK (FAK PY861), anti PYK2 protein (PYK2 protein), or anti phosphorylated PYK2 (PYK2 PY579) as described in’ Materials and Methods’. The apparent molecular weight of each major band was calculated by comparison with molecular weight standards and is presented in the margins (arrows).
Sub-cellular localization of FAK and PYK2 protein (right) was performed on oocytes collected prior to fertilization in vitro (A, C) or during anaphase/ telophase II (B, D) then labeled with anti-PYK2 protein and anti-FAK protein antibodies not targeted to phosphorylation sites in order to establish the sub-cellular distribution of the entire pool of these kinases in the oocyte. Bound antibodies were detected with alexa 488-anti-rabbit IgG (green). Chromatin was stained with ethidium homodimer (red) or Hoechst 33258 (blue). Magnification is indicated by the bar which represents 10 μm.
Immunofluorescence analysis demonstrated that before fertilization, FAK was localized in small punctuate structures that were distributed uniformly through the ooplasm (Figure 2A) while PYK2 was concentrated in the oocyte cortex (Figure 2, B). After fertilization, FAK remained associated with small punctuate structures but many of these seemed to aggregate in larger foci (Figure 2, C). PYK2 remained localized in the oocyte cortex after fertilization (Figure 2, D). Examination of zona-free oocytes fertilized in vitro revealed that a significant number of oocytes with sperm that were in contact, bound, or fused to the oocyte plasma membrane (12%) exhibited a striking concentration of PYK2 in the vicinity of the sperm (Figure 3). The images presented in Figure 3 also suggest that PYK2 may be concentrated in oocyte microvilli or other cell processes (see below).
Figure 3. Detection of PYK2 at sites of sperm-oocyte interaction.
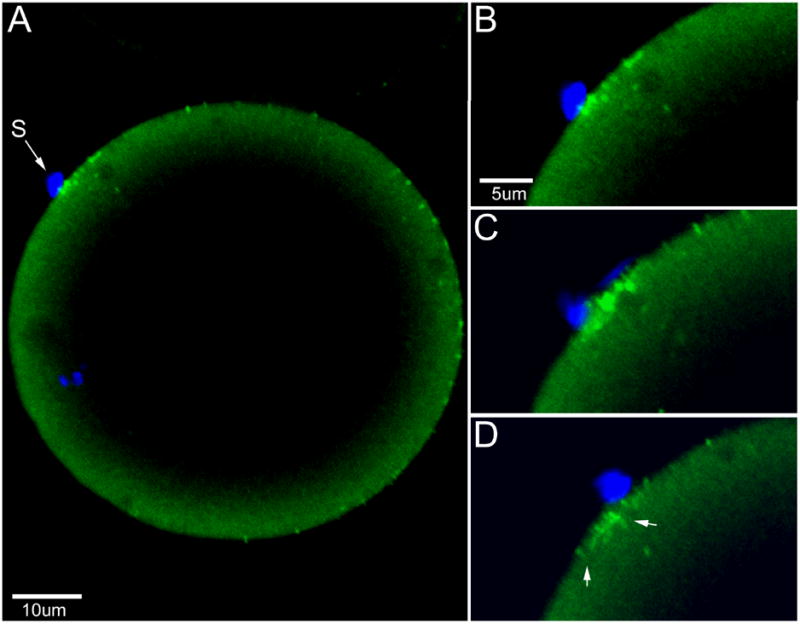
Zona-free oocytes fertilized in vitro and fixed at 45 m.p.i. were labeled with anti-PYK2 protein as in figure 2 and the nuclear stain Hoechst 33258 (A). Concentrations of bound anti-PYK2 antibody are evident underlying the bound sperm (S). Sequential Z-plane images enlarged at right (B-D) reveal the extent of the anti-PYK2 labeling which appears as elongated processes in some regions (panel D arrows) possibly reflecting the accumulation of PYK2 in microvilli. Magnification is indicated by the bars.
The effectiveness of the phosphorylation site-specific antibodies targeting PYK2 (anti-PYK2 PY579) and FAK (anti-FAK PY861) when used for immunofluorescence applications made it possible to demonstrate which regions of the oocyte were actively involved with PYK2 or FAK signaling during the process of fertilization. Analysis of zona-free oocytes undergoing in vitro fertilization revealed that PYK2 activation was occasionally detected in the oocyte plasma membrane or cortex underlying sperm that were in contact, bound, or fused or fixed during the sperm incorporation event (Figure 4, panels E1, E2). This pattern was detected in 14% of oocytes that had sperm oriented in a way that allowed careful examination (Table 1). Other images demonstrated that concentrations of activated PYK2 were frequently present in the cortex overlying fully incorporated sperm (Fig. 4 panel E3) and Table 1. Once oocytes entered anaphase or telophase of the second meiotic division, activated PYK2 remained concentrated in the cortex overlying the sperm head and appeared to spread laterally somewhat as the fertilization cone formed (Fig. 4F and Table 1). However, the concentration of activated PYK2 was usually limited to the region over the incorporated sperm or the meiotic spindle and did not involve the entire oocyte cortex.
Figure 4. Protein tyrosine kinase signaling at sites of sperm-oocyte contact and sperm incorporation.
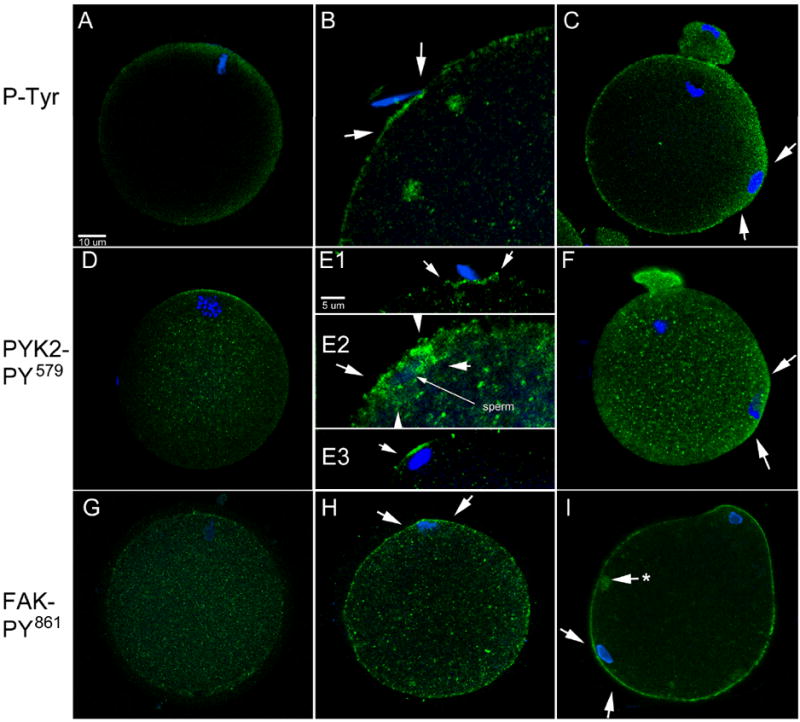
In order to determine whether PYK2 and FAK kinases were activated at the site of sperm-oocyte contact and sperm incorporation, zona-free oocytes processed as in Figure 1 were labeled with either the 4G10 anti-P-Tyr antibody (A-C), anti-PYK2 PY579 (D-F), or anti-FAK PY861 (G-I) to detect the activated (phosphorylated) forms of these kinases and allow comparison with the pattern detected with the anti-P-Tyr antibody. Panels A,D, and G represent MII oocytes. Panels B and E1 represent oocytes with sperm that were in contact, bound or in the process of fusion, panel E2 represents a sperm in the process of engulfment by the oocyte. Panels E3 and H represent oocytes with fully incorporated sperm underlying the oocyte plasma membrane. Panels C, F, and I represent oocytes with incorporated sperm that have formed a fertilization cone (arrows). (*) indicates a cytoplasmic accumulation of FAK PY861 which was commonly observed in oocytes activated by sperm. Magnification is indicated by the bars.
Since the images shown in Figure 3 appeared to show enrichment of PYK2 protein in cell processes resembling microvilli, further study of zona-intact oocytes was performed as microvilli were more easily found in oocytes not subjected to zona removal. Figure 5 shows the distribution of anti-PYK2 PY579 staining in what appear to be microvilli of a zona-intact oocyte fixed following IVF. Activated PYK2 is easily seen in the short cell processes extending from the oocyte surface and in the thin cell processes extending from the corona radiata cells into the zona pellucida (Figure 5B).
Figure 5. Detection of activated PYK2 in microvilli of zona-intact oocytes.
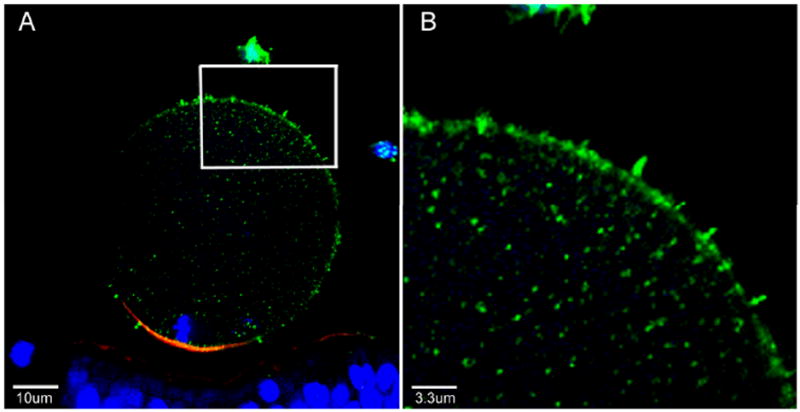
Cumulus and zona-intact oocytes fertilized in vitro for 2 hr were fixed and labeled with anti-PYK2-PY579 (green), alexa 568- phalloidin (red), and with Hoechst 33258 (blue) and imaged by confocal immunofluorescence. A region of a fertilized oocyte (sperm not shown) demonstrating multiple microvilli (inset) is expanded in panel B. Magnification is indicated by the bars.
The distribution of activated FAK in the oocyte exhibited some similarities and some differences from that of activated PYK2. Prior to fertilization, activated FAK was distributed evenly through the cytoplasm where it was concentrated in small punctuate structures (Fig. 4 panel G). Some concentration was detected over the meiotic spindle, but this was less noticeable and less frequent than that exhibited by activated PYK2 (Table 1). Activated FAK was not detected in association with sperm that were in contact, bound, or fused to the oocyte surface or in the process of being incorporated by the oocyte. Once the sperm was incorporated, activated FAK was much more easily detected and was concentrated throughout the entire oocyte cortex and not restricted to the region of the sperm head or fertilization cone as was activated PYK2. Activated FAK labeling over the small punctuate foci became more intense than in unfertilized oocytes and larger accumulations of these small foci were scattered through the ooplasm (Fig. 2, panels H, I).
In summary, PYK2 and FAK kinases exhibit different patterns of activation when examined by confocal immunofluorescence using phosphorylation site-specific antibodies. The widespread cortical activation of FAK differed from the pattern exhibited by PYK2 which was more restricted to the region of sperm engulfment, incorporation, and to the fertilization cone.
Relationship of PYK2 localization to filamentous actin distribution
The pattern of PYK2 and FAK labeling described above suggested that activated PYK2 and FAK might be closely associated with the cortical actin layer in oocytes and zygotes. In order to test for co-localization of activated PYK2 with the actin layer, oocytes were labeled with anti-PYK2-PY579 as well as alexa 568-conjugated phalloidin prior to examination by confocal microscopy. Activated PYK2 detected in the oocyte cortex was co-localized with actin filaments that form the cortical actin layer and could be detected with the alexa 568-phalloidin probe. The labeling of active PYK2 was most intense over the thickened ‘actin cap’ which formed over the meiotic spindle (Figure 6A-C) as well as over the expanding sperm nucleus (Figure 6D-F). Activated FAK was also co-localized with the actin layer in the oocyte cortex, but exhibited less tendency to become highly concentrated over the spindle and expanding sperm nucleus (not shown).
Figure 6. Co-localization of activated PYK2 and filamentous actin-rich regions of the fertilized oocyte.
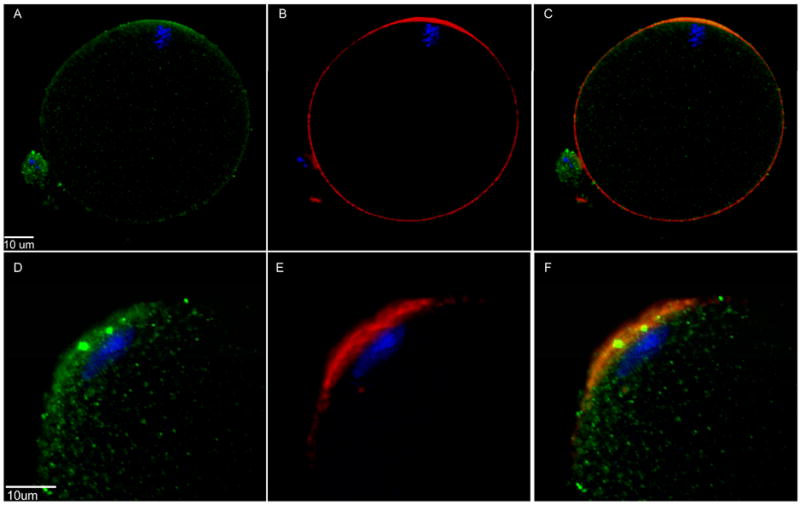
Oocytes fertilized in vitro were fixed at 90 m.p.i. and labeled with anti-PYK2 PY579 (green), alexa 568- phalloidin (red), and Hoechst 33258 (blue). Examples showing the actin-rich region overlying the MII spindle (A-C) and the actin-rich region overlying the newly incorporated sperm (D-F) demonstrate the co-localization of filamentous actin and phosphorylated PYK2 in panels where the two color channels were merged (C,F).
Discussion
Sperm-oocyte interaction occurs at many levels beginning with chemotactic signals that modify sperm swimming behavior, followed by binding interactions between the sperm and the extracellular matrix of the oocyte, and concluding with sperm binding to and fusing with the oocyte plasma membrane (Vacquier & Swanson 2011; Burnett et al. 2012; Bromfield & Nixon 2012; Gupta et al. 2012; Clark 2011). Some of the oocyte proteins such as CD9 and integrins that have been implicated in gamete binding or fusion (Glazar & Evans 2009; Ohnami et al. 2012) also have the potential to initiate signal transduction events in response to cell-cell or cell-substrate contact through FAK-family kinases (Berditchevski & Odintsova 1999; Powner et al. 2011; Ohnami et al. 2012). Such ‘outside-in’ signaling has only been established in marine invertebrate oocytes where gamete binding triggered plasma membrane depolarization leading to the fast polyspermy block (Longo et al. 1986; Longo et al. 1994). In mammalian oocytes, only the repetitive calcium transients initiated following gamete fusion have clearly been linked to specific signal transduction pathways important for oocyte activation (Carroll 2001; Runft et al. 2002; Parrington et al. 2007). The results presented in the present study demonstrate the accumulation of P-Tyr containing proteins in the oocyte plasma membrane and cortex directly underlying a small percentage of sperm that appeared to be in contact, bound, or fused with the oocyte plasma membrane. We interpret the accumulation of P-Tyr-containing proteins as indicative of a PTK signaling event resulting either from activation of a PTK, suppression of a phosphatase, or enhanced kinase-substrate availability due to docking interactions etc. To our knowledge, this is the first evidence for any protein kinase signaling event localized to the site of sperm-oocyte contact, binding, or fusion in mammalian systems. The fact that P-Tyr accumulations were not observed in the vicinity of every sperm head that was in contact with the oocyte surface indicates that the PTK signaling event does not automatically occur whenever the sperm and oocyte plasma membranes come in contact but seems instead either to occur only in a small percentage of sperm, or to occur very transiently. The data suggest a sequence of events wherein the oocyte and sperm plasma membranes reach a state that promotes PTK signaling which is quickly followed by sperm engulfment and incorporation. Since the light microscopic images presented here do not resolve whether the oocyte and sperm plasma membranes had begun to fuse, it is not possible to say whether PTK signaling was triggered by trans-membrane ‘outside-in’ signal transduction or by intracellular signaling following fusion of sperm and oocyte plasma membranes.
The identity of the PTK(s) that might be responsible for signaling at the site of sperm-oocyte contact, binding, or fusion clearly includes the FAK-family kinase PYK2 which was found to be concentrated and activated at the site of gamete interaction. PYK2 has a complex structure with domains that interact with cell surface signaling complexes involving integrins (Ivankovic-Dikic et al. 2000; Totani et al. 2006), cadherins (Allingham et al. 2007; Cain et al. 2010), and plexins (Basile et al. 2005) to initiate kinase activation which then requires phosphorylation by Src-family kinases (Dikic et al. 1996; Qian et al. 1997) and the presence of calcium and calmodulin (Wu et al. 2006) for full activation. Therefore, PYK2 might respond either to clustering of cell surface proteins during sperm-oocyte binding, to an increased level of free calcium in the ooplasm, or both (Liu et al. 2003; Wu et al. 2006). The trigger for elevation of free calcium in the vicinity of sperm-oocyte interaction could potentially occur by calcium influx through plasma membrane channels. In higher plants, for example, sperm-egg fusion is accompanied by rapid calcium influx near the site of fusion that precedes the global calcium transient and is required for sperm incorporation (Antoine et al. 2000) (Antoine et al. 2001). Another possibility is that once small fusion pores form between sperm and oocyte, sufficient PLCζ could diffuse into the oocyte to produce localized IP3 production and thus activation of PYK2. Further experiments under conditions that allow sperm-oocyte binding but block fusion could reveal the relationship between these events and PTK signaling.
Another interesting result demonstrated here was that active PYK2 was frequently observed to be concentrated in microvilli or other oocyte surface processes, a phenomenon that has been observed in somatic cells as well (Mitaka et al. 1997). Further insight into the potential function of PYK2 can be derived from more motile somatic cell types where PYK2 often plays an important role in reorganization of the actin cytoskeleton during cell junction turnover (Sun et al. 2011), cell process formation (Gil-Henn et al. 2007), contact (Hashido et al. 2006) and phagocytosis (Owen et al. 2007a). One might speculate that PYK2 activation might function during the organization and motility guidance of cell processes that engulf the fertilizing sperm as clearly shown by scanning electron microscopy (Tengowski 2004). The finding that active PYK2 was consistently found in the cortical actin cap overlying newly incorporated sperm heads suggests that PYK2 may also promote assembly or maintenance of this structure and formation of the fertilization cone. A role for PYK2 during mammalian sperm incorporation and fertilization cone formation would be consistent with functional data obtained in the zebrafish oocyte where PYK2 suppression impaired sperm incorporation and normal fertilization cone structure (Sharma & Kinsey 2013). Following sperm incorporation, activated PYK2 was increasingly concentrated over the meiotic spindle consistent with the earlier results describing MI rat oocytes (Meng et al. 2006). Together, these results suggest that PYK2 plays a role in the dynamic changes within the oocyte cortical cytoskeleton that are involved with incorporating the sperm and possibly during interaction between the cortex and maternal and paternal chromatin. PYK2 has frequently been reported to play a role in Rho activation (Okigaki et al. 2003; Schaller 2010; Vomaske et al. 2010) which may represent a mechanism by which kinase activity could impact actin-mediated events in the cortex.
The results presented here also demonstrated that FAK kinase was activated in the oocyte cortex after fertilization. However, FAK was not concentrated over the sperm head and meiotic spindle as was PYK2. Instead, FAK activation was more widely distributed throughout the entire oocyte cortex following sperm incorporation and during anaphase. Other studies have shown that PYK2 and FAK, while similar in structure, participate in distinct signaling networks and are specialized for distinct functions even though they can compensate for each other to some extent (Bruce-Staskal et al. 2002; Jiang et al. 2006; Owen et al. 2007b). It seems likely that FAK and PYK2 are responding to different signals within the oocyte and perhaps performing different functions even though they appear to be in the same region of the cell. The fact that PYK2 can transduce signals resulting from cell-cell contact as well as from changes in intracellular calcium levels suggest that this kinase may respond to sperm-oocyte binding or fusion and subsequently mediate the response by cortical cytoskeletal elements in the oocyte. Whether PYK2 impacts other aspects of oocyte activation and zygote development remains an open question.
Materials and Methods
Gamete handling and in vitro fertilization
Mice were housed in a temperature and light-controlled room on a 14L:10D light cycle and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. (Institute of Laboratory Animal Resources (U.S.) Committee on Care and Use of Laboratory Animals 1996; National Research Council (U.S.) 2011) All experimental procedures were approved by the University of Kansas Medical Center IACUC committee. Mice were euthanized by isoflurane (Abbott Animal-Health, North Chicago, IL) inhalation anesthesia followed by cervical dislocation. Female CF1 mice (Harlan Sprague-Dawley, Indianapolis, IN) six to seven weeks old were stimulated by i.p. injection of 5IU PMSG (PG600; Intervet Inc, Millsboro DE), followed 48 hrs later by 5IU hCG (Intervet Inc, Millsboro DE). Cumulus oocyte complexes were collected from the oviducts 15-16 hrs post-hCG, washed in FHM (HEPES buffered KSOM, Specialty Media Inc, Phillipsburgh NJ USA) containing 1mg/ml BSA and transferred to 500μl of mKSOMAA (Specialty Media Inc) containing 4mg/ml BSA, then overlain with sterile, embryo tested light mineral oil and incubated at 37°C in a humidified atmosphere of 6%CO2 in air. Sperm were collected from the cauda epididymis from mature B6D2F1 males (Harlan Sprague-Dawley, Indianapolis, IN). The sperm were transferred to a swim up column containing 750ul of mTyrodes (Summers et al. 2000) with 15mg/ml BSA, then incubated for 2.5 hr at 37°C, 6% CO2 to allow capacitation. Fertilization was begun by adding capacitated sperm to a final concentration of 2×105 /ml. For fertilization of zona-free oocytes, the cumulus cells were removed by treatment with hyaluronidase 0.3 mg/ml. The zona pellucida was removed by brief (15 second) incubation with acid Tyrodes (pH 1.5). Zona-free oocytes were washed several times with mKSOMAA containing 15mg/ml BSA and allowed to recover for 1 hr at 37°C in a humidified atmosphere of 6%CO2 in air (Gardner et al. 2007). The zona-free oocytes were then added to 10μl drops of medium under equilibrated oil and fertilized by addition of capacitated sperm to a final concentration of 2×104/ml. Unless otherwise stated, all reagents were purchased from Sigma Chemical Corp., St Louis, MO.
Fixation and immunofluorescence staining
Oocytes were fixed at 25°C for a minimum of 60 min in 2% paraformaldehyde, 0.5% saturated picric acid in PBS (Andersen et al. 1988). All fixatives, wash solutions, and blocking buffers were supplemented with 40 μM phenylarsine oxide and 100 μM sodium ortho-vanadate to inhibit phosphatase activity. After fixation, oocytes were washed three times in blocking buffer (PBS containing 0.1% Triton X-100; 3mg/ml bovine serum albumin and 0.15M glycine), then further incubated for 1 hr in blocking buffer. Primary antibodies suspended in blocking buffer were incubated with oocytes overnight at 4°C. Labeled oocytes were washed three times with blocking buffer prior to incubation with secondary antibody containing ethidium homodimer-2 (10 μM; Invitrogen #E3599) or Hoechst 33258 (10μg/ml) for 1 hr. Finally, oocytes were washed 3 times with blocking buffer without phenylarsine oxide, mounted under a cover slip in blocking buffer without glycerol or anti-fade reagents and imaged as soon as possible to avoid loss of oocyte structure. Antibodies used in this study included mouse anti-p-Tyr (mouse clone 4G10, #05-321, Millipore Inc), rabbit anti-PYK2 (P-3902, Sigma Aldrich, St. Louis, MO), rabbit antibodies against the phosphorylated PYK2 tyrosines 402 or 579, (#44-618 & 44-632; BioSource Invitrogen, Grand Island, NY); anti-FAK (C-20, Santa Cruz, Santa Cruz, CA), rabbit antibodies against the phosphorylated FAK tyrosines 576 and 861 (#44-652 & 04-626, BioSource Invitrogen, Grand Island, NY). Alexa 488-counjugated goat anti-rabbit or goat anti-mouse IgG (Molecular Probes Invitrogen, Grand Island, NY) were used as a secondary antibodies. Alexa-568-conjugated phalloidin (Molecular Probes) was used to detect filamentous actin. Oocytes were imaged at 63x on either a Nikon T2000 inverted confocal or 40x on a Zeiss LSM500 Pascal inverted confocal microscopes.
Western Blot Analysis
Oocytes collected as above were incubated in FHM containing 0.3mg/ml hyaluronidase to remove cumulus cells. The oocytes were then washed in FHM containing 4 mg/ml polyvinylalcohol instead of BSA and any attached cumulus cells removed by stripping with a pulled glass pipet. Oocytes were immediately solubilized in SDS-PAGE buffer containing phosphatase inhibitors (40 μM phenylarsine oxide, 100 μM sodium orthovanadate and 10 μM calyculin-A) at a final concentration of 10 oocytes/μl and stored at -70°C. Samples were resolved by electrophoresis on a 10% SDS gel and immunoblotting was performed as previously described (Sharma & Kinsey 2006).
Acknowledgments
We are indebted to Lily Zhang and Ling Chen for technical assistance. This work was supported by NICHD HD062860 to W.H.K.
Abbreviations
- IVF
In vitro fertilization
- PTK
protein tyrosine kinase
- P-Tyr
phosphotyrosine
- MII
metaphase-II
- FAK
focal adhesion kinase
- PYK2
protein tyrosine kinase-2
- SFK
Src-Family kinase
- mKSOMAA
modified potassium simplex-optimized medium with amino acids
- FHM
HEPES buffered mKSOM
- hCG
human chorionic gonadotropin
- eCG
equine chorionic gonadotropin
- BSA
bovine serum albumin
References
- Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. Journal of Immunology. 2007;179:4053–4064. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- Andersen J, Orntoft TF, Poulsen HS. Immunohistochemical demonstration of estrogen receptors (ER) in formalin-fixed, paraffin-embedded human breast cancer tissue by use of a monoclonal antibody to ER. J Histochem Cytochem. 1988;36:1553–1560. doi: 10.1177/36.12.2461414. [DOI] [PubMed] [Google Scholar]
- Antoine AF, Faure JE, Cordeiro S, Dumas C, Rougier M, Feijo JA. A calcium influx is triggered and propagates in the zygote as a wavefront during in vitro fertilization of flowering plants. Proc Natl Acad Sci U S A. 2000;97:10643–10648. doi: 10.1073/pnas.180243697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine AF, Faure JE, Dumas C, Feijo JA. Differential contribution of cytoplasmic Ca2+ and Ca2+ influx to gamete fusion and egg activation in maize. Nat Cell Biol. 2001;3:1120–1123. doi: 10.1038/ncb1201-1120. [DOI] [PubMed] [Google Scholar]
- Barraud-Lange V, Chalas BC, Serres C, Auer J, Schmitt A, Lefevre B, Wolf JP, Ziyyat A. Membrane transfer from oocyte to sperm occurs in two CD9-independent ways that do not supply the fertilizing ability of Cd9-deleted oocytes. Reproduction. 2012;44:53–66. doi: 10.1530/REP-12-0040. [DOI] [PubMed] [Google Scholar]
- Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25:6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Odintsova E. Characterization of integrin-tetraspanin adhesion complexes: role of tetraspanins in integrin signaling. Journal of Cell Biology. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromfield EG, Nixon B. The function of chaperone proteins in the assemblage of protein complexes involved in gamete adhesion and fusion processes. Reproduction. 2012 doi: 10.1530/REP-12-0316. Epub ahead of Print. [DOI] [PubMed] [Google Scholar]
- Bruce-Staskal PJ, Weidow CL, Gibson JJ, Bouton AH. Cas, Fak and Pyk2 function in diverse signaling cascades to promote Yersinia uptake. Journal of Cell Science. 2002;115:2689–2700. doi: 10.1242/jcs.115.13.2689. [DOI] [PubMed] [Google Scholar]
- Burnett LA, Washburn CA, Sugiyama H, Xiang X, Olson JH, Al-Anzi B, Bieber AL, Chandler DE. Allurin, an amphibian sperm chemoattractant having implications for mammalian sperm physiology. Int Rev Cell Mol Biol. 2012;295:1–61. doi: 10.1016/B978-0-12-394306-4.00007-1. [DOI] [PubMed] [Google Scholar]
- Cain RJ, Vanhaesebroeck B, Ridley AJ. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. Journal of Cell Biology. 2010;188:863–876. doi: 10.1083/jcb.200907135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. The initiation and regulation of Ca2+ signalling at fertilization in mammals. Semin Cell Dev Biol. 2001;12:37–43. doi: 10.1006/scdb.2000.0215. [DOI] [PubMed] [Google Scholar]
- Clark GF. The molecular basis of mouse sperm-zona pellucida binding: a still unresolved issue in developmental biology. Reproduction. 2011;142:377–381. doi: 10.1530/REP-11-0118. [DOI] [PubMed] [Google Scholar]
- Conover JC, Gwatkin RB. Pre-loading of mouse oocytes with DNA-specific fluorochrome (Hoechst 33342) permits rapid detection of sperm-oocyte fusion. J Reprod Fertil. 2008;82:681–690. doi: 10.1530/jrf.0.0820681. [DOI] [PubMed] [Google Scholar]
- Cross NL, Elinson RP. A fast block to polyspermy in frogs mediated by changes in the membrane potential. Developmental Biology. 1980;75:187–198. doi: 10.1016/0012-1606(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- Gadella BM, Evans JP. Membrane fusions during mammalian fertilization. Adv Exp Med Biol. 2011;713:65–80. doi: 10.1007/978-94-007-0763-4_5. [DOI] [PubMed] [Google Scholar]
- Gardner AJ, Williams CJ, Evans JP. Establishment of the mammalian membrane block to polyspermy: evidence for calcium-dependent and -independent regulation. Reproduction. 2007;133:383–393. doi: 10.1530/REP-06-0304. [DOI] [PubMed] [Google Scholar]
- Gil-Henn H, Destaing O, Sims NA, Aoki K, Alles N, Neff L, Sanjay A, Bruzzaniti A, De Camilli P, Baron R, Schlessinger J. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2(-/-) mice. Journal of Cell Biology. 2007;178:1053–1064. doi: 10.1083/jcb.200701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti AF, Carroll DJ, Abassi YA, Terasaki M, Foltz KR, Jaffe LA. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. Journal of Biological Chemistry. 1999;274:29318–29322. doi: 10.1074/jbc.274.41.29318. [DOI] [PubMed] [Google Scholar]
- Giusti AF, Xu WQ, Hinkle B, Terasaki M, Jaffe LA. Evidence that fertilization activates starfish eggs by sequential activation of a Src-like kinase and phospholipase Cgamma. Journal of Biological Chemistry. 2000;275:16788–16794. doi: 10.1074/jbc.M001091200. [DOI] [PubMed] [Google Scholar]
- Glahn D, Nuccitelli R. Voltage-clamp study of the activation currents and fast block to polyspermy in the egg of Xenopus laevis. Development Growth and Differentiation. 2003;45:187–197. doi: 10.1034/j.1600-0854.2004.00684.x. [DOI] [PubMed] [Google Scholar]
- Glazar AI, Evans JP. Immunoglobulin superfamily member IgSF8 (EWI-2) and CD9 in fertilisation: evidence of distinct functions for CD9 and a CD9-associated protein in mammalian sperm-egg interaction. Reprod Fertil Dev. 2009;21:293–303. doi: 10.1071/rd08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Bhandari B, Shrestha A, Biswal BK, Palaniappan C, Malhotra SS, Gupta N. Mammalian zona pellucida glycoproteins: structure and function during fertilization. Cell and Tissue Research. 2012;349:665–678. doi: 10.1007/s00441-011-1319-y. [DOI] [PubMed] [Google Scholar]
- Hashido M, Hayashi K, Hirose K, Iino M. Ca2+ lightning conveys cell-cell contact information inside the cells. EMBO Rep. 2006;7:1117–1123. doi: 10.1038/sj.embor.7400821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (U.S.) Committee on Care and Use of Laboratory Animals. NIH Publication. Bethesda, MD: 1996. Guide for the care and use of laboratory animals. [Google Scholar]
- Ivankovic-Dikic I, Gronroos E, Blaukat A, Barth BU, Dikic I. Pyk2 and FAK regulate neurite outgrowth induced by growth factors and integrins. Nat Cell Biol. 2000;2:574–581. doi: 10.1038/35023515. [DOI] [PubMed] [Google Scholar]
- Jiang X, Jacamo R, Zhukova E, Sinnett-Smith J, Rozengurt E. RNA interference reveals a differential role of FAK and Pyk2 in cell migration, leading edge formation and increase in focal adhesions induced by LPA in intestinal epithelial cells. J Cell Physiol. 2006;207:816–828. doi: 10.1002/jcp.20629. [DOI] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalive M, Faust JJ, Koeneman BA, Capco DG. Involvement of the PKC family in regulation of early development. Molecular Reproduction and Development. 2010;77:95–104. doi: 10.1002/mrd.21112. [DOI] [PubMed] [Google Scholar]
- Kim S, Spike C, Greenstein D. Control of oocyte growth and meiotic maturation in Caenorhabditis elegans. Adv Exp Med Biol. 2013;757:277–320. doi: 10.1007/978-1-4614-4015-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey WH. Intersecting roles of protein tyrosine kinase and calcium signaling during fertilization. Cell Calcium. 2012 doi: 10.1016/j.ceca.2012.11.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauchunas AR, Horner VL, Wolfner MF. Protein phosphorylation changes reveal new candidates in the regulation of egg activation and early embryogenesis in D. melanogaster. Developmental Biology. 2012;370:125–134. doi: 10.1016/j.ydbio.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Smyth J, Wu H, Fukami K, Takenawa T, Fissore RA. Evidence that activation of Src family kinase is not required for fertilization-associated [Ca2+]i oscillations in mouse eggs. Reproduction. 2004;127:441–454. doi: 10.1530/rep.1.00128. [DOI] [PubMed] [Google Scholar]
- Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–241. doi: 10.1242/dev.124.1.233. [DOI] [PubMed] [Google Scholar]
- Levi M, Maro B, Shalgi R. The conformation and activation of Fyn kinase in the oocyte determine its localisation to the spindle poles and cleavage furrow. Reproduction, Fertility and Development. 2011;23:846–857. doi: 10.1071/RD11033. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang GY, Hou XY, Xu TL. Two types of calcium channels regulating activation of proline-rich tyrosine kinase 2 induced by transient brain ischemia in rat hippocampus. Neurosci Lett. 2003 Sep 18;348:127–130. doi: 10.1016/s0304-3940(03)00618-9. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Cook S, McCulloh DH, Ivonnet PI, Chambers EL. Stages leading to and following fusion of sperm and egg plasma membranes. Zygote. 1994;2:317–331. doi: 10.1017/s0967199400002148. [DOI] [PubMed] [Google Scholar]
- Longo FJ, Lynn JW, McCulloh DH, Chambers EL. Correlative ultrastructural and electrophysiological studies of sperm-egg interactions of the sea urchin, Lytechinus variegatus. Dev-Biol. 1986;118:155–166. doi: 10.1016/0012-1606(86)90083-7. [DOI] [PubMed] [Google Scholar]
- Mahbub Hasan AK, Sato K, Sakakibara K, Ou Z, Iwasaki T, Ueda Y, Fukami Y. Uroplakin III, a novel Src substrate in Xenopus egg rafts, is a target for sperm protease essential for fertilization. Dev Biol. 2005 Oct 15;286:483–492. doi: 10.1016/j.ydbio.2005.08.020. [DOI] [PubMed] [Google Scholar]
- McCulloh DH, Chambers EL. Fusion of membranes during fertilization. Increases of the sea urchin egg’s membrane capacitance and membrane conductance at the site of contact with the sperm. J-Gen-Physiol. 1992;99:137–175. doi: 10.1085/jgp.99.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Developmental Biology. 2007;306:241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Carroll DJ, Kinsey WH. Protein tyrosine kinase signaling during oocyte maturation and fertilization. Mol Reprod Dev. 2011a;78:831–845. doi: 10.1002/mrd.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis LK, Hong X, Christenson LK, Kinsey WH. Fer tyrosine kinase is required for germinal vesicle breakdown and meiosis-I in mouse oocytes. Mol Reprod Dev. 2011b;78:33–47. doi: 10.1002/mrd.21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Jaffe LA. SH2 domain-mediated activation of an SRC family kinase is not required to initiate Ca2+ release at fertilization in mouse eggs. Reproduction. 2005;129:557–564. doi: 10.1530/rep.1.00638. [DOI] [PubMed] [Google Scholar]
- Meng XQ, Zheng KG, Yang Y, Jiang MX, Zhang YL, Sun QY, Li YL. Proline-rich tyrosine kinase2 is involved in F-actin organization during in vitro maturation of rat oocyte. Reproduction. 2006;132:859–867. doi: 10.1530/rep.1.01212. [DOI] [PubMed] [Google Scholar]
- Mitaka T, Shindoh M, Mochizuki Y, Sasaki H, Ishino M, Matsuya M, Ninomiya T, Sasaki T. Restricted expression of cell adhesion kinase-beta in rat tissues. American Journal of Pathology. 1997;150:267–281. [PMC free article] [PubMed] [Google Scholar]
- Miyado K, Yoshida K, Yamagata K, Sakakibara K, Okabe M, Wang X, Miyamoto K, Akutsu H, Kondo T, Takahashi Y, Ban T, Ito C, Toshimori K, Nakamura A, Ito M, Miyado M, Mekada E, Umezawa A. The fusing ability of sperm is bestowed by CD9- containing vesicles released from eggs in mice. Proc Natl Acad Sci U S A. 2008;105:12921–12926. doi: 10.1073/pnas.0710608105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse M, Ishikawa R, Sakaya H, Moriyama H, Hoshi M, Matsumoto M. Novel conserved structural domains of acrosome reaction-inducing substance are widespread in invertebrates. Molecular Reproduction and Development. 2011;78:57–66. doi: 10.1002/mrd.21274. [DOI] [PubMed] [Google Scholar]
- National Research Council (U.S.) Guide for the care and use of laboratory animals. Washington D.C.: Committee for the Update of the guide for the care and use of laboratory animals; 2011. p. 220. [Google Scholar]
- Nolan K, Lacoste J, Parsons JT. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnami N, Nakamura A, Miyado M, Sato M, Kawano N, Yoshida K, Harada Y, Takezawa Y, Kanai S, Ono C, Takahashi Y, Kimura K, Shida T, Miyado K, Umezawa A. CD81 and CD9 work independently as extracellular components upon fusion of sperm and oocyte. Biol Open. 2012;1:640–647. doi: 10.1242/bio.20121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Takahashi K, Yamashita N. Ionic currents through the membrane of the mammalian oocyte and their comparison with those in the tunicate and sea urchin. J Physiol. 1977;267:465–495. doi: 10.1113/jphysiol.1977.sp011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okigaki M, Davis C, Falasca M, Harroch S, Felsenfeld DP, Sheetz MP, Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc Natl Acad Sci U S A. 2003 Sep 16;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. Journal of Cell Biology. 2007a;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen KA, Thomas KS, Bouton AH. The differential expression of Yersinia pseudotuberculosis adhesins determines the requirement for FAK and/or Pyk2 during bacterial phagocytosis by macrophages. Cell Microbiol. 2007b;9:596–609. doi: 10.1111/j.1462-5822.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- Parrington J, Davis LC, Galione A, Wessel G. Flipping the switch: how a sperm activates the egg at fertilization. Developmental Dynamics. 2007;236:2027–2038. doi: 10.1002/dvdy.21255. [DOI] [PubMed] [Google Scholar]
- Powner D, Kopp PM, Monkley SJ, Critchley DR, Berditchevski F. Tetraspanin CD9 in cell migration. Biochemical Society Transactions. 2011;39:563–567. doi: 10.1042/BST0390563. [DOI] [PubMed] [Google Scholar]
- Qian D, Lev S, van Oers NS, Dikic I, Schlessinger J, Weiss A. Tyrosine phosphorylation of Pyk2 is selectively regulated by Fyn during TCR signaling. Journal of Experimental Medicine. 1997 Apr 7;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: Where it all begins. Developmental Biology. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. Journal of Cell Science. 2010;123:1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. Fertilization triggers localized activation of Src-family protein kinases in the zebrafish egg. Dev Biol. 2006;295:604–614. doi: 10.1016/j.ydbio.2006.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. Regionalized calcium signaling in zebrafish fertilization. Int J Dev Biol. 2008;52:561–570. doi: 10.1387/ijdb.072523ds. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. PYK2: A calcium-sensitive protein tyrosine kinase activated in response to fertilization of the zebrafish oocyte. Developmental Biology. 2013;373:130–140. doi: 10.1016/j.ydbio.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker SA. Interactions between mitogen-activated protein kinase and protein kinase C signaling during oocyte maturation and fertilization in a marine worm. Mol Reprod Dev. 2009;76:708–721. doi: 10.1002/mrd.21032. [DOI] [PubMed] [Google Scholar]
- Summers MC, McGinnis LK, Lawitts JA, Raffin M, Biggers JD. IVF of mouse ova in a simplex optimized medium supplemented with amino acids. Human Reproduction. 2000;15:1791–1801. doi: 10.1093/humrep/15.8.1791. [DOI] [PubMed] [Google Scholar]
- Sun CK, Ng KT, Lim ZX, Cheng Q, Lo CM, Poon RT, Man K, Wong N, Fan ST. Proline-rich tyrosine kinase 2 (Pyk2) promotes cell motility of hepatocellular carcinoma through induction of epithelial to mesenchymal transition. PLoS One. 2011;6:e18878. doi: 10.1371/journal.pone.0018878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann K, Larman MG, Saunders CM, Lai FA. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction. 2004;127:431–439. doi: 10.1530/rep.1.00169. [DOI] [PubMed] [Google Scholar]
- Tengowski MW. Microscopic techniques for studying sperm-oocyte interaction during fertilization and early embryonic development. Methods Mol Biol. 2004;253:165–199. doi: 10.1385/1-59259-744-0:165. [DOI] [PubMed] [Google Scholar]
- Tokmakov A, Iwasaki T, Itakura S, Sato K, Shirouzu M, Fukami Y, Yokoyama S. Regulation of Src kinase activity during Xenopus oocyte maturation. Developmental Biology. 2005;278:289–300. doi: 10.1016/j.ydbio.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Tokmakov AA, Iwasaki T, Sato K, Fukami Y. Analysis of signal transduction in cell-free extracts and rafts of Xenopus eggs. Methods. 2010;51:177–182. doi: 10.1016/j.ymeth.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Totani L, Piccoli A, Manarini S, Federico L, Pecce R, Martelli N, Cerletti C, Piccardoni P, Lowell CA, Smyth SS, Berton G, Evangelista V. Src-family kinases mediate an outside-in signal necessary for beta2 integrins to achieve full activation and sustain firm adhesion of polymorphonuclear leucocytes tethered on E-selectin. Biochemical Journal. 2006;396:89–98. doi: 10.1042/BJ20051924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townley IK, Schuyler E, Parker-Gur M, Foltz KR. Expression of multiple Src family kinases in sea urchin eggs and their function in Ca2+ release at fertilization. Developmental Biology. 2009;327:465–477. doi: 10.1016/j.ydbio.2008.12.032. [DOI] [PubMed] [Google Scholar]
- Vacquier VD, Swanson WJ. Selection in the rapid evolution of gamete recognition proteins in marine invertebrates. Cold Spring Harb Perspect Biol. 2011;3:a002931. doi: 10.1101/cshperspect.a002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomaske J, Varnum S, Melnychuk R, Smith P, Pasa-Tolic L, Shutthanandan JI, Streblow DN. HCMV pUS28 initiates pro-migratory signaling via activation of Pyk2 kinase. Herpesviridae. 2010;1:2. doi: 10.1186/2042-4280-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronina E, Wessel GM. Activator of G-protein signaling in asymmetric cell divisions of the sea urchin embryo. Dev Growth Differ. 2006;48:549–557. doi: 10.1111/j.1440-169X.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Fukutomi K, Kubo H, Ohta M, Takayama-Watanabe E, Onitake K. Identification of egg-jelly substances triggering sperm acrosome reaction in the newt, Cynops pyrrhogaster. Molecular Reproduction and Development. 2009;76:399–406. doi: 10.1002/mrd.20959. [DOI] [PubMed] [Google Scholar]
- Wu SS, Jacamo RO, Vong SK, Rozengurt E. Differential regulation of Pyk2 phosphorylation at Tyr-402 and Tyr-580 in intestinal epithelial cells: roles of calcium, Src, Rho kinase, and the cytoskeleton. Cell Signal. 2006;18:1932–1940. doi: 10.1016/j.cellsig.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Wu W, Kinsey W. Fertilization triggers activation of Fyn kinase in the zebrafish egg. Int J Devel Biol. 2000;44:837–841. [PubMed] [Google Scholar]
- Xiong WC, Macklem M, Parsons JT. Expression and characterization of splice variants of PYK2, a focal adhesion kinase-related protein. Journal of Cell Science. 1998;111:1981–1991. doi: 10.1242/jcs.111.14.1981. [DOI] [PubMed] [Google Scholar]


