Abstract
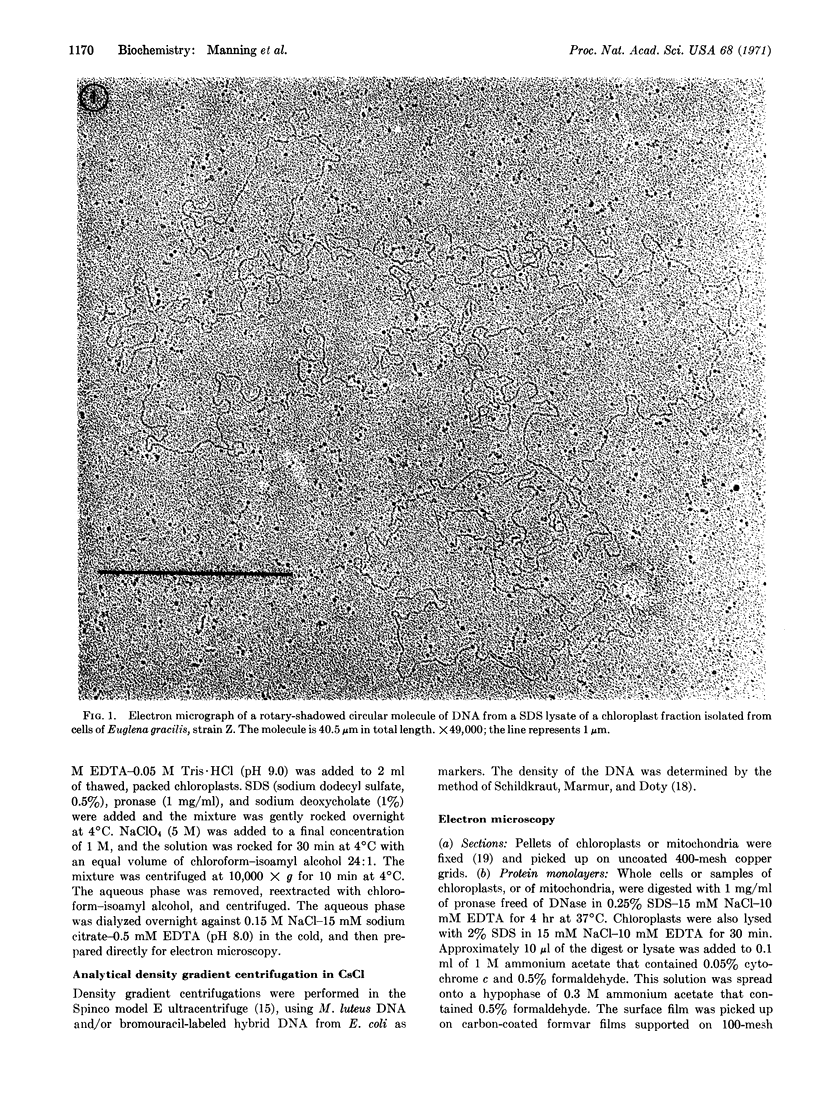
Chloroplast DNA of the protozoan flagellate, Euglena gracilis, exists as circular molecules, 40 μm in contour length, as shown by electron microscopy and buoyant density analyses.
Keywords: electron microscopy, density gradient centrifugation, mitochondrial DNA
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAWERMAN G., EISENSTADT J. M. DEOXYRIBONUCLEIC ACID FROM THE CHLOROPLASTS OF EUGLENA GRACILIS. Biochim Biophys Acta. 1964 Nov 15;91:477–485. doi: 10.1016/0926-6550(64)90077-5. [DOI] [PubMed] [Google Scholar]
- CARO L. G. THE MOLECULAR WEIGHT OF LAMBDA DNA. Virology. 1965 Feb;25:226–236. doi: 10.1016/0042-6822(65)90201-1. [DOI] [PubMed] [Google Scholar]
- EDELMAN M., SCHIFF J. A., EPSTEIN H. T. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA. XII. TWO TYPES OF SATELLITE DNA. J Mol Biol. 1965 Apr;11:769–774. doi: 10.1016/s0022-2836(65)80034-1. [DOI] [PubMed] [Google Scholar]
- Edelman M., Cowan C. A., Epstein H. T., Schiff J. A. STUDIES OF CHLOROPLAST DEVELOPMENT IN EUGLENA, VIII. CHLOROPLAST-ASSOCIATED DNA. Proc Natl Acad Sci U S A. 1964 Nov;52(5):1214–1219. doi: 10.1073/pnas.52.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D., Kleinschmidt A. K. Single-strand breaks in duplex DNA of coliphage T7 as demonstrated by electron microscopy. J Mol Biol. 1965 Nov;14(1):271–278. doi: 10.1016/s0022-2836(65)80246-7. [DOI] [PubMed] [Google Scholar]
- Green B. R., Burton H. Acetabularia chloroplast DNA: electron microscopic visualization. Science. 1970 May 22;168(3934):981–982. doi: 10.1126/science.168.3934.981. [DOI] [PubMed] [Google Scholar]
- Inman R. B. Some factors affecting electron microscopic length of deoxyribonucleic acid. J Mol Biol. 1967 Apr 28;25(2):209–216. doi: 10.1016/0022-2836(67)90138-6. [DOI] [PubMed] [Google Scholar]
- KISLEV N., SWIFT H., BOGORAD L. NUCLEIC ACIDS OF CHLOROPLASTS AND MITOCHONDRIA IN SWISS CHARD. J Cell Biol. 1965 May;25:327–344. doi: 10.1083/jcb.25.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawiec S., Eisenstadt J. M. Ribonucleic acids from the mitochondria of bleached Euglena gracilis Z. I. Isolation of mitochondria and extraction of nucleic acids. Biochim Biophys Acta. 1970 Sep 17;217(1):120–131. doi: 10.1016/0005-2787(70)90128-0. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- MACHATTIE L. A., THOMAS C. A., Jr DNA FROM BACTERIOPHAGE LAMBDA: MOLECULAR LENGTH AND CONFORMATION. Science. 1964 May 29;144(3622):1142–1144. doi: 10.1126/science.144.3622.1142. [DOI] [PubMed] [Google Scholar]
- RAY D. S., HANAWALT P. C. PROPERTIES OF THE SATELLITE DNA ASSOCIATED WITH THE CHLOROPLASTS OF EUGLENA GRACILIS. J Mol Biol. 1964 Sep;9:812–824. doi: 10.1016/s0022-2836(64)80187-x. [DOI] [PubMed] [Google Scholar]
- RAY D. S., HANAWALT P. C. SATELLITE DNA COMPONENTS IN EUGLENA GRACILIS CELLS LACKING CHLOROPLASTS. J Mol Biol. 1965 Apr;11:760–768. doi: 10.1016/s0022-2836(65)80033-x. [DOI] [PubMed] [Google Scholar]
- RIS H., PLAUT W. Ultrastructure of DNA-containing areas in the chloroplast of Chlamydomonas. J Cell Biol. 1962 Jun;13:383–391. doi: 10.1083/jcb.13.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGER R., ISHIDA M. R. CHLOROPLAST DNA IN CHLAMYDOMONAS. Proc Natl Acad Sci U S A. 1963 Oct;50:725–730. doi: 10.1073/pnas.50.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sharpless T. K., Butow R. A. Phosphorylation sites, cytochrome complement, and alternate pathways of coupled electron transport in Euglena gracilis mitochondria. J Biol Chem. 1970 Jan 10;245(1):50–57. [PubMed] [Google Scholar]
- Stutz E. The kinetic complexity of Euglena gracilis chloroplasts DNA. FEBS Lett. 1970 May 11;8(1):25–28. doi: 10.1016/0014-5793(70)80216-2. [DOI] [PubMed] [Google Scholar]
- Suyama Y., Miura K. Size and structural variations of mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):235–242. doi: 10.1073/pnas.60.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R., Birnstiel M. Kinetic complexity of chloroplastal deoxyribonucleic acid and mitochondrial deoxyribonucleic acid from higher plants. Biochem J. 1969 May;112(5):777–786. doi: 10.1042/bj1120777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz G., Kellner G. Molecular characteristics of chloroplast DNA of acetabularia cells. J Ultrastruct Res. 1968 Jul;24(1):109–115. doi: 10.1016/s0022-5320(68)80020-6. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Gross N. J. The form and size of mitochondrial DNA of the red bean, Phaseolus vulgaris. Proc Natl Acad Sci U S A. 1968 Sep;61(1):245–252. doi: 10.1073/pnas.61.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. D., Luck D. J. Hybridization of mitochondrial ribosomal RNA. J Mol Biol. 1969 Apr;41(2):211–224. doi: 10.1016/0022-2836(69)90386-6. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L., Bogorad L. Evidence for variation in the quantity of DNA among plastids of Acetabularia. J Cell Biol. 1970 Feb;44(2):361–375. doi: 10.1083/jcb.44.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock C. L., Fernández-Morán H. Electron microscopy of DNA conformations in spinach chloroplasts. J Mol Biol. 1968 Feb 14;31(3):627–631. doi: 10.1016/0022-2836(68)90435-x. [DOI] [PubMed] [Google Scholar]




