Abstract
Background
Terminal QRS complex distortion on admission is a simple and reliable predictor of infarct size in patients with acute myocardial infarction (AMI). It is uncertain, however, whether this reflects reduced myocardial perfusion of the infarct area and a larger area of the myocardium at risk. This study was conducted to investigate whether terminal QRS distortion complex on admission is a reliable predictor of reduced residual flow and a larger area of the myocardium at risk compared to patients who are admitted without a terminal QRS distortion.
Methods
We evaluated the relationship between terminal QRS complex distortion and residual flow to the infarct zone and risk area in 46 anterior AMI patients undergoing primary angioplasty. 99mTc-sestamibi imaging was performed at baseline and 5-9 days after angioplasty. The study population was divided into those with (Group I, n=16) and without (Group II, n=30) terminal QRS complex distortion.
Results
Baseline characteristics were similar between the two groups. The area of the myocardium at risk was higher in Group I (59.9±15.3%) than in Group II (48.6±13.7%, p<0.05; mean+SD) while the nadir measurement of the residual flow was lower in Group I (0.10±0.07) than in Group II (0.16±0.09, p<0.05). Although the final infarct size was significantly higher in Group I (40.8±17.2%) than in Group II (27.1±18.1%, p<0.05), the myocardial salvage index did not differ significantly between the two groups.
Conclusion
Terminal QRS complex distortion seems to be associated with less residual flow to the infarct zone, a larger risk area and greater infarct size in patients with anterior AMI.
Keywords: Myocardial infarction, QRS distortion, Angioplasty
INTRODUCTION
Primary angioplasty is widely used as standard therapy for acute myocardial infarction (AMI), but a significant proportion of these patients does not benefit from this procedure1-3). Diagnostic criteria to identify these patients could lead to the design of more effective therapeutic regimens. Terminal QRS complex distortion on the admission electrocardiogram has been reported to reflect profound myocardial ischemia, larger infarcts and generally worse prognoses compared to those without terminal QRS complex distortion in AMI patients who are given thrombolytic therapy4-8). Patients with terminal QRS complex distortion who have larger infarcts would therefore benefit less from primary angioplasty9, 10). Residual flow to the infarct zone attenuates the severity of myocardial ischemia during AMI11, 12) and may reduce distortion of the terminal portion of the QRS complex. It is not yet known, however, whether terminal QRS complex distortion reflects reduced myocardial perfusion of the infarct area and larger risk to the myocardium prior to primary angioplasty13). We, therefore, have tested the hypothesis that terminal QRS complex distortion on the admission electrocardiogram is related to reduced residual flow to the infarct zone, a larger risk area and greater infarct size in anterior AMI patients undergoing primary angioplasty.
MATERIALS AND METHODS
Study patients
The study population consisted of 46 consecutive patients with anterior AMI who were treated with primary angioplasty at our institution. The inclusion criteria were typically chest pain lasting more than 30 min, presentation within 12 h of the onset of symptoms, and ST-segment elevation ≥ 0.1 mV in ≥ 2 limb leads or ≥ 0.2 mV in ≥ 2 consecutive precordial leads. Patients previously administered thrombolytic agents for index infarction, and those with a prior myocardial infarction or bypass surgery, cardiogenic shock, chronic left bundle branch block, ventricular rhythm or negative T waves in the leads with ST elevation, were excluded from the study. Written informed consent was obtained from each patient, in accordance with the rules of the Institutional Ethics Committee, which approved the study.
Primary angioplasty
Right and left coronary angiograms were obtained in all patients, and primary angioplasty was performed with the goal of ≤30% residual diameter stenosis Stents were deployed using the standard technique. Heparin was administered as an initial bolus of 10,000 U during the procedure, followed by continuous infusion for 3 days. All patients were treated with aspirin, and all patients treated with stents were administered ticlopidine.
Radionuclide studies
Immediately prior to coronary angiography, each patient was intravenously injected with 740 MBq of 99mTc-sestamibi. Imaging acquisition occurred within 6 hours of tracer injection. A follow-up 99mTc-sestamibi study was performed 5~9 days after primary angioplasty. For tomographic imaging studies, we obtained 64 projections of 20 sec each over 180°, using a dual head rotating gamma camera. Images were reconstructed using standard back projection algorithms.
Definitions and analysis
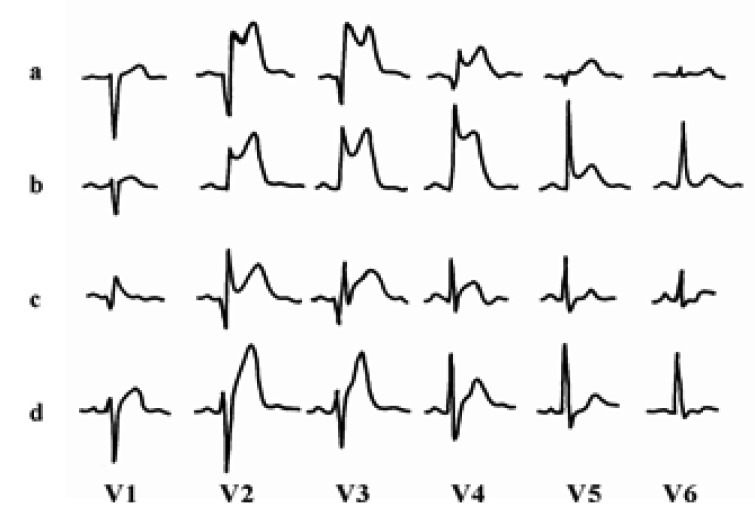
Scintigraphic results were analyzed by an experienced physician (D.H.M.) who was blinded to the clinical data. Short axis slices of the left ventricle were normalized to the peak counts in the heart. Circumferential count profiles were generated for 5 short axis slices from apex to base. Risk area (perfusion defect size on the acute study) and final infarct size (perfusion defect size on the follow-up study) were determined with a threshold of 60% of maximal counts and expressed as a percentage of the left ventricle12, 14). Nadir (residual flow) was estimated from the initial image by the lowest ratio of minimal to maximal counts. The myocardial salvage index was quantified as the change in defect size adjusted for the risk area and calculated as (risk area - final infarct size)/risk area. Distortion of the terminal portion of the QRS complex was defined as the presence of the following in ≥ 2 adjacent leads (Figure 1)4-8) 1) emergence of the J point at ≥ 50% of the R-wave amplitude in leads with initial QR configuration; or 2) absence of S waves in leads without Q waves (Rs configuration).
Figure 1.
Admission electrocardiograms of four patients with anterior wall acute myocardial infarction (paper speed 25 mm/s, 1 mV=10 mm): QRS distortion (+) pattern (a, b) and QRS distortion (-) pattern (c, d).
Statistics
All data are expressed as mean ± SD for continuous variables, and as frequencies for categorical variables. Continuous variables were compared by an unpaired Student's t-test and categoricalvariables by a chi-square test. A probability of p<0.05 was considered to be statistically significant.
RESULTS
The study population was divided into patients with (Group I, n=16) and without (Group II, n=30) terminal QRS complex distortion. We observed few significant differences in baseline characteristics between the two groups (Table 1). However, preinfarct angina, defined as antecedent angina within 24 h prior to the onset of AMI, was present in 4/16 patients (25%) in Group I and 18/30 patients (60%) in Group II (p<0.05). In addition, patients in Group I had significantly higher mean peak levels of serum creatine kinase (4,500±1,430 U/L) than patients in Group II (2,900±1,840 U/L, p<0.01).
Table 1.
Baseline Characteristics
LAD, left anterior descending coronary artery; *p<0.05
The procedural success rate (≤30% diameter stenosis and final TIMI flow ≥ grade 2) was 93.8% in Group I and 100% in Group II. Angiographic characteristics did not differ significantly between the two groups (Table 2). Final TIMI 3 flow tended to be less common in Group I than in Group II, but this difference did not achieve statistical significance (p=0.253). No patients experienced re-infarction during the study period.
Table 2.
Angiographic Characteristics
Radionuclide assays showed that the myocardium risk area for patients in Group I (59.9±15.3%) was significantly greater than in Group II (48.6±13.7%, p<0.05), and the nadir measurement of residual flow was significantly lower in Group I (0.10±0.07) than in Group II (0.16±0.09, p<0.05). We also found that the final infarct size was significantly larger in Group I (40.8±17.2%) than in Group II (27.1±18.1%, p<0.05), but the myocardial salvage index did not differ significantly between the two groups (0.28±0.19 for Group I, 0.43±0.31 for Group II, p=0.087)(Table 3).
Table 3.
Radionuclide Results
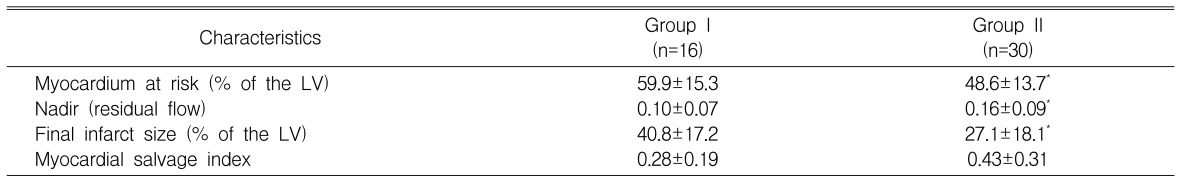
LV, left ventricle; *p<0.05
DISCUSSION
We have shown here that distortion of the terminal portion of the QRS complex on the admission electrocardiogram is associated with reduced residual flow to the infarct zone, a larger risk area and a greater infarct size in patients with anterior AMI treated with primary angioplasty. These findings suggest that residual flow to the infarct area may play a crucial role in preventing terminal QRS complex distortion.
Clinical outcome after AMI is largely determined by infarct size, thus the primary goal of treating patients with AMI should be to reduce infarct size. Final infarct size depends on the duration of ischemia, residual flow to the infarct zone and the size of myocardial area at risk. It is difficult, however, to estimate myocardial risk areas and to predict final infarct size in AMI patients undergoing primary angioplasty. It has been shown that, in AMI patients on thrombolytic therapy, the terminal QRS complex distortion on the admission electrocardiogram is associated with greater mortality rates, larger infarct sizes and greater prevalence of left ventricular dysfunction4-8), thus suggesting that an admission electrocardiogram may provide information about infarct size and clinical outcome in AMI patients undergoing reperfusion therapy. Factors that determine terminal QRS complex distortion in these patients, however, are still poorly understood.
During myocardial ischemia, the terminal QRS complex reflects the activation wave in the local Purkinje fibers, which is less sensitive to ischemia than the contracting myocytes15-17). Thus, profound myocardial ischemia would have to occur to alter the terminal QRS complex sufficiently to affect the Purkinje fibers. Residual flow to the infarct zone, either through collateral vessels or by antegrade flow, has been shown to be a powerful predictor of infarct size11, 12). Therefore, it seems likely that patients with terminal QRS complex distortion have less residual flow to the infarct zone and greater myocardium at risk during AMI. Preinfarction angina seems to limit the size of the infarct resulting from a subsequent coronary occlusion. Interestingly, we found that terminal QRS complex distortion was less common, and final infarct size (26.6±19.7 vs. 38.3±16.5%, p<0.05) was smaller, in patients with preinfarct angina. These results are in agreement with those in previous reports, showing that the presence of preinfarct angina is associated with a better in-hospital outcome, a reduced incidence of heart failure and smaller infarct size in AMI patients treated with reperfusion therapy18, 19).
There are several limitations to this study. First, it represents the experience of a single medical center with a relatively small number of patients, and thus may not be entirely representative of the general population. In addition, this was a selected group of patients, those with hemodynamic stability, because initial 99mTc-sestamibi imaging could be obtained only from these patients.
CONCLUSIONS
The present study showed that terminal QRS complex distortion was associated with less residual flow to the infarct zone, a larger risk area and greater infarct size in patients with anterior AMI.
ACKNOWLEDGEMENT
This study was supported by a grant from the Cardio-Vascular Research Foundation, Seoul, Korea.
References
- 1.van 't Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Grines CL, Browne KF, Marco J, Rothbaum D, O'Keefe J, Hartzler GO, Overlie P, Donohue B, Chelliah N, Timmis GC, Vlietstra R, Strzelecki M, Puchrowicz-Ochocki S, O'Neill WW. Predictors of in-hospital and 6-month outcome after acute myocardial infarction in the reperfusion era. J Am Coll Cardiol. 1995;25:370–377. doi: 10.1016/0735-1097(94)00367-y. [DOI] [PubMed] [Google Scholar]
- 3.Michaels AD, Gibson CM, Barron HV. Microvascular dysfunction in acute myocardial infarction: focus on the roles of platelet and inflammatory mediators in the no-reflow phenomenon. Am J Cardiol. 2000;85:50B–60B. doi: 10.1016/s0002-9149(00)00811-0. [DOI] [PubMed] [Google Scholar]
- 4.Holland RP, Brooks H. The QRS complex during myocardial ischemia: an experimental analysis in the porcine heart. J Clin Invest. 1976;57:541–550. doi: 10.1172/JCI108309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnbaum Y, Sclarovsky S, Blum A, Mager A, Gabbay U. Prognostic significance of the initial electrocardiographic pattern in a first acute anterior wall myocardial infarction. Chest. 1993;103:1681–1687. doi: 10.1378/chest.103.6.1681. [DOI] [PubMed] [Google Scholar]
- 6.Birnbaum Y, Herz I, Sclarovsky S, Zlotikamien B, Chetrit A, Olmer L, Barbash GI. Prognostic significance of the admission electrocardiogram in acute myocardial infarction. J Am Coll Cardiol. 1996;27:1128–1132. doi: 10.1016/0735-1097(96)00003-4. [DOI] [PubMed] [Google Scholar]
- 7.Birnbaum Y, Maynard C, Wolfe S, Mager A, Strasberg B, Rechavia E, Gates K, Wagner GS. Terminal QRS distortion on admission is better than ST-segment measurements in predicting final infarct size and assessing the potential effect of thrombolytic therapy in anterior wall acute myocardial infarction. Am J Cardiol. 1999;84:530–534. doi: 10.1016/s0002-9149(99)00372-0. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum Y, Kloner RA, Sclarovsky S, Cannon CP, McCabe CH, Davis VG, Zaret BL, Wachers FJ, Braunwald E. Distortion of the terminal portion of the QRS on the admission electrocardiogram in acute myocardial infarction and correlation with infarct size and long-term prognosis (Thrombolysis In Myocardial Infarction 4 Trial) Am J Cardiol. 1996;78:396–403. doi: 10.1016/s0002-9149(96)00326-8. [DOI] [PubMed] [Google Scholar]
- 9.Mager A, Sclarovsky S, Herz I, Zlotikamien B, Strasberg B, Birnbaum Y. QRS complex distortion predicts no reflow after emergency angioplasty in patients with anterior wall myocardial infarction. Coron Artery Dis. 1998;9:199–205. doi: 10.1097/00019501-199809040-00005. [DOI] [PubMed] [Google Scholar]
- 10.Lee CW, Hong MK, Yang HS, Choi SW, Kim JJ, Park SW, Park SJ. Determinants and prognostic implications of terminal QRS complex distortion in patients treated with primary angioplasty for acute myocardial infarction. Am J Cardiol. 2001;88:210–213. doi: 10.1016/s0002-9149(01)01627-7. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons RJ, Miller TD, Christian TF. Infarct size measured by single photon emission computed tomographic imaging With 99mTc-sestamibi: a measure of the efficacy of therapy in acute myocardial infarction. Circulation. 2000;101:101–108. doi: 10.1161/01.cir.101.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Milavetz JJ, Giebel DW, Christian TF, Schwartz RS, Holmes DR, Jr, Gibbons RJ. Time to therapy and salvage in myocardial infarction. J Am Coll Cardiol. 1998;31:1246–1251. doi: 10.1016/s0735-1097(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum Y, Mahaffey KW, Criger DA, Gates KB, Barbash GI, Barbagelata A, Clemmensen P, Sgarbossa EB, Gibbons RJ, Rahman MA, Califf RM, Granger CB, Wagner GS. Grade III ischemia on presentation with acute myocardial infarction predicts rapid progression of necrosis and less myocardial salvage with thrombolysis. Cardiology. 2002;97:166–174. doi: 10.1159/000063334. [DOI] [PubMed] [Google Scholar]
- 14.Schömig A, Kastrati A, Dirschinger J, Mehilli J, Schricke U, Pache J, Martinoff S, Neumann FJ, Schwaiger M. Coronary stenting plus platelet glycoprotein IIb/IIIa blockade compared with tissue plasminogen activator in acute myocardial infarction. N Engl J Med. 2000;343:385–391. doi: 10.1056/NEJM200008103430602. [DOI] [PubMed] [Google Scholar]
- 15.David D, Naito M, Michelson E, Watanabe Y, Chen CC, Morganroth J, Shaffenburg M, Blenko T. Intramyocardial conduction: a major determinant of R-wave amplitude during acute myocardial ischemia. Circulation. 1982;65:161–167. doi: 10.1161/01.cir.65.1.161. [DOI] [PubMed] [Google Scholar]
- 16.Spekhorst H, Sippens-Groenewegen A, David GK, Jase MJ, Dunning AJ. Body surface mapping during percutaneous transluminal coronary angioplasty: QRS changes indicating regional myocardial conduction delay. Circulation. 1990;81:840–849. doi: 10.1161/01.cir.81.3.840. [DOI] [PubMed] [Google Scholar]
- 17.DeHaan RL. Differentiation of the atrioventricular conduction system of the heart. Circulation. 1961;24:458–470. doi: 10.1161/01.cir.24.2.458. [DOI] [PubMed] [Google Scholar]
- 18.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001;104:3158–3167. doi: 10.1161/hc5001.100039. [DOI] [PubMed] [Google Scholar]
- 19.Yellon DM, Dana A. The preconditioning phenomenon: a tool for the scientist or a clinical reality? Circ Res. 2000;87:543–550. doi: 10.1161/01.res.87.7.543. [DOI] [PubMed] [Google Scholar]