Abstract
Adulthood hypertension can be prenatally programmed by maternal dietary protein deprivation. We have shown that the sympathetically-mediated pressor response to physical stress is exaggerated in prenatally programmed hypertensive rats (PPH). The mechanisms underlying this abnormal responsiveness remain undetermined. The renin-angiotensin system is known to effect sympathetic nerve activity. Therefore, the purpose of this study was to determine whether inhibition of the renin-angiotensin system attenuates the enhanced sympathetic and pressor responses to physical stress in PPH. Changes in renal sympathetic nerve activity and blood pressure in response to i) hindlimb contraction; ii) hindlimb stretch; and iii) hindlimb intra-arterial capsaicin administration were assessed in control and PPH rats chronically treated (from age 3 wks) with either vehicle or the angiotensin-converting enzyme inhibitor enalapril. Conscious resting systolic arterial pressure was significantly greater in PPH (142±5 mmHg) than control (128±2 mmHg) after vehicle treatment (P<0.05). Resting systolic pressure was reduced by enalapril treatment in PPH (125±2mmHg) but had no effect in control (128±2 mmHg). The pressor and renal sympathetic responses to muscle contraction and stretch were significantly higher in decerebrate PPH than decerebrate control in vehicle treated groups. Responses to capsaicin were variable. Enalapril significantly attenuated the enhanced contraction-induced elevations in mean pressure (vehicle=45±6 mmHg; enalapril=21±3 mmHg) and renal sympathetic activity (vehicle=175±22%; enalapril=89±23%) in PPH. Its effects were similar on responses to stretch in PPH but were equivocal during capsaicin administration. The results suggest that the renin-angiotensin system contributes to the enhancement of the renal sympathetic and pressor responses to physical stress in PPH.
Keywords: prenatal programming, hypertension, low protein diet, autonomic nervous system, blood pressure, heart rate, exercise pressor reflex, renin-angiotensin system
INTRODUCTION
Small for gestational age infants subjected to prenatal insults are at risk for developing hypertension in adulthood1–4. Animal models using rats have demonstrated that maternal dietary protein deprivation, prenatal administration of glucocorticoids and uteroplacental insufficiency lead to small for gestational age neonates that develop hypertension as adults5–7.These models have been utilized to study the pathophysiology of the hypertension. However, the mechanism(s) underlying the predisposition of offspring with a suboptimal prenatal environment to develop hypertension as adults is unclear. Current evidence suggests that the sympathetic nervous system may play a crucial role since renal sympathetic denervation normalizes baseline blood pressure in hypertensive adult offspring of mothers administered glucocorticoids or having reduced uterine perfusion during pregnancy8–10. Moreover, we have recently demonstrated in adult rats that the prenatal programming of hypertension (PPH) induces sympathetic overactivity in response to physical stress11.
The renin-angiotensin system (RAS) has also been implicated in the prenatal programming of hypertension. Despite plasma angiotensin II levels being within normal range in adult offspring after prenatal insult 6, 12, 13, the hypertension that develops as a result of prenatal programming is normalized by either administration of angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor blockers14–17.Further, it has been demonstrated that intrarenal RAS activity is increased and renal angiotensin II receptor type 1 (AT1) abundance enhanced with prenatal programming 6, 12, 13. With regard to the latter, AT1receptor expression has also been shown to be elevated in the brain of adult offspring whose mothers were fed a low protein diet as compared to control suggesting that increases in central RAS activity may likewise play a role in the prenatal programming of hypertension16.
Despite the probable roles of the RAS and the sympathetic nervous system in the development of chronic high blood pressure in PPH, a direct link between the two has not been established previously. In order to better understand the pathophysiology of the disease, determining if such a link exists is important. For example, it is possible that either an increase in central or peripheral RAS activity may enhance sympathetic outflow to the kidney which could affect renal function including renal sodium transport5, 9, 18 leading to hypertension. In addition, RAS mediated elevations in sympathetic nerve activity (SNA) could increase vasoconstrictor tone contributing to high blood pressure. The purpose of this study was, therefore, to examine the role of the RAS in the generation of enhanced sympathetic responsiveness in PPH which has been shown to occur during acute physical stress. The renal sympathetic nerve activity (RSNA) response to physical stress was assessed in controls and PPH rats chronically treated (from 3 wks of age) with either vehicle or the ACE inhibitor enalapril in their drinking water. It was hypothesized that disruption of RAS activity would attenuate the exaggerated RSNA response to acute physical stress in PPH rats.
MATERIALS AND METHODS
For a complete description of the Materials and Methods see the online-only Data Supplement.
Animals
Pregnant Sprague Dawley rats were fed either a control 20% protein diet (Teklad) or an isocaloric 6% protein diet (Teklad) from day 12 of gestation until delivery of their offspring 11, 19, 20. After weaning (~3 weeks of age), offspring from the 20% and 6% protein groups received either vehicle (0.4% ethanol) or enalapril (100mg/L) in their drinking water until the time of experimentation (19–26 weeks of age). This is comparable to a regimen used by others to study the effect of RAS inhibition on blood pressure with prenatal programming15. The procedures outlined were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center. All experiments were performed in accordance with the United States Department of Health and Human Services National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preliminary Measurements
Animals were placed in metabolic cages to measure 24 hour urine output after 3 days of acclimatization. Non-invasive blood pressure measurements were obtained in conscious rats at ~12 weeks of age using a CODA blood pressure system. Plasma angiotensin II levels were assessed using an en enzyme immunoassay kit.
Acute Surgical Procedures
Isoflurane anesthetized rats were instrumented for mechanical ventilation as well as the measurement of blood pressure (left common carotid artery), heart rate (electrocardiograph), SNA (renal nerve) and hindlimb muscle tension (triceps surae muscles). In addition, the circulation of the right hindlimb was isolated for the intra-arterial administration of chemicals. After instrumentation, animals were decerebrated pre-collicularly and isoflurane anesthesia discontinued.
Experimental Procedures
Physical stress was induced by activation of the exercise pressor reflex (EPR) as well as its individual functional components; the muscle mechanoreflex and metaboreflex. These stressors were chosen as they significantly enhance SNA, arterial blood pressure (ABP) and heart rate (HR) when preferentially stimulated21. The EPR was stimulated by contracting the triceps surae muscles. Isolated L4 and L5 ventral roots were stimulated for 30 s (40 Hz, 0.1 ms at 3 times motor threshold: the minimum current required to produce a muscle twitch). The muscle mechanoreflex was preferentially engaged by stretching the triceps surae muscles of the hindlimb for 30s using a calibrated 9.5 mm rack and pinion system. To selectively activate the muscle metaboreflex, capsaicin was administered into the arterial supply of the hindlimb (0.3 and 1.0 µg/100 µL). In all animals, the heart was excised and weighted post mortem. The tibia was harvested and its length measured.
Statistical Analyses
Data were analyzed using two-way analysis of variance with a post hoc Student Newman Keuls test utilized when appropriate. In Table 1, the factor “diet” indicates comparisons between control and PPH whereas the factor “treatment” indicates comparisons between vehicle (i.e. ethanol) and enalapril. An unpaired t-test was performed to compare plasma angiotensin II concentration between groups. The significance level was set at P< 0.05. All values are expressed as means ± SEM.
Table 1.
Morphometric characteristics and baseline hemodynamics.
| Variable | Control | PPH | Diet | Treatment | Interaction | ||
|---|---|---|---|---|---|---|---|
| Ethanol | Enalapril | Ethanol | Enalapril | ||||
| (n=10) | (n=10) | (n=8) | (n=10) | ||||
| Body weight (g) | 438 ± 16 | 427 ± 16 | 366 ± 20 | 349 ± 17 | P<0.0001 | P=0.4140 | P=0.8799 |
| Heart weight/body weight (mg g−1) | 2.70 ± 0.04 | 2.52 ± 0.06 | 2.70 ± 0.04 | 2.62 ± 0.07 | P=0.3982 | P=0.0269 | P=0.3108 |
| Heart weight/tibial length (mg mm−1) | 28.7 ± 0.7 | 25.9 ± 1.0 | 25.1 ± 0.8 | 23.0 ± 0.8 | P=0.0007 | P=0.0074 | P=0.6892 |
| Under anesthesia | (n=10) | (n=10) | (n=8) | (n=10) | |||
| MAP(mmHg) | 88 ± 5 | 97 ± 5 | 92 ± 6 | 82 ± 6 | P=0.3197 | P=0.8975 | P=0.0874 |
| HR (beats min−1) | 346 ± 5 | 362 ± 8 | 346 ± 9 | 356 ± 9 | P=0.7097 | P=0.0961 | P=0.7124 |
| Baseline signal to noise ratio for RSNA | 4.1 ± 0.9 | 5.0 ± 0.8 | 3.8 ± 0.9 | 4.1 ± 0.5 | P=0.4267 | P=0.4630 | P=0.7626 |
| After decerebration | (n=10) | (n=10) | (n=8) | (n=10) | |||
| MAP(mmHg) | 93 ± 7 | 74 ± 4 | 84 ± 5 | 65 ± 4 | P=0.0855 | P=0.0008 | P=0.9691 |
| HR (beats min−1) | 410 ± 18 | 430 ± 12 | 388 ± 10 | 407 ± 15 | P=0.1290 | P=0.1776 | P=0.9749 |
| Baseline signal to noise ratio for RSNA | 6.6 ± 1.1 | 5.7 ± 1.2 | 5.4 ± 1.2 | 6.7 ± 1.2 | P=0.9271 | P=0.8519 | P=0.3918 |
Values are means ± S.E.M.
RESULTS
Characterization of the Prenatal Programming of Hypertension
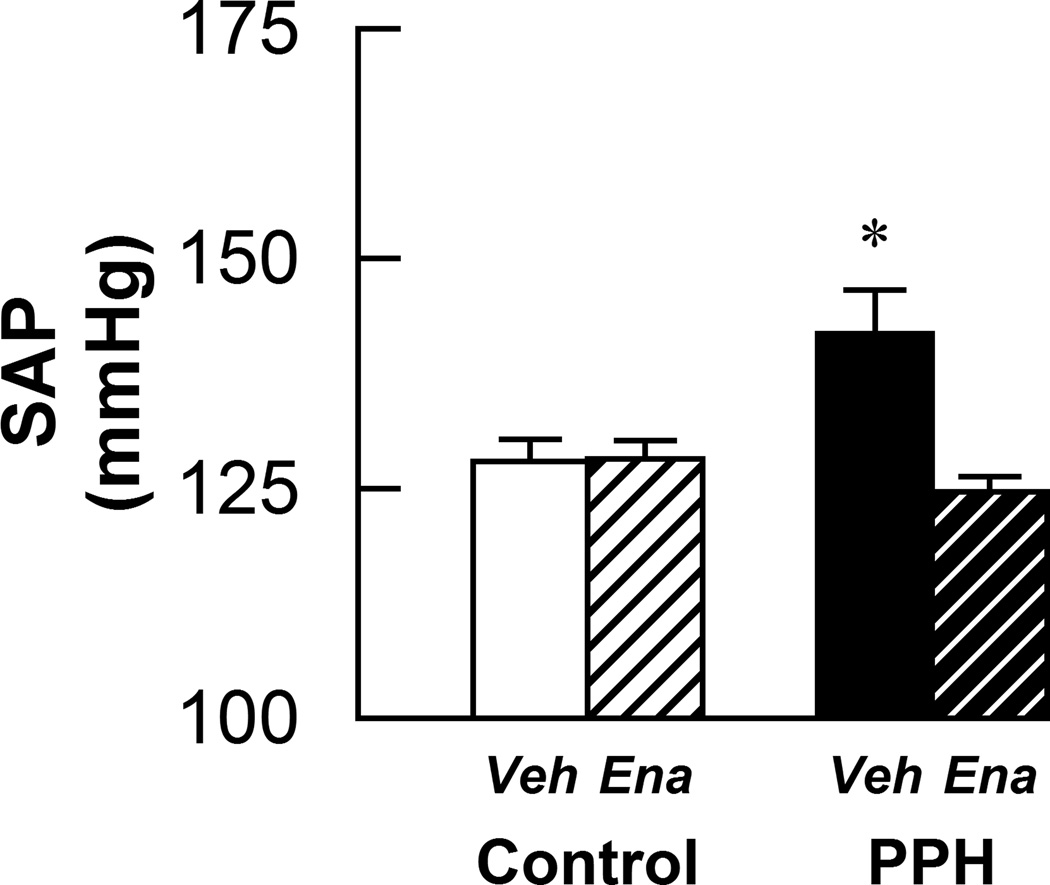
In agreement with previous findings11, systolic arterial pressure (SAP) in the conscious state was significantly higher in vehicle-treated PPH compared to all other groups (Fig. 1). Treatment with enalapril significantly attenuated the increase in SAP in conscious PPH but had no effect on controls (Fig. 1). Table 1 summarizes the morphometric characteristics and baseline hemodynamics of the animals studied. Body weight was lower in rats whose mothers received a low-protein diet (i.e. PPH). Heart weight/body weight ratios were lower in enalapril treated animals although not significantly so in PPH. Heart weight/tibial length ratios were greater in vehicle-treated control animals than in all other groups. Under anesthesia, mean arterial pressure (MAP), HR and RSNA baseline signal/noise ratio were not significantly different. Similar findings were obtained for these variables after decerebration although rats treated with enalapril had lower baseline MAP. There was no difference in plasma angiotensin II concentrations between control and PPH (7.3 ± 1.8 vs. 10.0 ± 1.8 pg/mL, respectively). There was also no difference in daily urine output between control or PPH with (12.6 ± 1.8 vs. 11.5 ± 1.8 mL/day, respectively) or without (13.2 ± 0.6 vs. 9.1 ± 0.7 mL/day, respectively) enalapril treatment.
Figure 1.
Conscious systolic arterial pressure (SAP) measured by tail cuff in control-vehicle (n=10), control-enalapril (n=10), PPH-vehicle (n=10) and PPH-enalapril (n=6) rats. Veh: vehicle-treated; Ena: enalapril-treated. * P < 0.05vs control and enalapril treated PPH.
The Effect of Enalapril on the Responses to EPR Activation
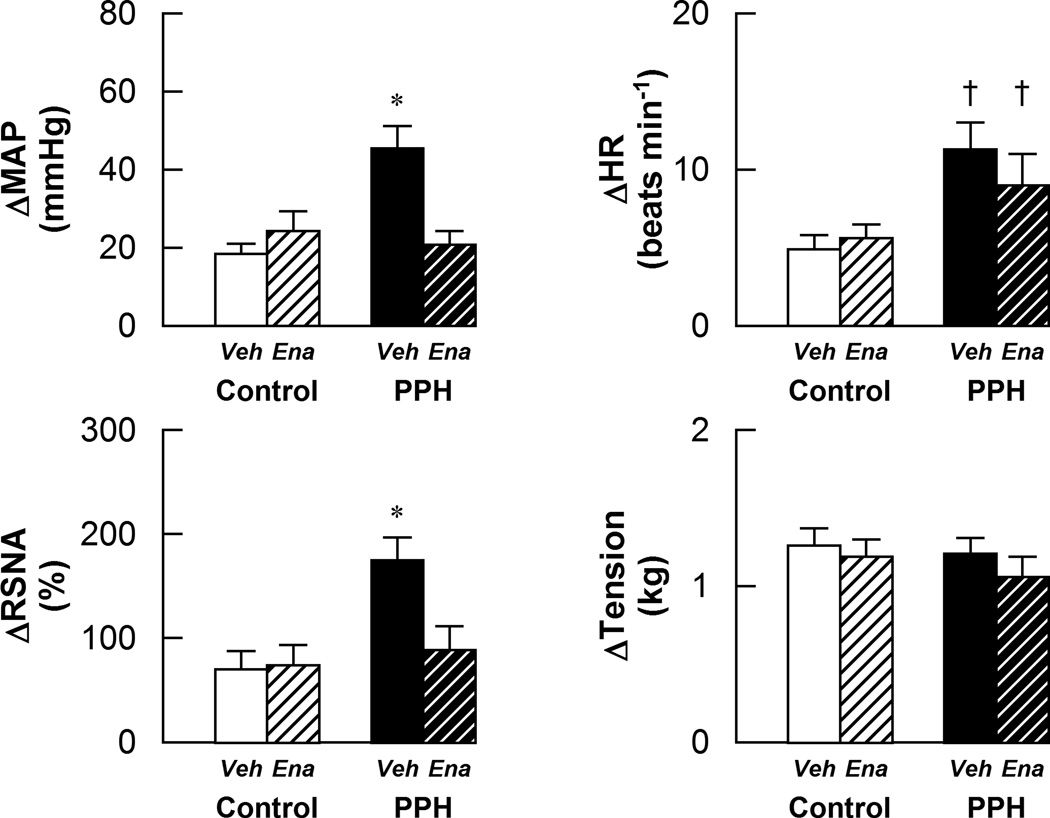
As previously reported11, stimulation of the EPR evoked significantly greater increases in MAP, HR and RSNA in vehicle-treated PPH compared to control animals (Fig.2). Enalapril treatment did not affect the sympathetic and cardiovascular responses to EPR stimulation in control. In contrast, enalapril treatment significantly attenuated the increases in MAP and RSNA, but not HR, in response to EPR activation in PPH. The tension developed during muscle contraction was similar between all groups.
Figure 2.
Cardiovascular and sympathetic responses to activation of the EPR in control-vehicle (n=10), control-enalapril (n=10), PPH-vehicle (n=8) and PPH-enalapril (n=10) rats. Veh: vehicle-treated; Ena: enalapril-treated. * P < 0.05vs control and enalapril treated PPH. † P < 0.05 vs control.
The Effect of Enalapril on the Responses to Mechanoreflex Activation
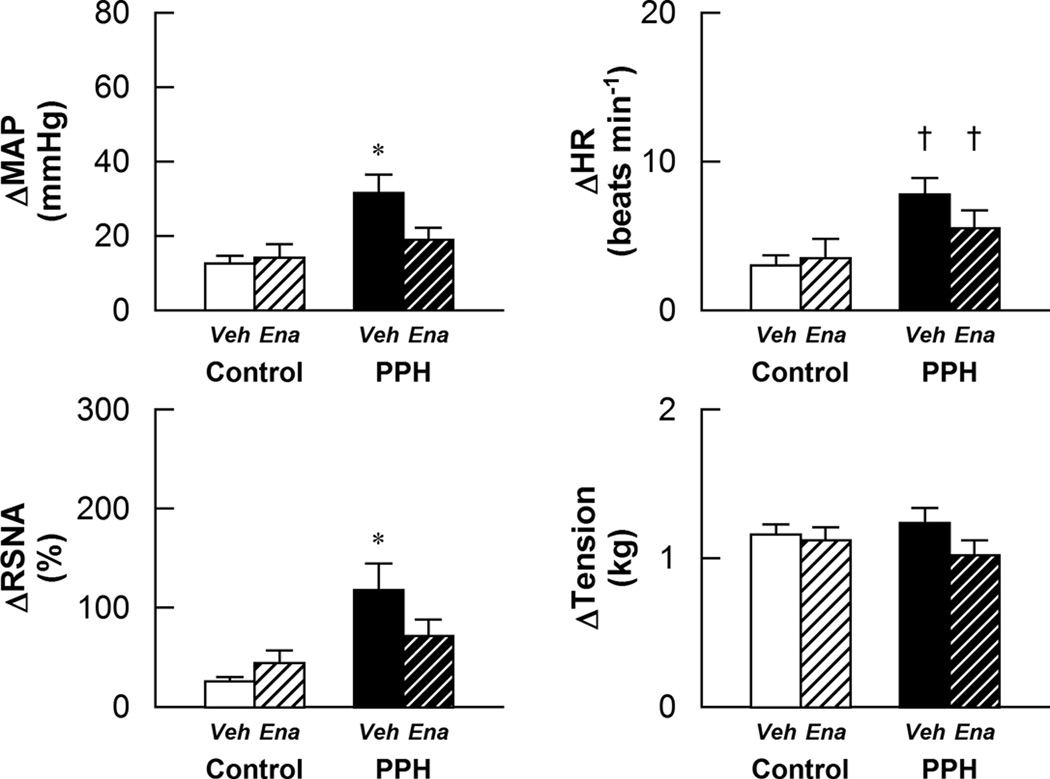
The sympathetically mediated cardiovascular responses to stimulation of the mechanically-sensitive component of the EPR by passive muscle stretch were exaggerated in vehicle treated PPH as compared to control (Fig. 3). In PPH, but not control, the MAP and RSNA responses to muscle stretch were significantly reduced by enalapril treatment. In contrast, enalapril had no significant effect on the HR response to this maneuver. The tension developed during muscle stretch was similar between all groups.
Figure 3.
Cardiovascular and sympathetic responses to activation of the mechanically sensitive component of the EPR in control-vehicle (n=10), control-enalapril (n=10), PPH-vehicle (n=8) and PPH-enalapril (n=10) rats. Veh: vehicle-treated; Ena: enalapril-treated. * P < 0.05 vs control and enalapril treated PPH. † P < 0.05 vs control.
The Effect of Enalapril on the Responses to Metaboreflex Activation
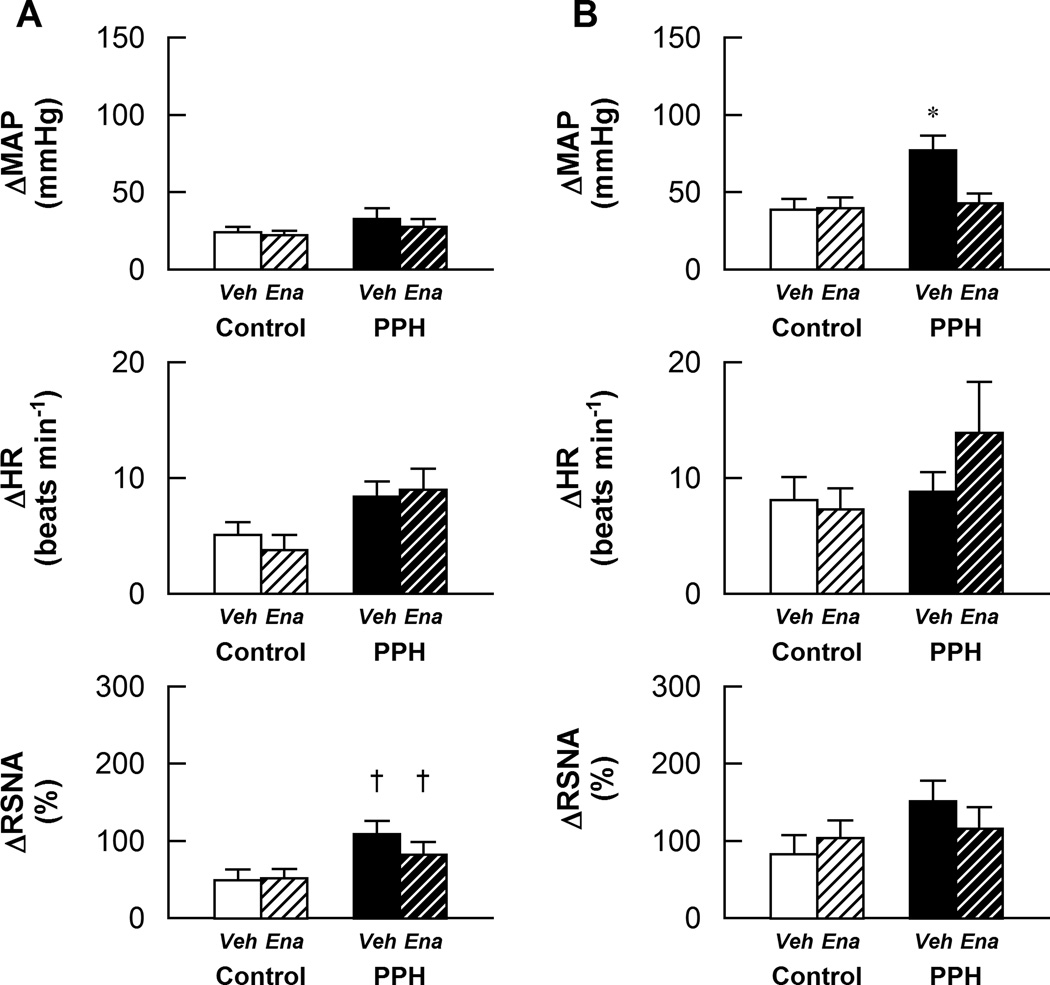
The RSNA response to activation of chemically-sensitive afferent fibers in skeletal muscle via the intra-arterial administration of 0.3 µg/100 µL of capsaicin were larger in PPH compared to control. Enalapril treatment had no effect on this exaggerated response (Fig. 4A).MAP and HR responses at this dose of capsaicin were not different between groups and were unaffected by enalapril treatment. In contrast, at the higher concentration of capsaicin (1.0 µg/100 µL), the pressor response was significantly greater in vehicle-treated PPH as compared to control. This augmented MAP response was significantly attenuated by enalapril treatment (Fig.4B).Similarly, the RSNA response was larger in vehicle-treated PPH than control although statistical significance was not reached (p=0.08). The HR responses were not significantly different between groups at this dose of capsaicin. Neither the RSNA nor HR responses to 1.0 µg/100 µL of capsaicin were significantly affected by treatment with enalapril in either control or PPH animals.
Figure 4.
Cardiovascular and sympathetic responses to activation of the metabolically sensitive component of the EPR in control-vehicle (n=10), control-enalapril (n=10), PPH-vehicle (n=8) and PPH-enalapril (n=10) rats. A: Responses to the intra-arterial administration of 0.3 µg/100µL capsaicin in the hindlimb; B: Responses to the intra-arterial administration of 1.0 µg/100µLcapsaicin in the hindlimb. Veh: vehicle-treated; Ena: enalapril-treated. * P < 0.05vs control and enalapril treated PPH.† P < 0.05 vs control.
DISCUSSION
The significant findings of the present investigation were i) chronic ACE inhibition with enalapril lowered elevated baseline SAP in conscious PPH; ii) enalapril significantly attenuated the exaggerated increases in RSNA and MAP in response to activation of the EPR and mechanoreflex as well as the augmented MAP response to stimulation of the metaboreflex (at the higher dose of capsaicin) in decerebrate PPH; iii) the HR responses to stimulation of the EPR, mechanoreflex and metaboreflex in decerebrate PPH were largely unaffected by enalapril treatment; and iv) enalapril had no effect on baseline SAP in conscious healthy controls nor the sympathetically mediated cardiovascular response to physical stress in decerebrate controls. These findings support the contention that the renin-angiotensin system plays a significant role not only in the generation of raised basal blood pressures but also in the development of the enhanced renal sympathetic and pressor responses to physical stress in prenatally programmed adult hypertensive rats.
In agreement with the current findings, previous studies have provided evidence the RAS mediates, at least in part, the hypertension with prenatal programming 14, 16, 17. For example, it has been demonstrated that both ACE inhibition14, 15 and angiotensin II receptor blockade17 normalize baseline blood pressure in conscious PPH16. In addition, ACE inhibition has been shown to correct abnormal increases in blood pressure variability and alterations in arterial baroreflex control of HR in PPH16. We previously demonstrated that the sympathetically mediated cardiovascular response to physical stress (e.g. stimulation of the EPR, mechanoreflex and metaboreflex) is markedly exaggerated in PPH 11. In the current study, inhibition of ACE was shown to normalize, in large part, these responses as well. The results are novel in that they establish, for the first time, a direct link between the RAS and altered sympathetic regulation in PPH.
Given this link, the question arises as to how RAS activation evokes exaggerations in RSNA in PPH. A definitive answer remains elusive although several possibilities exist. The control of SNA resides in autonomic centers within the brain. When considering the systemic RAS, circulating angiotensin II can cross the blood brain barrier (via fenestrated capillaries in certain areas of the brain)and serves to increase SNA22. In humans, for example, systemic infusion of angiotensin II has been shown to enhance SNA23. However, plasma angiotensin II levels were found to be comparable between control and PPH animals in the current study. Thus, it is unlikely that higher plasma angiotensin II levels acted centrally to augment SNA. This is similar to others who examined offspring at 28 days of age and found no difference in angiotensin II levels between PPH and control animals13. That being stated, Playdys et al. 16have demonstrated greater expression of the angiotensin II receptor, AT1,in several areas of the brain including the vascular organ of the lamina terminalis, the subfornical organ and the nucleus tractus solitarii (NTS)in PPH compared to control rats. Of these areas, the NTS is a major central site for sympathetically mediated cardiovascular regulation both at rest and during exercise24. An increase expression of AT1 receptors within the NTS could evoke exaggerated responses in PPH via this mechanism despite the lack of difference in circulating angiotensin II compared to controls.
Independent of the systemic RAS and circulating angiotensin II, there is a brain RAS system that may also play a significant role in generating stress induced SNA overactivity in PPH25. As evidence, intracerebroventricular administration of losartan (an AT1 receptor antagonist) lowers baseline blood pressure in PPH but has no effect on control rats 16. Enalapril may affect this central RAS system independent of its actions on circulating angiotensin II. For example, enalapril has been shown to decrease the expression of mRNA for the AT1 receptor in the brain stem as well as the cerebral cortex in stroke-prone spontaneously hypertensive rats26.It is possible that enalapril induced reductions in AT1 expression in the brain stem could account for the attenuation of the exaggerated RSNA and MAP responses to physical stress in PPH demonstrated in the current study. It should be noted, however, that some researchers suggest that enalapril does not cross the blood brain barrier as oral treatment with the ACE inhibitor appears to have no effect on the intracerebral RAS in spontaneously hypertensive rats27.In contrast, others have suggested that inflammation-induced increases in blood brain barrier permeability, which has been shown in diabetic rats, allows passage of enalapril into the brain28.
It is likewise possible that the results reported in this study are due to the actions of enalapril on other components of the RAS. For example, the enhanced RSNA responsiveness to physical stress could result from renal injury induced by prenatal programming. Studies in patients with chronic kidney disease have shown that there is an increase in SNA which is likely due to stimulation of renal afferent nerves as patients with a bilateral nephrectomy have comparable levels of SNA as controls with normal renal function29. Indeed, injection of as little as 50 µl of 10% phenol into a rat kidney can increase blood pressure via an increase in SNA30.Moreover, the increase in blood pressure and secretion of norepinephrine from the posterior hypothalamic nuclei with phenol injection is not present in rats that undergo renal denervation30. In patients, the increase in SNA with chronic kidney disease can be abrogated by blockade of the RAS31–33. Adult rat offspring whose mothers were fed a low protein diet during pregnancy develop renal injury with glomerulosclerosis and interstitial fibrosis34.Thus, it is possible that the exaggerated increases in SNA observed in the current study, which were largely attenuated by treatment with enalapril, could be mediated by activation of renal afferent nerves.
The effects of enalapril treatment on the function of the EPR in this investigation are noteworthy. In our previous study we found that the EPR and each of its functional components were enhanced by programming11. That being stated, the magnitude to which metaboreflex function was accentuated in programmed rats was far less than that of the mechanoreflex and only manifested at the highest dose of capsaicin administered. Similar results were obtained in the current study. Collectively, the data suggest that prenatal programming may alter mechanoreflex function to a greater degree than the metaboreflex. Given these findings, it is not surprising that, with regard to the metaboreflex, the lone effect of enalapril was to normalize the MAP response to the highest of dose of capsaicin administered in PPH. In contrast, enalapril treatment effectively normalized mechanoreflex mediated increases in MAP and RSNA in PPH. Thus, it is logical to suggest that the ability of enalapril to largely correct EPR dysfunction is mediated by its effect on the mechanically-sensitive component of the reflex. Of note, urine output (and presumably by extension water intake) was similar between controls and PPH, the latter of which had significantly lower body weights. Thus, it is acknowledged that PPH animals may have inadvertently been given more enalapril per kg of body weight. This may have contributed to the enalapril-induced reductions in the sympathetic and pressor responses to stress reported in PPH and the absence of such reductions in control rats. Despite this possibility, the findings clearly demonstrate that chronic administration of enalapril mitigates EPR overactivity in PPH.
Surprisingly, HR responses to activation of the EPR were unaffected by enalapril treatment. These findings suggest that enalapril differentially modulates sympathetic outflow to the heart and kidneys. Practically, this is an important point to consider when selecting pharmacological treatment for EPR overactivity in PPH. Clearly, other treatments (e.g. beta-blockade) would be required to abrogate augmented sympathetic activity to the heart. Regardless, the demonstrated ability of enalapril to largely correct EPR dysfunction in PPH is significant and suggests that it may serve as a viable treatment for EPR overactivity in this form of hypertension. This is a significant finding as the cardiovascular response to exercise in hypertension is exaggerated and mediated, in part, by an overactive EPR11, 35, 36. This enhanced circulatory responsiveness to physical activity increases the risk for occurrence of an adverse cardiovascular event (e.g. myocardial infarction, stroke) during or immediately following a bout of exercise37, 38. Thus, effectively treating EPR dysfunction in hypertension, as was demonstrated with enalapril in the current study, may reduce the risks associated with physical activity in this disease.
It should be noted that chronic exposure to enhanced SAP can induce pathological hypertrophic cardiac remodeling leading to heart failure 39. It is commonly accepted that the EPR is exaggerated in cardiomyopathic rats, although the function of its individual components (the mechanoreflex and metaboreflex) remains an area of controversy 40. Therefore, it was important to establish in this study that the alterations in EPR, mechanoreflex and metaboreflex function in PPH and the effects of enalapril on this function were independent of the development of heart failure. To this end, both heart weight to body weight and heart weight to tibial length ratios were greatest in vehicle treated control animals suggesting that cardiac hypertrophy did not develop in PPH. As such, the stress-induced changes in RSNA, MAP and HR in hypertensive animals before and after enalapril treatment were likely not confounded by the presence of heart failure.
Supplementary Material
PERSPECTIVES.
These data demonstrate, for the first time, that the RAS plays a crucial role in the generation of the exaggerated renal sympathetic and pressor responses to physical stress due to prenatal insults. This enhanced responsiveness likely contributes to the pathogenesis of hypertension in PPH. These studies may have clinical relevance for patients who are born small for gestational age and who are predisposed to developing hypertension.
NOVELTY AND SIGNIFICANCE.
What Is New?
We have shown previously that the sympathetic response to physical stress is exaggerated in adult prenatally programmed hypertensive rats (induced by maternal dietary protein restriction during gestation).The mechanisms underlying this abnormal sympathetic responsiveness to physical stress remain undetermined. The present study assessed a possible role for the renin-angiotensin system in the generation of this hyper-responsiveness by directly measuring renal sympathetic activity during physical stress in normotensive and prenatally programmed hypertensive rats chronically treated with or without the angiotensin converting enzyme inhibitor enalapril.
What Is Relevant?
Enalapril decreased resting blood pressure in adult prenatally programmed hypertensive rats. Importantly, the sympathetic and pressor responses to physical stress were likewise attenuated by treatment with enalapril in these animals while angiotensin converting enzyme inhibition had no effect in normotensive rats.
Summary
This investigation has demonstrated a direct link between the renin-angiotensin system and altered sympathetic regulation in adult rats subjected to dietary protein restriction during gestation. The results suggest that, in response to physical stress, the renin-angiotensin system contributes to the generation of sympathetic overactivity in adult prenatally programmed hypertensive rats. This enhanced sympathetic responsiveness can be normalized by inhibiting the renin-angiotensin system with enalapril.
Acknowledgments
SOURCES OF FUNDING
This research was supported by grants from the National Institutes of Health Heart, Lung and Blood Institute (HL-088422 to SAS), the National Institutes of Diabetes and Digestive and KidneyDK41612 (MB) ,DK078596 (MB) and the O’Brien Center P30DK 079328 (Peter Igarashi PI - Core B MB co PI).
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES
None.
REFERENCES
- 1.Barker DJ. The fetal origins of adult hypertension. J Hypertens Suppl. 1992;10:S39–S44. [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C. Low birth weight and hypertension. BMJ. 1988;297:134–135. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. Bmj. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 5.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 2010;298:F235–F247. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kett MM, Denton KM. Renal programming: Cause for concern? Am J Physiol Regul Integr Comp Physiol. 2011;300:R791–R803. doi: 10.1152/ajpregu.00791.2010. [DOI] [PubMed] [Google Scholar]
- 7.Ojeda N, Grigore D, Alexander B. Developmental programming of hypertension: Insight from animal models of nutritional manipulation. Hypertension. 2008;52:44–50. doi: 10.1161/HYPERTENSIONAHA.107.092890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 9.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol. 2008;295:F29–F34. doi: 10.1152/ajprenal.00123.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojeda NB, Johnson WR, Dwyer TM, Alexander BT. Early renal denervation prevents development of hypertension in growth-restricted offspring. Clin Exp Pharmacol Physiol. 2007;34:1212–1216. doi: 10.1111/j.1440-1681.2007.04754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuno M, Siddique K, Baum M, Smith SA. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension. 2013;61:180–186. doi: 10.1161/HYPERTENSIONAHA.112.199356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dagan A, Gattineni J, Habib S, Baum M. Effect of prenatal dexamethasone on postnatal serum and urinary angiotensin ii levels. Am J Hypertens. 2010;23:420–424. doi: 10.1038/ajh.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2004;287:F262–F267. doi: 10.1152/ajprenal.00055.2004. [DOI] [PubMed] [Google Scholar]
- 14.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- 15.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary na and ace inhibition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R80–R84. doi: 10.1152/ajpregu.00309.2004. [DOI] [PubMed] [Google Scholar]
- 16.Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin ii in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- 17.Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000;98:269–275. [PubMed] [Google Scholar]
- 18.Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule na+/h+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1230–R1235. doi: 10.1152/ajpregu.00669.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagan A, Habib S, Gattineni J, Dwarakanath V, Baum M. Prenatal programming of rat thick ascending limb chloride transport by low-protein diet and dexamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R93–R99. doi: 10.1152/ajpregu.91006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manning J, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal bsc1 and tsc in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2002;283:F202–F206. doi: 10.1152/ajprenal.00358.2001. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 22.Fink GD. Long-term sympatho-excitatory effect of angiotensin ii: A mechanism of spontaneous and renovascular hypertension. Clin Exp Pharmacol Physiol. 1997;24:91–95. doi: 10.1111/j.1440-1681.1997.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin ii on muscle sympathetic nerve activity in humans. Am J Physiol. 1991;261:R690–R696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- 24.Toney GM, Mifflin SW. Time-dependent inhibition of hindlimb somatic afferent transmission within nucleus tractus solitarius: An in vivo intracellular recording study. Neuroscience. 1995;68:445–453. doi: 10.1016/0306-4522(95)00156-d. [DOI] [PubMed] [Google Scholar]
- 25.Davisson R. Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol. 2003;285:R498–R511. doi: 10.1152/ajpregu.00190.2003. [DOI] [PubMed] [Google Scholar]
- 26.Nishida Y, Takahashi Y, Sugahara-Kobayashi M, Ishikawa K, Asai S. Decreased expression of angiotensin ii type 1 and type 2 receptors in the brain after long-term administration of antihypertensive drugs in stroke-prone spontaneously hypertensive rat. J Pharmacol Sci. 2008;106:663–666. doi: 10.1254/jphs.sc0080027. [DOI] [PubMed] [Google Scholar]
- 27.Jouquey S, Mathieu MN, Hamon G, Chevillard C. Effect of chronic treatment with trandolapril or enalapril on brain ace activity in spontaneously hypertensive rats. Neuropharmacology. 1995;34:1689–1692. doi: 10.1016/0028-3908(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 28.Vargas R, Rincon J, Pedreanez A, Viera N, Hernandez-Fonseca JP, Pena C, Mosquera J. Role of angiotensin ii in the brain inflammatory events during experimental diabetes in rats. Brain Res. 2012;1453:64–76. doi: 10.1016/j.brainres.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Converse RL, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 30.Ye S, Gamburd M, Mozayeni P, Koss M, Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens. 1998;11:723–728. doi: 10.1016/s0895-7061(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 31.Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Enalapril and losartan reduce sympathetic hyperactivity in patients with chronic renal failure. J Am Soc Nephrol. 2003;14:425–430. doi: 10.1097/01.asn.0000045049.72965.b7. [DOI] [PubMed] [Google Scholar]
- 32.Ligtenberg G, Blankestijn PJ, Oey PL, Klein IH, Dijkhorst-Oei LT, Boomsma F, Wieneke GH, van Huffelen AC, Koomans HA. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N Engl J Med. 1999;340:1321–1328. doi: 10.1056/NEJM199904293401704. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqi L, Oey PL, Blankestijn PJ. Aliskiren reduces sympathetic nerve activity and blood pressure in chronic kidney disease patients. Nephrol Dial Transplant. 2011;26:2930–2934. doi: 10.1093/ndt/gfq857. [DOI] [PubMed] [Google Scholar]
- 34.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 35.Aoki K, Sato K, Kondo S, Pyon CB, Yamamoto M. Increased response of blood pressure to rest and handgrip in subjects with essential hypertension. Jpn Circ J. 1983;47:802–809. doi: 10.1253/jcj.47.802. [DOI] [PubMed] [Google Scholar]
- 36.Mizuno M, Murphy MN, Mitchell JH, Smith SA. Skeletal muscle reflex-mediated changes in sympathetic nerve activity are abnormal in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300:H968–H977. doi: 10.1152/ajpheart.01145.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoberg E, Schuler G, Kunze B, Obermoser AL, Hauer K, Mautner HP, Schlierf G, Kubler W. Silent myocardial ischemia as a potential link between lack of premonitoring symptoms and increased risk of cardiac arrest during physical stress. Am J Cardiol. 1990;65:583–589. doi: 10.1016/0002-9149(90)91034-4. [DOI] [PubMed] [Google Scholar]
- 38.Mittleman MA, Siscovick DS. Physical exertion as a trigger of myocardial infarction and sudden cardiac death. Cardiol Clin. 1996;14:263–270. doi: 10.1016/s0733-8651(05)70279-4. [DOI] [PubMed] [Google Scholar]
- 39.Takimoto E, Champion H, Li M, Belardi D, Ren S, Rodriguez E, Bedja D, Gabrielson K, Wang Y, Kass D. Chronic inhibition of cyclic gmp phosphodiesterase 5a prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 40.Smith SA, Mammen PP, Mitchell JH, Garry MG. Role of the exercise pressor reflex in rats with dilated cardiomyopathy. Circulation. 2003;108:1126–1132. doi: 10.1161/01.CIR.0000084538.40542.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.