Abstract
The posterior cingulate cortex is a highly connected and metabolically active brain region. Recent studies suggest it has an important cognitive role, although there is no consensus about what this is. The region is typically discussed as having a unitary function because of a common pattern of relative deactivation observed during attentionally demanding tasks. One influential hypothesis is that the posterior cingulate cortex has a central role in supporting internally-directed cognition. It is a key node in the default mode network and shows increased activity when individuals retrieve autobiographical memories or plan for the future, as well as during unconstrained ‘rest’ when activity in the brain is ‘free-wheeling’. However, other evidence suggests that the region is highly heterogeneous and may play a direct role in regulating the focus of attention. In addition, its activity varies with arousal state and its interactions with other brain networks may be important for conscious awareness. Understanding posterior cingulate cortex function is likely to be of clinical importance. It is well protected against ischaemic stroke, and so there is relatively little neuropsychological data about the consequences of focal lesions. However, in other conditions abnormalities in the region are clearly linked to disease. For example, amyloid deposition and reduced metabolism is seen early in Alzheimer’s disease. Functional neuroimaging studies show abnormalities in a range of neurological and psychiatric disorders including Alzheimer’s disease, schizophrenia, autism, depression and attention deficit hyperactivity disorder, as well as ageing. Our own work has consistently shown abnormal posterior cingulate cortex function following traumatic brain injury, which predicts attentional impairments. Here we review the anatomy and physiology of the region and how it is affected in a range of clinical conditions, before discussing its proposed functions. We synthesize key findings into a novel model of the region’s function (the ‘Arousal, Balance and Breadth of Attention’ model). Dorsal and ventral subcomponents are functionally separated and differences in regional activity are explained by considering: (i) arousal state; (ii) whether attention is focused internally or externally; and (iii) the breadth of attentional focus. The predictions of the model can be tested within the framework of complex dynamic systems theory, and we propose that the dorsal posterior cingulate cortex influences attentional focus by ‘tuning’ whole-brain metastability and so adjusts how stable brain network activity is over time.
Keywords: posterior cingulate cortex, attention: functional connectivity, default mode network, metastability
Introduction
The posterior cingulate cortex (PCC) forms part of the posteromedial cortex. It is highly anatomically connected (Hagmann et al., 2008), has a high baseline metabolic rate (Raichle et al., 2001), and is a central part of the default mode network (DMN) (Buckner et al., 2008). Despite its importance in health and disease, the PCC is notably absent from many systems-level models of brain function, and there is no clear consensus about its function (Leech et al., 2012).
One influential hypothesis is that the PCC has a central role in supporting internally directed cognition (Raichle et al., 2001; Buckner et al., 2008). The PCC shows increased activity when individuals retrieve autobiographical memories or plan for the future, as well as during unconstrained ‘rest’ when activity in the brain can be thought of as cognitively ‘free-wheeling’ (Gusnard et al., 2001; Addis et al., 2007; Mason et al., 2007). However, other evidence suggests that the PCC plays a more direct role in regulating the focus of attention (Gusnard and Raichle, 2001; Hampson et al., 2006; Hahn et al., 2007), perhaps controlling the balance between internally and externally focused thought (Leech et al., 2011). In addition, activity in the PCC varies with arousal state, and its interactions with other brain networks may be important for conscious awareness (Vogt and Laureys, 2005).
An important consideration is the degree to which the PCC is functionally homogeneous. Although it covers a relatively large area of cortex it is often discussed as having a unitary function. Functional MRI studies have repeatedly shown relative deactivation during many types of cognitively demanding tasks, such as a visual discrimination task (Singh and Fawcett, 2008). However, recent work suggests that focusing on overall changes in relative blood flow can be misleading as the region shows a complex functional organization (Vogt et al., 2006; Margulies et al., 2009; Dastjerdi et al., 2011; Leech et al., 2011). For example, we have recently found evidence for ‘echoes’ or traces of the activity of multiple functionally discreet large-scale brain networks within the PCC (Leech et al., 2012).
The PCC also shows abnormal structure and function in many diseases (Zhang and Raichle, 2010). For example, early amyloid deposition and reduced metabolism is seen in Alzheimer’s disease (Johnson et al., 1998; Greicius et al., 2004; Buckner et al., 2005). Functional neuroimaging studies also show abnormalities in a range of neurological and psychiatric disorders including schizophrenia, autism, depression and attention deficit hyperactivity disorder (ADHD), as well as ageing (Buckner et al., 2008; Greicius, 2008). Our own work has consistently shown abnormal PCC function following traumatic brain injury that relates to the pattern of cognitive impairment (Bonnelle et al., 2011, 2012; Sharp et al., 2011). These results suggest that an accurate description of the function of the PCC will be important to the understanding of a wide range of diseases.
Here we review the anatomy and physiology of the PCC, including its atypical structural connectivity and elevated metabolism, before discussing its possible functions. We go on to discuss the impact of disease on the PCC, discussing this in the context of current theories of brain network function. We then synthesize key findings and propose a novel model of PCC function. We distinguish dorsal and ventral parts of the PCC and propose that the function of these subcomponents can be explained by considering: (i) arousal state; (ii) whether attention is focused internally or externally; and (iii) the breadth of attentional focus (ABBA: Arousal, Balance and Breadth of Attention model). We then outline a mechanistic explanation of elements of the model that uses a complex dynamic systems approach to studying brain function. We propose that the dorsal PCC plays a role in tuning the metastability of intrinsic connectivity networks, which allows control of how variable neural activity is across time in these networks, and so influences attentional focus.
Anatomy
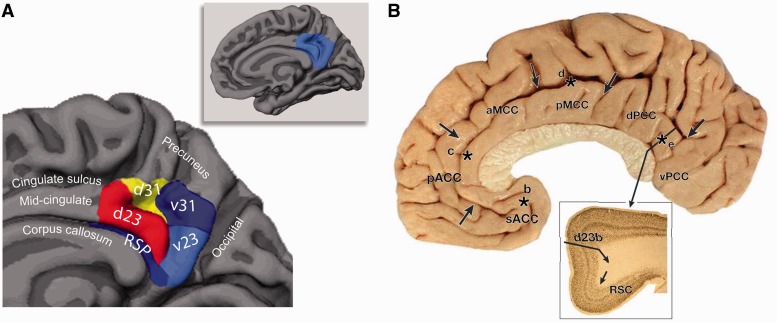
The PCC is situated in the medial part of the inferior parietal lobe and lies within the posteromedial cortex, which also includes the precuneus and retrosplenial cortex (Fig. 1) (Parvizi et al., 2006). Papez (1937) thought of the PCC as a single functional entity, forming part of a limbic system specialized for emotional processing. However, Brodmann (1909) had already distinguished between anterior and posterior cingulate divisions on the basis of cytoarchitectonics. More recently Vogt (1993, 2009) has proposed a further major subdivision: the midcingulate region, containing a motor field with direct corticospinal projections. Here, we employ Vogt’s model, in which the PCC consists of Brodmann areas 23 and 31. It is bounded superiorly by the marginal ramus of the cingulate sulcus, inferiorly by the corpus callosum, posteriorly by the parieto-occipital sulcus and anteriorly by Brodmann area 24 in the midcingulate region. The precuneus (area 7 m) lies posterior and superior to the PCC, and the retrosplenial cortex (Brodmann areas 29 and 30) adjacent, along the ventral bank of the cingulate sulcus. The retrosplenial cortex and the PCC together form the retrosplenial gyrus.
Figure 1.
PCC anatomy. (A) An illustration of the approximate locations of PCC subdivisions based on cytoarchitectonics, with associated Brodmann labels. This is based on Vogt et al. (2009). Area v23 and the posterior portion of area 31 correspond approximately to ventral PCC (labelled v23 and v31, in cold colours). Area d23 and the anterior portion of area 31 correspond to the dorsal PCC (labelled d23 and d31, in warm colours). RSP = retrosplenial cortex. (B) An anatomical midline section, highlighting different cingulate cortical regions: the anterior cingulate (ACC), mid-cingulate (MCC) and PCC. Inset: A section through the dorsal posterior cingulate cortex (adapted from Vogt, 2005; reproduced with permission).
Cytoarchitectonically, the PCC is characterized as paralimbic cortex, exhibiting a transitional cell architecture between typical six layered isocortex and the primitive allocortex of the core limbic structures such as the hippocampus (Mesulam, 1998). Together with the parahippocampal gyrus and retrosplenial cortex, Mesulam (1998) viewed the PCC as part of the hippocampocentric subdivision of the paralimbic zone. Subregions of the PCC have distinct cytoarchitectonics, with an anterior and dorsal subregion (dorsal PCC) situated superior to the splenium of the corpus callosum (areas d23a, d23b, 23d, anterior 31) and a ventral part (ventral PCC) posterior to the splenium (v23a and v23b, posterior 31) (Vogt et al., 2006) (Fig. 1).
Structural connections in non-human primates
Tract-tracing studies have accurately delineated the connections of the PCC in the non-human primate (Vogt and Pandya, 1987; Vogt et al., 1987; Kobayashi and Amaral, 2007). The PCC is reciprocally connected to other parts of the posteromedial cortex, with the densest connections to neurons within the same cytoarchitectonic region (Parvizi et al., 2006). Outside the posteromedial cortex, the PCC and adjacent retrosplenial cortex are highly connected to other paralimbic and limbic structures. Reciprocal connections to the medial temporal lobe are seen particularly from the ventral PCC and adjacent retrosplenial cortex (Kobayashi and Amaral, 2007). Both regions show dense connections to the hippocampal formation and parahippocampal cortex (Kobayashi and Amaral, 2007). In contrast, these connections are much less prominent for the dorsal PCC. Similarly, strong connections are present from the ventral PCC and retrosplenial cortex to the ventromedial prefrontal cortex, including subgenual parts of the anterior cingulate cortex (Vogt and Pandya, 1987; Parvizi et al., 2006). These connections are either absent or much less prominent for the dorsal PCC. Unlike the rodent, the general anatomical organization of the PCC is broadly preserved between non-human primates and humans (Vogt et al., 1995, 2001).
The PCC (both ventral and dorsal) also shows prominent connections to areas of heteromodal association cortex in the frontal, temporal and parietal lobes. Strong reciprocal connections are seen to the dorsolateral prefrontal cortex (Brodmann area 46) and the frontal poles (Brodmann areas 10/11), as well as less prominent connections to Brodmann areas 9/46, 8 and 9 (Parvizi et al., 2006). Both the dorsal PCC and ventral PCC also show connections to dorsal parts of the anterior cingulate cortex (24 a and b). In addition, the PCC projects to other heteromodal regions outside the frontal lobe, with prominent projections to the macaque homologue of the inferior parietal cortex (area PE) and posterior regions of the superior temporal sulcus (area PO) (Parvizi et al., 2006).
Dense connections also exist from the PCC to subcortical regions, including the thalamus and striatum (Vogt et al., 1987; Yeterian and Pandya, 1988; Kunishio and Haber, 1994; Romanski et al., 1997; Parvizi et al., 2006). Projections to the thalamus show an unusual organization, with corticothalamic fibres from the PCC innervating a continuous strip from the front to the back of the dorsal thalamus. These tracts cross the anterior dorsal, anterior ventral, anterior medial, superficial lateral dorsal, ventral lateral, ventral anterior, lateral posterior and lateral pulvinar nuclei (Parvizi et al., 2006). The reverse thalamocortical projections to the PCC are organized differently, although the structure still receives input from a large number of thalamic nuclei (Yeterian and Pandya, 1988). In line with other parts of the posteromedial cortex, the PCC shows no evidence of connections to primary sensory or primary motor areas (Parvizi et al., 2006), an observation that serves to emphasize that the region occupies a position remote from low-level sensory or motor processing.
Structural connections in humans
Structural connectivity of the PCC in humans is less well established than in non-human primates; however, recent advances in MRI have allowed the white matter connectivity of the PCC to be studied in vivo in humans (Wakana et al., 2004; Concha et al., 2005; Hagmann et al., 2008; Greicius et al., 2009). Techniques such as diffusion tensor imaging and diffusion spectrum imaging can be used to map white matter connections. For example, diffusion tensor imaging tractography confirms the presence of structural connections from the retrosplenial cortex and ventral PCC to the medial temporal lobes, as well as connections from the more dorsal PCC to the ventromedial prefrontal cortex along the cingulum bundle (Greicius et al., 2009). These techniques have also been used to provide a more comprehensive description of the structural connectivity of the whole brain (Hagmann et al., 2008). Graph-theoretic analyses of the structural connectivity show how highly connected the PCC is, relative to other brain regions, providing evidence for a role as a hub for information processing (Hagmann et al., 2008).
Functional neuroimaging and the posterior cingulate cortex
Metabolism
One of the most striking physiological features of the PCC is its high rate of metabolism. In the human, cerebral blood flow and metabolic rate are ∼40% greater than average within the PCC and adjacent precuneus (Raichle et al., 2001). This may in part relate to the region’s interactions with the thalamus (Van Groen et al., 1993; Vogt and Laureys, 2005). The metabolism of the PCC is responsive to cognitive state (e.g. whether a demanding cognitive task such as making a perceptual decision and motor response is required). However, fluctuations in perfusion produced by changes in cognitive state are relatively small compared to the high levels of baseline activity (Raichle et al., 2001). Cognitive exertion only leads to a fall in PCC perfusion of ∼6% (Pfefferbaum et al., 2011). Therefore, even when a task results in a relative fall in PCC activity, the region still shows consistently higher levels of blood flow compared with almost all other brain regions (Pfefferbaum et al., 2011).
Functional connectivity of the posterior cingulate cortex
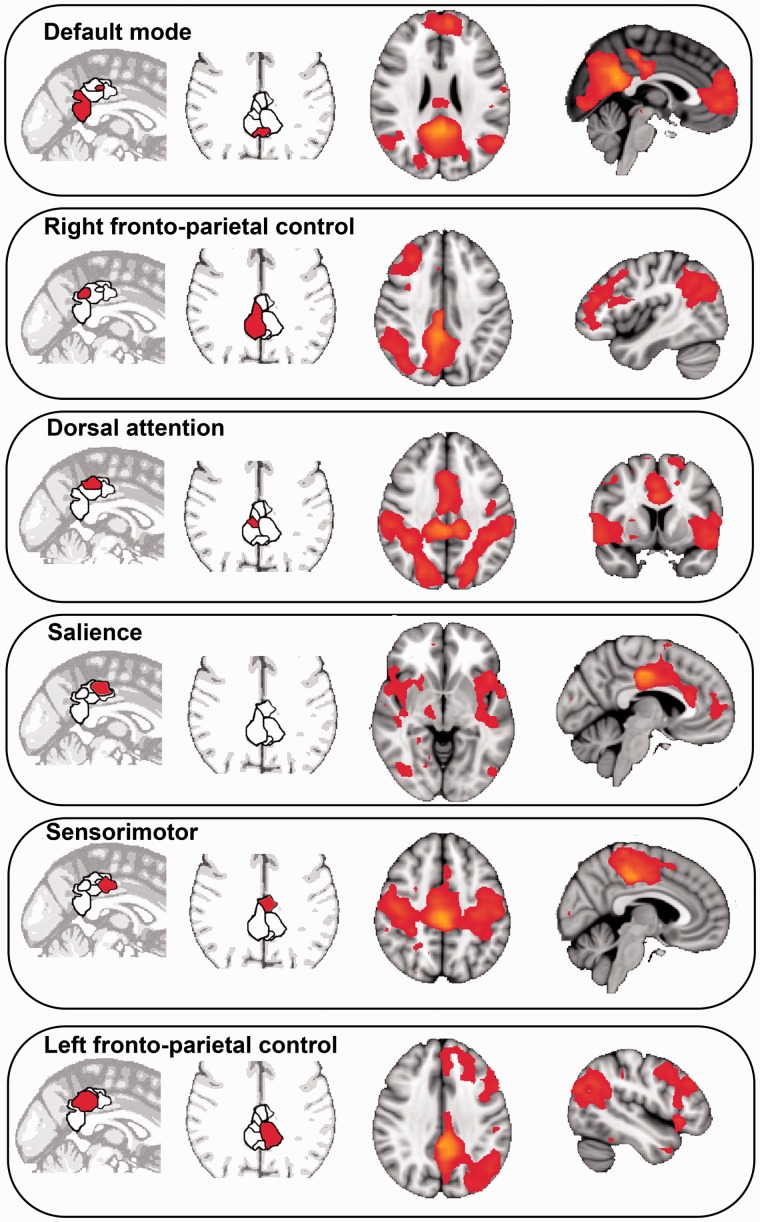
The structural connectivity of the PCC is closely related to its functional connectivity with other brain regions, which can be measured with functional MRI (Hagmann et al., 2008; Margulies et al., 2009; Leech et al., 2011). Functional connectivity can be used to define intrinsic connectivity networks, which consist of networks of brain regions that share common spatio-temporal patterns of activity and that are observed across different populations and across a range of tasks (Fig. 2) (Beckmann et al., 2005; Smith et al., 2009). In keeping with its extensive structural connectivity, the PCC shows a complex pattern of functional connectivity (Vincent et al., 2006; Margulies et al., 2009; Leech et al., 2012), which provides evidence for its interaction with distinct intrinsic connectivity networks across the brain (Leech et al., 2012).
Figure 2.
The functional connectivity of the PCC. The dorsal PCC sits at the nexus of multiple intrinsic connectivity networks. Activity ‘echoes’ intrinsic connectivity networks in the rest of the brain. Sagittal and axial slices showing distinct but spatially overlapping regions in the PCC, defined in a data-driven way by their distinct activity pattern. These regions show functional connectivity with the networks illustrated in the sagittal and axial slices on the right of the panel. The networks are labelled in relation to their resemblance to well-defined intrinsic connectivity networks. Adapted from Leech et al. (2011, 2012).
The default mode network and interactions with other intrinsic connectivity networks
The dominant functional characterization of the PCC arises out of its central role within the DMN (Greicius et al., 2009; Margulies et al., 2009; Leech et al., 2011). In addition to the PCC, the main nodes in the DMN are the ventromedial prefrontal cortex, lateral inferior parietal lobes and medial temporal structures (Raichle et al., 2001). The DMN shows highly correlated activity at ‘rest’, as well as rapid and reactive deactivation during most tasks where attention is directed externally (Shulman et al., 1997; Gusnard et al., 2001; Raichle et al., 2001; Buckner et al., 2008). As many experimental tasks involve an external focus of attention, this has led to the DMN being labelled as a ‘task-negative’ network (Fox et al., 2005). However, this is misleading as increased DMN activity is observed in many situations where attention is internally directed, such as episodic memory retrieval, planning for the future, and daydreaming (Spreng, 2012). In the healthy brain, the magnitude of reactive changes in PCC activity is related to cognitive load (Singh and Fawcett, 2008), and a failure of appropriate deactivation is associated with inefficient cognitive function in both the healthy and damaged brain (e.g. Weissman et al., 2006; Sonuga-Barke and Castellanos, 2007; Bonnelle et al., 2011, 2012; Crone et al., 2011). Therefore, dynamic control of DMN activity appears important for efficient cognitive function.
The dorsal PCC shows strong connectivity with the DMN, but also to a wide range of other intrinsic connectivity networks, including frontal and parietal regions involved in cognitive control (Vincent et al., 2006; Margulies et al., 2009; Leech et al., 2011). In contrast to the DMN/‘task-negative network’, this fronto-parietal network has been termed a ‘task-positive network’, as a result of the activation of these regions in many experimental situations (Fox et al., 2005; Kelly et al., 2008). Again, this term is potentially confusing because parts of the ‘task-positive’ network co-activate with the DMN when attention is directed internally (Gerlach et al., 2011). Large parts of a fronto-parietal network are engaged in the top-down control of visual attention and eye movements. These regions are generally termed the dorsal attentional network (Corbetta et al., 2008) and include the intraparietal sulci, parts of the superior parietal lobules, the temporal lobe MT complex and the frontal eye fields. In addition to the dorsal attention network, a second network involved in executive control, the fronto-parietal control network (FPCN), has been defined (Vincent et al., 2008). This involves parts of the frontal pole and dorsolateral prefrontal cortex, the rostral anterior cingulate cortex/pre-supplementary motor area, the anterior insula and parts of the inferior parietal lobule. Functional connectivity analysis suggests that the FPCN also contains the dorsal PCC (Leech et al., 2011, 2012; Spreng, 2012). Several functional connectivity studies suggest that there are distinct lateralized networks within the FPCN (Damoiseaux et al., 2006) containing lateralized dorsal PCC nodes (Leech et al., 2011, 2012). In addition, the FPCN can be further fractionated, with the rostral anterior cingulate/pre-supplementary motor area and anterior insulae forming a subnetwork involved in rapidly responding to transient behaviourally salient events, which has been termed the ‘salience network’ (Seeley et al., 2007).
Co-ordination between these intrinsic connectivity networks is important for efficient cognitive function, and a major challenge is to understand how these networks dynamically interact. For example, during demanding tasks where attention is directed to external information, activity increases in the dorsal attention network and FPCN, and decreases within the DMN in a tightly coupled way. This produces an anti-correlated pattern over time between the DMN and dorsal attention network/FPCN, and increases in the magnitude of this anti-correlation is associated with improved behavioural responses (Kelly et al., 2008).
Our work has shown that different parts of the PCC show a complex pattern of interaction with other intrinsic connectivity networks (Leech et al., 2011, 2012). The ventral PCC shows increased connectivity with the DMN during internally focused states (such as at rest), whereas the dorsal PCC shows increased functional connectivity within the DMN and increased anti-correlation with the dorsal attention network/FPCN when the external attentional task requirements are high; a result supported by assessment of local field potential in ventral and dorsal PCC regions (Dastjerdi et al., 2011). In a similar vein, we have shown that subregions within the PCC ‘echo’ many well characterized intrinsic connectivity networks (Fig. 2) (Leech et al., 2012). For example, one region within the ventral PCC showed strong functional connectivity with the DMN, whilst part of the dorsal PCC showed selective functional connectivity across a range of networks, including both a right and a left FPCN, parts of the dorsal attention network, a sensori-motor network, and a separate salience network. This result suggests that the dorsal PCC exhibits a ‘transitional’ pattern of connectivity (Vincent et al., 2006; Margulies et al., 2009), linking networks that are functionally distinct but that require co-ordinated changes in activity to allow for efficient cognitive function.
The posterior cingulate cortex and disease
The PCC and DMN show abnormalities in a wide range of neurological and psychiatric disorders (Buckner et al., 2008; Zhang and Raichle, 2010). Therefore, a detailed understanding of the effects of disease on the PCC is likely to be important. Here, we provide a selective review of PCC abnormalities in a range of disease states, focusing mainly on studies that investigate brain networks (summarized in Table 1). We aim to provide examples of the common patterns of abnormality seen within the PCC, and discuss how these relate to network dysfunction, clinical symptoms and disease mechanism.
Table 1.
Patterns of disease-related abnormality in the posterior cingulate cortex
| Condition | Posterior cingulate cortex abnormality (method) | Associated clinical, cognitive and psychiatric impairment | Study |
|---|---|---|---|
| Focal Lesions | PCC, RSC and fornix lesions | Memory impairment | Valenstein et al., 1987; Rudge and Warrington, 1991 |
| Ventral PCC and RSC lesion | Topographic disorientation | Takahashi et al., 1997; Katayama et al., 1999; Alsaadi et al., 2000 | |
| Left PCC and forceps major | Impairment of multi-tasking | Burgess et al., 2000 | |
| Alzheimer’s disease | Reduced metabolism (SPECT and FDG PET) | Minoshima et al., 1997; Johnson et al., 1998; Buckner et al., 2005 | |
| Amyloid deposition (PIB PET) | Buckner et al., 2005 | ||
| Reduced functional connectivity (resting functional MRI) | Greicius et al., 2004 | ||
| Atrophy (T1 MRI) | Buckner et al., 2005, 2009 | ||
| Healthy ageing | Reduced functional connectivity (resting functional MRI) | Information processing speed, executive function, memory | Andrews-Hanna et al., 2007; Damoiseaux et al., 2008 |
| Abnormal task-induced activity (task functional MRI) | Impaired working memory performance | Sambataro et al., 2010; Prakash et al., 2012; Spreng and Schacter, 2012 | |
| Reduced task-dependent PCC deactivation (task functional MRI) | Impaired working memory performance | Prakash et al., 2012 | |
| White matter tract damage in PCC connections (MRI DTI) | Andrews-Hanna et al., 2007 | ||
| Amyloid deposition (PIB PET) associated with reduced functional connectivity (resting functional MRI) | Hedden et al., 2009 | ||
| Traumatic brain injury | Reduced metabolism (FDG PET) | Nakashima et al., 2007 | |
| Reduced perfusion (ASL MRI) | Kim et al., 2010 | ||
| Abnormal functional connectivity (functional MRI) | Correlation with sustained attention impairment | Bonnelle et al., 2011; Hillary et al., 2011; Mayer et al., 2011; Sharp et al., 2011; Stevens et al., 2012 | |
| Reduced task-dependent PCC deactivation (task functional MRI) | Predicted by structural damage to the Salience Network | Bonnelle et al., 2012 | |
| White matter tract damage in PCC connections (MRI DTI) | Correlation with sustained attention impairment | Bonnelle et al., 2011 | |
| Autistic spectrum disorder | Reduced metabolism (FDG PET) | Haznedar et al., 2000 | |
| Altered cytoarchitectonic structure (post-mortem) | Oblak et al., 2011 | ||
| Reduced GABA A receptor density and benzodiazepine binding sites (post-mortem autorad) | Oblak et al., 2011 | ||
| Reduced functional connectivity (resting functional MRI) | Cherkassky et al., 2006 | ||
| Abnormal task-induced activity (task functional MRI) | Cingulate responses correlate with autistic symptoms | Pierce et al., 2004; Chiu et al., 2008 | |
| Reduced task-dependent PCC deactivation (task functional MRI) | Impaired social function | Kennedy et al., 2006 | |
| Schizophrenia | Decreased metabolism (FDG PET) | Haznedar et al., 2004 | |
| Increased thalamic/PCC correlations in metabolic rate (FDG PET) | Mitelman et al., 2005a, b | ||
| Abnormal receptor binding (PM autoradiography/PET) | Correlation of CB1 receptor binding and psychotic symptoms (Wong et al., 2010) | Newell et al., 2005, 2006, 2007; Wong et al., 2010 | |
| Reduced volume (T1 MRI) | Worse in poor outcome (Mitelman et al., 2005a, b) | Sowell et al., 2000; Hulshoff Pol et al., 2001; Mitelman et al., 2005a, b | |
| White matter tract damage in the cingulum (MRI DTI) | Correlation with working memory and attention (Kubicki et al., 2003) | Kubicki et al., 2003, 2005; Samartzis et al., 2013; Sun et al., 2003 | |
| Reduced functional connectivity (resting functional MRI) | Liang et al., 2006 | ||
| Increased functional connectivity (resting functional MRI) | Correlation with working memory performance and psychopathology (Whitfield-Gabrieli et al., 2009) | Zhou et al., 2007; Whitfield-Gabrieli et al., 2009 | |
| Reduced anti-correlations with FPCN regions (resting functional MRI) | Zhou et al., 2007 | ||
| Altered task-dependent PCC deactivation (task functional MRI) | Correlation with working memory performance and psychopathology | Harrison et al., 2007; Whitfield-Gabrieli et al., 2009 | |
| ADHD | Increased volume (T1 MRI) | Meta-analysis of voxel based morphometry studies | Nakao et al., 2011 |
| Reduced functional connectivity (resting functional MRI) | Castellanos et al., 2008; Uddin et al., 2009 | ||
| Reduced anti-correlations with FPCN regions (resting functional MRI) | Castellanos et al., 2008 | ||
| Reduced task-dependent PCC deactivation (task functional MRI) | Deactivation increased by psychostimulant medication and motivation | Peterson et al., 2009; Liddle et al., 2011 | |
| Depression | Increased metabolism (PET) | Ho et al., 1996; Mayberg et al., 1999 | |
| Altered functional connectivity (resting functional MRI) | Zhou et al., 2007; Bluhm et al., 2009; Berman et al., 2011; Zhu et al., 2012 | ||
| Increased metabolism after fluoxetine treatment (PET) | Associated with good response | Mayberg et al., 1999, 2000 | |
| Increased metabolism after DBS to the subgenual cingulate region | Associated with good response | Mayberg et al., 2005; Lozano et al., 2008 |
DBS = deep brain stimulation; FDG = fluorodeoxyglucose; PIB = Pittsburgh Compound B; RSC = retrosplenial cortex; SPECT = single photon emission computed tomography.
Focal damage
There is surprisingly little neuropsychological research about the cognitive consequences of focal lesions on the PCC. Strokes around the posteromedial cortex produce an amnestic syndrome, which may result in part from damage to both the retrosplenial cortex and PCC (Valenstein et al., 1987). However, the damage produced by this mechanism is almost always extensive and usually affects white matter structures known to be involved in memory function, such as the fornix. In addition, other parts of the posteromedial cortex including the retrosplenial cortex are usually damaged, and this region is also strongly linked to memory function. Although the rostral branches of the posterior cerebral artery supply the ventral PCC and the caudal branches of the anterior cerebral artery supply the dorsal PCC, focal ischaemic lesions to these vascular territories have rarely been reported.
Relatively focal damage to the right ventral PCC and retrosplenial cortex has been shown to produce an impairment of spatial cognition that has been termed topographic disorientation. This is thought to be secondary to memory dysfunction rather than to a perceptual problem (Takahashi et al., 1997; Katayama et al., 1999; Alsaadi et al., 2000). For example, Takahashi and colleagues (1997) report three patients with right lateralized damage to the ventral part of the cingulate gyrus. The patients found it difficult to recall spatial routes, despite normal or near normal perceptual function. This appeared to be due to problems recalling the relationships between remote spatial locations. Other forms of damage in the posteromedial cortex also produce memory disturbance, as well as high-level visual perceptual deficits. For example, Rudge and Warrington (1991) have reported a series of patients with tumours of the splenium of the corpus callosum. These produced a marked amnestic syndrome as well as a form of visual agnosia characterized by the inability to integrate information into a single gestalt. As with stroke damage, the effects of these tumours extended well beyond the PCC, and memory impairments could be attributed to fornix damage. PCC damage also disrupts many aspects of multi-tasking, including learning task rules, remembering to implement new rules, and the ability to follow distinct plans in different parts of a task (Burgess et al., 2000).
Neurodegeneration
In contrast to its relatively infrequent focal damage, the PCC is commonly affected by neurodegenerative disease (Buckner et al., 2008). Reduced metabolism in the PCC is an early feature of Alzheimer’s disease, and is often present before a definitive clinical diagnosis (Minoshima et al., 1997; Johnson et al., 1998). This abnormality is usually part of a more distributed pattern of metabolic dysfunction that includes medial temporal lobe structures and the anterior thalamus. These metabolic changes might reflect damage in remote but connected regions (Johnson et al., 1998). For example, hypometabolism of the PCC is seen after remote experimental damage to parts of the rhinal cortex (Meguro et al., 1999).
The metabolic abnormality of the PCC in Alzheimer’s disease is associated with amyloid deposition and brain atrophy in a spatial distribution that strikingly reflects the nodes of the DMN (Buckner et al., 2005). Functional connectivity within the DMN is reduced in early Alzheimer’s disease, a change that particularly affects the connection between the PCC and the hippocampus (Greicius et al., 2004). Altered patterns of PCC functional connectivity also reflect APOE genetic status, which is a risk factor for Alzheimer’s disease. In healthy young people carrying the APOE E4 allele, increased DMN functional connectivity is observed; a change that might reflect increased neural activity required to overcome network inefficiency that is secondary to the effects of this genotype (Filippini et al., 2009). Amyloid deposition is prominently seen in the PCC, and this may be because the high metabolic activity in brain network hubs predisposes to the neuropathological cascade that leads to Alzheimer’s disease (Buckner et al., 2009).
Recently, interest has focused on the way in which neurodegenerative diseases spread ‘prion-like’ through the brain. Proteins such as amyloid-β and TDP-43 that are causatively linked to neurodegeneration have been shown to spread trans-synaptically in their abnormal form (Frost and Diamond, 2010). This provides a potential explanation for the spatial distribution of Alzheimer’s disease pathology within the DMN, as the transmission of abnormal protein would be constrained by the organization of white matter connections. In general, there is a striking similarity between patterns of atrophy seen in different neurodegenerative diseases, and the structure and connectivity patterns seen in healthy intrinsic connectivity networks (Seeley et al., 2009). In the case of Alzheimer’s disease, patterns of atrophy are predicted by the topology of white matter connectivity (Raj et al., 2012), providing an explanation for why the highly connected PCC is affected early in the course of the disease.
Healthy ageing
Abnormalities of PCC function are also consistent findings in studies of ageing (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008). For example, in ‘resting’ functional MRI data, PCC functional connectivity to other parts of the DMN reduces with age, and this correlates with cognitive impairment (Andrews-Hanna et al., 2007). Functional abnormalities in the PCC are also observed during task performance (Sambataro et al., 2010; Prakash et al., 2012; Spreng and Schacter, 2012). When attention is directed externally, ageing is associated with a failure of the PCC deactivation that is usually tightly coupled to increases in cognitive load (Prakash et al., 2012). This change may not necessarily reflect an age-related abnormality within the PCC itself. In the study of Prakash et al. (2012), the region’s activity during other cognitive tasks was shown to be normal. Instead, the abnormal pattern of deactivation may reflect abnormal control of the PCC/DMN (Spreng and Schacter, 2012). These functional abnormalities might be caused by either structural changes within the PCC, abnormalities in its white matter connections, or abnormalities in the brain regions with which it interacts. White matter tract damage to the connections of the DMN correlates with age-related changes in PCC functional connectivity (Andrews-Hanna et al., 2007), and older individuals with high levels of amyloid deposition in the PCC but who are cognitively normal show reduced levels of PCC functional connectivity (Hedden et al., 2009).
Traumatic brain injury
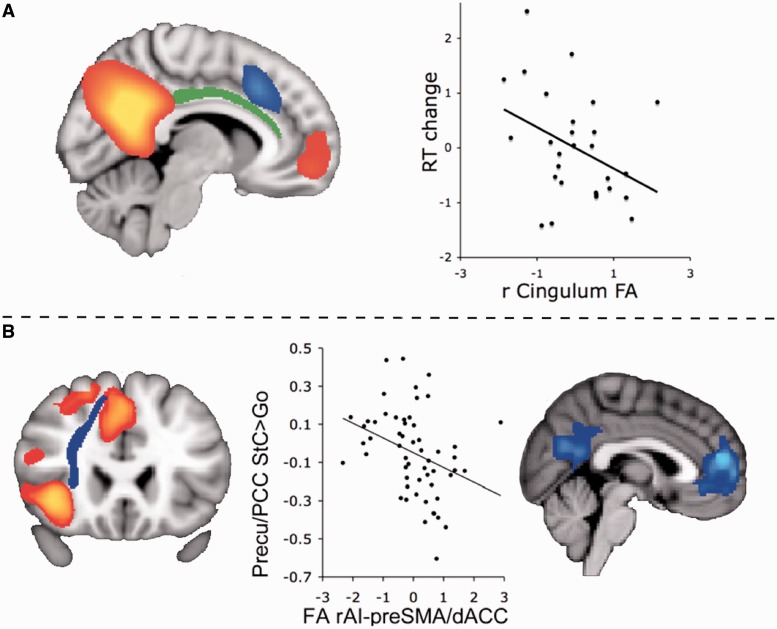
Our own work and others have consistently shown abnormalities in the PCC following traumatic brain injury (Kim et al., 2010; Mayer et al., 2011; Sharp et al., 2011; Bonnelle et al., 2011, 2012). Head injuries frequently produce diffuse axonal injury, which disconnects brain regions and produces cognitive impairment (Kinnunen et al., 2011). This is associated with reduced metabolism within the PCC (Nakashima et al., 2007). We studied the clinical significance of these abnormalities by investigating patients with traumatic brain injury performing a simple choice reaction time task (Bonnelle et al., 2011). Traumatic brain injury often leads to impairments of sustained attention, and we observed that cognitive performance declined over time in our patients. Behaviour was normal at the start of the experiment, but slower and more variable by the end. The pattern of functional connectivity from the PCC/precuneus to the rest of the DMN predicted this decline in performance, and importantly this predictive information was present in the initial period of the experiment, when behaviour was normal. We also investigated the structure of the cingulum bundles, which connect the PCC to the anterior part of the DMN. Increasing damage to the right cingulum bundle (green area in Fig. 3A) was correlated with impairments of sustained attention. These results suggest that disruption of structural and functional connectivity within the DMN contribute to impairments of sustained attention (Fig. 3A).
Figure 3.
Traumatic brain injury and the PCC. (A) Functional connectivity from the PCC/precuneus to the rest of the DMN (shown in red/yellow) predicts sustained attention impairment. In addition, damage to the right cingulum bundle (green) is correlated with sustained attention following traumatic brain injury, here measured as reaction time (RT) change over the course of a choice reaction time task. Part of the salience network is also shown for orientation in blue. (B) Reduced structural integrity of the salience network between pre-supplementary motor area/dorsal anterior cingulate cortex (pre-SMA/dACC) and right anterior insula (rAI) leads to functional abnormality within the PCC after traumatic brain injury. Activation within the salience network during successful stopping on the Stop Signal Task is shown in red-yellow in the left panel. The white matter tract connecting these regions is shown in blue. In the middle panel the structural integrity of the rAI–preSMA/dACC tract is plotted against deactivation of the ventral PCC during successful stopping on the Stop Signal Task (Stop correct versus Go trial contrast). Fractional anisotropy (FA) derived from diffusion tensor imaging data is used to quantify structural integrity of the tract. The right panel shows regions where activity during stopping is predicted by the structural integrity of the rAI–preSMA/dACC tract using a whole brain analysis.
Patients with traumatic brain injury can also have difficulty switching from relatively automatic to controlled responses, and this can result in perseverative behaviour. As in healthy ageing, this cognitive problem was associated with a failure of task-dependent deactivation within the PCC (Bonnelle et al., 2012). We studied this using the Stop Signal Task, where subjects are required to inhibit a motor response to an unexpected Stop cue. A subgroup of patients with traumatic brain injury showed impaired motor inhibition related to a failure to rapidly deactivate the ventral PCC. As in healthy individuals (Weissman et al., 2006), this pattern suggests that the inability to maintain efficient control of DMN function is associated with lapses in the moment-to-moment regulation of attention. The extent of this predominantly ventral PCC abnormality in patients with traumatic brain injury was strikingly predicted by the structural integrity of the white matter tract connecting the right anterior insula to the dorsal anterior cingulate cortex/pre-supplementary motor area, core nodes of the salience network (blue area in Fig. 3A; red area in Fig. 3B). These results suggest that interactions between the salience network and the DMN are important for the rapid re-allocation of attentional resources. Therefore, the failure to efficiently control PCC/DMN activity is associated with attentional lapses, and this can result from damage to the integrity of the salience network.
Autism
Metabolic and functional abnormalities of the PCC are seen in autistic spectrum disorders. Patients show reductions in metabolism measured by fluorodeoxyglucose PET (Haznedar et al., 2000), as well as abnormal functional responses measured with functional MRI (Pierce et al., 2004; Cherkassky et al., 2006; Kennedy et al., 2006; Chiu et al., 2008). In general, studies have also demonstrated reductions in functional connectivity (Horwitz et al., 1988). These reductions are particularly prominent in the PCC (Cherkassky et al., 2006), although more complex patterns of functional connectivity abnormality have also been reported (Monk et al., 2009). Abnormalities in cingulate responses during interpersonal interaction have been shown to correlate with the severity of patients’ autistic symptoms (Chiu et al., 2008), and a failure to show task dependent deactivation in the PCC has been shown to correlate with social function (Kennedy et al., 2006). Post-mortem studies provide evidence for cytoarchitectonic abnormalities within the PCC of patients with autistic spectrum disorders, as well as reduced levels of GABA A receptors (Oblak et al., 2011) and benzodiazepine binding sites (Oblak et al., 2011), compatible with an abnormality in local circuit inhibitory processing.
Schizophrenia
The PCC has also been shown to exhibit abnormal brain activity in a number of psychiatric conditions including schizophrenia, depression and ADHD (for reviews see Greicius, 2008; Broyd et al., 2009). Schizophrenia is a psychotic disorder that produces disordered perception and cognitive function resulting in hallucinations, delusions, and disorganized speech and behaviour (Liddle, 1987). Much of the symptomatology is characterized by a failure to distinguish clearly between internal and external events and a lack of insight. Two PET studies have shown abnormalities of PCC metabolism in schizophrenia. Glucose metabolism measured using fluorodeoxyglucose PET was decreased in schizophrenics (Haznedar et al., 2004), and the correlation between glucose metabolism in the pulvinar and the PCC was abnormally high in unmedicated patients (Mitelman et al., 2005a, b). In this last study, thalamic interactions with the frontal lobes were reduced in the same patients, suggesting a differential effect of schizophrenia on thalamo-cortical connections. Post-mortem autoradiography has also demonstrated abnormalities of NMDA, cannabinoid and GABAergic receptor binding in schizophrenics in the PCC (Newell et al., 2005, 2006, 2007). For example, cannabinoid receptors are elevated in the PCC (Newell et al., 2006), and in vivo PET neuroimaging of the cannabinoid receptor CB1 show that levels in patients correlate with the likelihood of psychotic symptoms (Wong et al., 2010).
Schizophrenic patients show abnormalities in the structure of the PCC, and in its white matter connections. Reduction of PCC volume has been reported (Sowell et al., 2000; Hulshoff Pol et al., 2001), which is more pronounced in those patients with poor outcome (Mitelman et al., 2005a, b). Disconnection of large-scale brain networks may be important in the aetiology of the condition, and a large number of studies have reported white matter abnormalities in the cingulum bundle, which connects the PCC to other limbic structures (Kubicki et al., 2003, 2005; Sun et al., 2003; Samartzis et al., 2013). Many functional MRI studies have also provided evidence for abnormal PCC function. Increases and decreases of ‘resting’ functional connectivity have been reported in both patients and their first-degree relatives (Liang et al., 2006; Zhou et al., 2007; Whitfield-Gabrieli et al., 2009). Interestingly, a weakening of the anti-correlation of activity between the PCC/DMN and the FPCN is observed (Zhou et al., 2007). The PCC also responds abnormally during task performance. For example, less task dependent deactivation has been reported in patients and their relatives, an abnormality that correlated with both behavioural performance on the task and psychopathology (Whitfield-Gabrieli et al., 2009).
The network abnormalities observed in schizophrenia may be directly related to psychotic symptoms (Carhart-Harris et al., 2012a, b, c). The psychedelic drug psiloscybin is a candidate for a drug model of psychosis, and has a marked effect on PCC activity (Carhart-Harris et al., 2012a, b, c). The drug induces an altered state of consciousness, which is strongly related to abnormal metabolism and PCC functional connectivity (Carhart-Harris et al., 2012a, b, c), and reduces the strength of anti-correlations between the DMN and FPCN (Carhart-Harris et al., 2012a, b, c). As these networks frequently contribute in opposing ways to internally and externally directed cognition, this might plausibly represent a weakening of the separation between internal and external thought, which might lead directly to psychosis.
Depression
Recently a number of MRI studies have shown abnormal PCC functional connectivity in major depression, although the abnormalities reported are variable (Bluhm et al., 2009; Zhou et al., 2010; Berman et al., 2011; Zhu et al., 2012). One study reported increased PCC functional connectivity, accompanied by increased anti-correlation with parts of the FPCN (Zhou et al., 2010). Another showed decreased functional connectivity from the PCC to the caudate in patients with depression before starting medication (Bluhm et al., 2009). Several studies have investigated interactions between the PCC and the sub-genual cingulate region, Brodmann’s area 25 in the frontal lobe, an area implicated in the aetiology of depression. The sub-genual cingulate region is highly connected to the PCC, forming part of the anterior node of the DMN. Both regions are metabolically overactive in treatment-resistant major depression (Ho et al., 1996; Mayberg et al., 1999), and deep brain stimulation of the adjacent white matter can lead to alleviation of refractory depressive symptoms (Mayberg et al., 2005). Increased correlation of activity between the PCC and the sub-genual cingulate is seen in depression, and correlates with excessive rumination, a behavioural feature of depression (Berman et al., 2011).
Interactions between the PCC and the sub-genual cingulate region may also be an important influence on treatment response. Both regions show metabolic changes after anti-depressant treatment, which predicts clinical response (Mayberg et al., 1999, 2000, 2005). Responders to fluoxetine showed increased PCC activity, accompanied by decreases in the sub-genual cingulate (Mayberg et al., 1999, 2000). Similarly, responders to deep brain stimulation showed increased glucose metabolism and regional cerebral blood flow in the PCC, accompanying a more complex pattern of alterations in the sub-genual cingulate target region (Mayberg et al., 2005; Lozano et al., 2008). The successful treatment of refractory depression using deep brain stimulation demonstrates the therapeutic value of developing a detailed understanding of abnormal patterns of brain network activity. Moving forwards, a detailed understanding of the brain’s intrinsic connectivity networks and the role of hub regions such as the PCC could greatly assist development of targeted interventions using deep brain stimulation aimed at normalizing network activity (Kringelbach et al., 2011).
Attention deficit hyperactivity disorder
It has been proposed that ADHD is a disorder of the DMN, where uncontrolled activity disrupts other neural systems leading to attentional lapses (Sonuga-Barke and Castellanos, 2007). A recent meta-analysis of structural MRI studies in almost 400 patients with ADHD shows an increased size of the left PCC, providing some evidence for a developmental abnormality affecting the PCC (Nakao et al., 2011). PCC function is abnormal in ADHD (Castellanos et al., 2008; Uddin et al., 2009; Fisher et al., 2011; for a review see Castellanos and Proal, 2012). Reductions in the anti-correlation between the PCC/precuneus and the FPCN are found in both adults with ADHD and in children before the start of medication (Castellanos et al., 2008; Sun et al., 2012). In addition, functional connectivity is also reduced within the DMN (Castellanos et al., 2008), and resting state activity has been shown to be useful in classifying children with and without ADHD (Zhu et al., 2008).
Psychostimulant medication improves attentional impairments in ADHD, and has a specific effect on PCC activity. A reduction of the normal task-dependent PCC deactivation is seen in patients not taking their medication, and this abnormality is normalized by psychostimulants (Peterson et al., 2009). This effect of stimulant medication on the PCC has been replicated in two further studies (Liddle et al., 2011; Schulz et al., 2012). One of these provided evidence that this PCC effect may be specific to stimulant treatments, as it is not seen with the non-stimulant medication atomoxetine (Schulz et al., 2012). The other varied the level of motivation subjects had for doing a Go/No-Go task (Liddle et al., 2011). As expected, when motivation levels were low, the stimulant methylphenidate normalized DMN deactivation associated with performance of a Go/No-Go task. However, increasing levels of motivation also normalized DMN deactivation, and adding the drug in this situation produced no further benefit. This suggests that the DMN is able to function normally in patients with ADHD, but that its regulatory influences are impaired (Liddle et al., 2011).
ADHD has a strong hereditary component, and a number of candidate genes have been identified (Faraone et al., 2005). One of these, synaptosomal-associated protein 25 (SNAP25), is a neuronal protein implicated in neurotransmitter release. Intriguingly, in healthy children SNAP25 polymorphisms are associated with (i) varying levels of working memory capacity; (ii) altered PCC structure; and (iii) varying task-dependent PCC deactivation patterns on a working memory task. This suggests that genetic predisposition to ADHD might be mediated through an effect on PCC function (Soderqvist et al., 2010).
Summary of disease and the posterior cingulate cortex
Across a range of neurological and psychiatric disorders the PCC is repeatedly found to be structurally and functionally abnormal. In some cases, such as Alzheimer’s disease, there is clear evidence for structural pathology within the PCC. However, in many cases abnormal function is likely to be the consequence of remote pathology or distributed network pathology (Spreng and Schacter, 2012). For example, the failure of appropriate PCC deactivation as cognitive load increases has been reported in healthy ageing, traumatic brain injury, ADHD, autism and schizophrenia. This PCC abnormality appears linked to lapses of attention. Disruption of the controlling influence of the salience network, rather than damage to the PCC itself, may be critical factor (Bonnelle et al., 2012). As suggested in the following sections, the PCC is likely to function as an integrative hub, mediating information flow around the brain. Therefore, many of the functional abnormalities observed within the PCC may be best viewed as a form of diaschisis, where the effects of remote and often diffuse damage accumulate as abnormal function within the PCC. Although these changes may be non-specific, their predictable and localized nature may make them particularly useful as biomarkers for guiding treatment.
Current theories of the role of the posterior cingulate cortex in cognitive function
As we have discussed, abnormalities within the PCC are often associated with cognitive impairments, which include memory function (e.g. Alzheimer’s disease, traumatic brain injury, depression), attention (e.g. Alzheimer’s disease, traumatic brain injury, ADHD) and problems maintaining the balance between internal and external thought (e.g. schizophrenia, traumatic brain injury, autism). These observations suggest that the region plays a key role in cognitive function. Early theories emphasized a role for the whole of the cingulate in emotion (Papez, 1937). However, a single function for the whole cingulate is not compatible with more recent work. Although the PCC responds to emotional stimuli (Maddock et al., 2003), it is clear that the role of the region in cognition extends well beyond emotional processing (Vogt, 2009). The region has extensive connections to heteromodal association and paralimbic areas suggesting that it is well placed to integrate and influence higher-level information processing. There are several competing theories about the nature of this role.
Arousal and awareness
The PCC is implicated as a key structure for both arousal and awareness (Laureys et al., 2004; Vogt and Laureys, 2005; Boly et al., 2008). Relatively high levels of PCC metabolism and functional connectivity are associated with the normal conscious state. In contrast, in low states of arousal and awareness, including deep sedation, PCC activity measured by both absolute blood flow and functional connectivity is reduced (Fiset et al., 1999). PCC metabolism and connectivity is also low in the vegetative state and increases as patients regain consciousness (Laureys et al., 1999, 2000; Vanhaudenhuyse et al., 2010; Heine et al., 2012).
Activity in the PCC is also sensitive to changes in awareness associated with sleep. Stepwise reductions of connectivity between the PCC and prefrontal regions track the changes in vigilance level that occur in different sleep states (Horovitz et al., 2008, 2009; Larson-Prior et al., 2009; Samann et al., 2011). Similarly, under anaesthesia, propofol reduces both PCC metabolism and functional connectivity more than many other brain regions (Fiset et al., 1999). More sophisticated analyses of causal interactions between brain regions (effective connectivity) suggest that the reduction in conscious level observed with propofol sedation is associated with a loss of the normal top-down cortico-cortical communication from the dorsal anterior cingulate cortex to the PCC (Boly et al., 2012). Hence, alterations in arousal and awareness across many different states are associated with changes in the neural activity of the PCC and its interactions with other brain regions.
Internally directed thought
Probably the dominant current theory of PCC function is that it is directly involved in some aspect of internally directed thought (Binder et al., 1999; Buckner et al., 2008). One early hypothesis was that the PCC plays a role in supporting the semantic elements of ‘stimulus-independent’ thought (Binder et al., 1999). Activity in the PCC was higher when subjects report more ‘stimulus-independent’ thoughts, a state where mental activity is unconstrained by external stimuli. Binder et al. (1999) proposed that the PCC, along with other DMN regions such as lateral inferior parietal regions, are continually involved in the conceptual processing associated with this type of freewheeling mental activity. In this state semantic knowledge is retrieved, maintained in awareness and manipulated for the purposes of problem solving and planning. A related account is that the PCC supports internally directed thought more generally (Buckner et al., 2008). In support of this account, increased brain activity in the PCC is seen in individuals who daydream more (Mason et al., 2007), and when thoughts are explicitly directed internally, for example when autobiographical memories are retrieved or when individuals plan for the future (Gusnard et al., 2001; Addis et al., 2007). Such internally directed cognition has been particularly related to ventral parts of the PCC, as well as to the retrosplenial cortex (Maguire and Mummery, 1999; Maddock et al., 2003).
Controlling the balance between internal and external attention
As discussed above, the PCC shows a cytoarchitectonic structure that is intermediate between the isocortical brain regions organized to hierarchically process perceptual input, and more primitive limbic allocortical and hypothalamic regions that are primarily involved in internal homeostasis as well as mnemonic processing. Mesulam (1998) proposed the theory that paralimbic regions, including the PCC, link the processing of information derived from internal and external worlds and that the PCC is part of a hippocampocentric subdivision of the paralimbic zone, that provides a link between the hippocampal formation and higher-level cortices. Viewed in this context, the role of the PCC could extend beyond supporting internal thought, and rather play a more active role in controlling the balance between an internal and external focus of attention.
Functional imaging studies provide support for Mesulam’s (1998) theory. Activity within the PCC has clearly been shown to correlate with the efficiency of cognitive processing in various contexts (Hampson et al., 2006; Weissman et al., 2006; Gilbert et al., 2007; Hahn et al., 2007; Kelly et al. 2008; Singh and Fawcett, 2008; Sharp et al., 2011). One explanation is that a failure to suppress PCC activity is associated with the intrusion of internal mentation into task performance (Weissman et al., 2006; Sonuga-Barke and Castellanos, 2007). However, in certain situations increased PCC activity or its functional connectivity is associated with improved behavioural performance, even when attention is externally directed (Hampson et al., 2006; Hahn et al., 2007; Sharp et al., 2011). For example, activity in the PCC, as well as other parts of the DMN, increases with faster responses to unpredictable but not predictable stimuli (Hahn et al., 2007), and increased functional connectivity of the PCC within the DMN is associated with faster reaction times to external stimuli in the healthy brain and following traumatic brain injury (Hampson et al., 2006; Sharp et al., 2011). These functional imaging results are difficult to reconcile with a simple relationship in which PCC activity is always the result of internally directed thought. Instead, they are more consistent with an alternative hypothesis; that parts of the PCC have a direct role controlling the balance between an internal and external attentional focus. The connections of the dorsal PCC are consistent with such a role, as they show a ‘transitional’ pattern of connectivity, linking the region to other intrinsic connectivity networks.
Environmental change detection
The PCC may be actively involved in maintaining a vigilant attentional state (Shulman et al., 1997; Gilbert et al., 2007; Hahn et al., 2007), or in signalling behaviourally relevant changes in the environment (Pearson et al., 2011). These theories could explain the positive relationship between increasing PCC activity and efficient externally directed behaviour, as a vigilant state or an efficient signal of a behaviourally relevant environmental change would be expected to result in a more rapid behavioural response.
A role for the PCC in environmental change detection is supported by elegant electrophysiological work in non-human primates (Hayden et al., 2008, 2009; Pearson et al., 2009). The PCC exhibits a sustained suppression of cell firing when attention is externally focused, with relatively small reductions of activity associated with slower reaction times (Hayden et al., 2009). Some neurons also show transient phasic increases in firing, which are associated with behaviourally salient information appearing in the environment (Hayden et al., 2009). These phasic responses appear to indicate a change in the environment that is important for learning, and provide a signal that is complementary to the reward signals coded by midbrain dopaminergic cells (McCoy et al., 2003). In humans, PCC activity has been shown to increase during attentional biasing to targets that are of high motivational value, which is accompanied by an increased functional connectivity to areas within the intraparietal sulcus involved in spatial attention (Mohanty et al., 2008). These phasic and tonic elements of PCC function appear to be independent, and in monkeys the firing of PCC neurons over time both integrate the history of rewards experienced, and importantly predict the likelihood that there will be a change in behavioural strategy in the future (Pearson et al., 2009). This work provides evidence that the PCC integrates the consequences of behaviour over time, and provides a signal for strategic changes in behaviour when the consequences of previous actions are suboptimal (Hayden et al., 2008; Pearson et al., 2009).
In keeping with this theory is our observation that ‘echoes’ of a range of intrinsic connectivity networks are present in the activity of spatially overlapping but distinct parts of the PCC (Fig. 2) (Leech et al., 2012). This shows that the PCC can code complex patterns of neural activity that are present in large numbers of remote brain regions, a feature that would be expected of a brain hub involved in integrating sources of information. Once activity across the PCC is controlled using a multivariate approach, the results show that parts of the dorsal PCC show high functional connectivity to the FPCN (Leech et al., 2011, 2012). In addition, activity in this dorsal PCC subregion is modulated by attentional state. When subjects are waiting for an external cue to action and broad vigilance is required, activity in this region is high. In contrast, when action is initiated and attention must be focused on the externally cued task, activity in this region falls.
An integrated model of posterior cingulate function
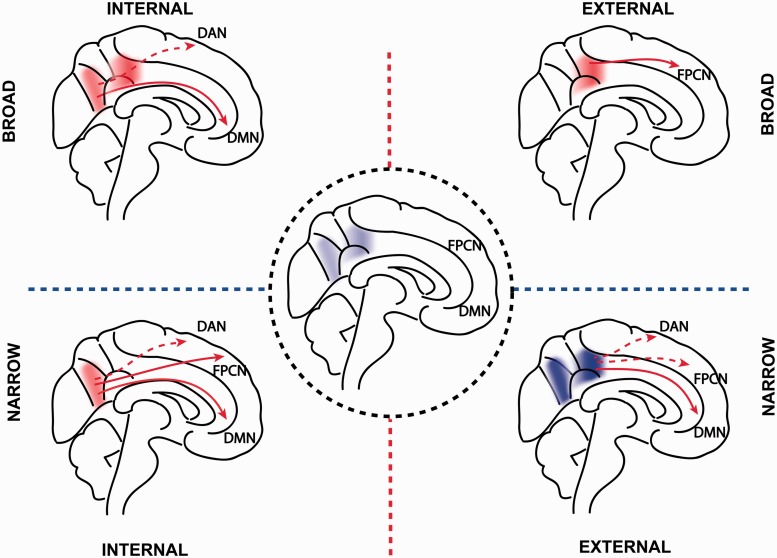
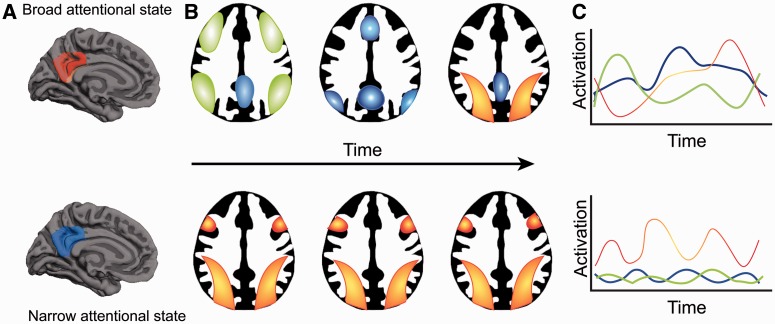
Current experimental findings are not well described by a simple model of the PCC as an isolated cortical region engaged only in internally directed thought. Instead, we propose an integrated model that synthesizes elements from the main competing theories, and provides insight into the patterns of PCC abnormality observed in different disease states (the ‘Arousal, Balance and Breadth of Attention’ model, ABBA). We suggest that to understand PCC function one needs to consider three dimensions: (i) the state of arousal; (ii) the balance between internally and externally focused attention; and (iii) the breadth of attention. This conceptualization allows for the multiple competing sources of data to be integrated into a single theoretical model. We discuss each dimension in the next section, and the main features of the model are illustrated in Fig. 4 with further explanation in the text.
Figure 4.
Illustration of the proposed theoretical account of the PCC, combining the three dimensions of (i) arousal; (ii) internal/external focus; and (iii) breadth of attentional focus. The central panel illustrates the PCC during a low state of arousal, where there is little interaction with other networks. Each panel surrounding this illustrates one of four possible states from the interaction of an internal/external and a broad/narrow focus of attention. Interaction between the PCC and intrinsic connectivity networks are shown as arrows: solid arrows signify increased functional connectivity, broken arrows greater anti-correlation. DAN = dorsal attention network. Red areas within the PCC signify relatively increased neural activity, blue areas relatively decreased activity. For the purposes of illustration, dorsal and ventral PCC regions are shown as separated areas.
Posterior cingulate function in three dimensions
Arousal
The PCC is highly sensitive to arousal state, which is important in interpreting the abnormalities seen in patients with reduced levels of consciousness. We illustrate this at the centre of Fig. 4, by showing a low level of PCC activation and functional connectivity when arousal is low. It seems likely that a minimum level of arousal is required for the PCC to efficiently interact with other brain regions, and that the cortico-cortical interactions required for awareness are not possible below this level. We envisage that changes in the level of arousal lead to alterations of activity across the whole PCC (both dorsal and ventral subregions). In contrast, changes in the balance of internal/external attention and the breadth of attentional focus produce regional alterations of activity and connectivity within the PCC.
Internal/external thought
In line with previous work (Vogt et al., 2006), we propose that the dorsal and ventral subregions of the PCC have distinct functions. Because of its structural connections to the rest of the DMN, including the retrosplenial cortex and hippocampal formations, the ventral PCC is predominantly involved in supporting various aspects of internally directed thought, including the retrieval of episodic and semantic memories. In contrast, the dorsal PCC has broad connectivity to other intrinsic connectivity networks, particularly networks involved in cognitive control. Therefore, the dorsal PCC is involved in detecting and responding to environmental events that may require a change in behaviour and that are not part of the current cognitive set. We envisage that dynamic interactions between the subdivisions of the PCC and other intrinsic connectivity networks are important for regulating the balance between internal and external attentional focus (Leech et al., 2011). The inability to regulate the balance of internal versus external attention appears to be important in a range of clinical disorders. For example, the inability to deactivate the PCC (either the result of local damage or through modulating fronto-parietal brain networks) during externally-focused tasks, may result in distracting internally-focused information processing. This may impair a range of executive abilities in traumatic brain injury, ADHD, Alzheimer’s disease and normal ageing and is consistent with a range of psychiatric symptoms in schizophrenia and depression.
Attentional focus
Attention can be viewed as varying along a spectrum from narrow to broad (Wachtel, 1967; Eriksen and Yeh, 1985). For example, in the case of visual processing one might focus exclusively on a single visual event, or allow a range of stimuli across the visual field to enter consciousness (Andrews-Hanna et al., 2010). More generally, a variable attentional focus can apply across different sensory modalities, as well as to internal or external thought. We propose that the PCC is involved in ‘tuning’ the focus of attention (see ‘Dynamical systems’ section below). Many of the clinical disorders associated with PCC abnormalities also have symptoms that are consistent with an inability to regulate the breadth of attention. Impairments in integrating information reported in autism, some types of dementia, depression and schizophrenia may relate to breadth of attention (either inappropriately wide or too narrow), and may be a consequence of dysfunction of the PCC because of either local or remote damage.
External/narrow attentional focus
Most cognitive neuroscience studies require subjects to have a narrow external focus. The N-back task is representative of this type of task (Fig. 4). Here subjects are rapidly presented with new information that must be held in mind (working memory) and used to guide choices. This is associated with increased activation of the dorsal attention network and FPCN, and coupled deactivation in both the dorsal PCC and ventral PCC (Leech et al., 2011). Although PCC activity is relatively reduced, the dorsal PCC shows increased functional connectivity to the rest of the DMN and greater anti-correlation with networks that are activated by the task, including the FPCN and dorsal attention network (Leech et al., 2011).
External/broad attentional focus
Broadening the focus of attention potentially allows an individual to be more adaptive, as unexpected events can be captured by attention more rapidly (Fig. 4). Cognitively this corresponds to an ability to monitor and respond to behaviourally relevant environmental stimuli that occur outside the current cognitive task, and then rapidly change cognitive state. At present, there is relatively little functional neuroimaging data on this state. However, we predict that future studies will show that this state is associated with increased dorsal PCC activity, as well as increased functional connectivity between the dorsal PCC and FPCN.
Internal/narrow attentional focus
Attention can also be directed internally, for example when attempting to retrieve autobiographical information from memory (Fig. 4). This is associated with activation in the ventral PCC and retrosplenial cortex (Vann et al., 2009). These regions co-activate with parts of the FPCN during internally directed cognition, illustrating that these brain regions do not always show anti-correlated activity (Spreng and Grady, 2010; Spreng, 2012). Increased functional connectivity between the ventral PCC and the FPCN has also been shown in the pattern of brain activity in expert meditators, which might be viewed as another example of a focused internal state (Brewer et al., 2011). Again, there is relatively little evidence directly assessing the pattern of functional activation and connectivity in this state. However, based on our model, we predict that this state will involve increased ventral PCC activity, increased ventral PCC functional connectivity to the DMN and FPCN, and greater anti-correlation to the dorsal attention network. We predict that the interaction between the ventral PCC and FPCN will be involved in the maintenance of a narrow internal focus.
Internal/broad attentional focus
A broad internal focus of attention occurs in the freewheeling cognitive state, which is usually thought of as accompanying subjects at ‘rest’ (Fig. 4). Given the inherent difficulties in assessing the content of unconstrained thought, this state is challenging to study empirically. However, ‘resting state’ functional MRI shows that activity across the PCC is relatively high. Functional connectivity within the DMN is also high, whereas the ventral PCC shows an anti-correlated pattern of activity to the dorsal attention network (Fox et al., 2005; Leech et al., 2011).
A dynamic systems approach to understanding posterior cingulate function
So far we have discussed the connectivity of the PCC in a rather static way. An important additional consideration is how its connectivity changes over time, i.e. its dynamic properties. From a network perspective, when attention is focused we expect activity and functional connectivity to be relatively stable over time. Brain regions involved in supporting task performance will show increased activity, which will fluctuate relatively little. This corresponds to a narrow attentional focus, where the influence of events outside the attentional focus is limited. In contrast, if an individual is in a relatively unfocused attentional state, network activity will be less stable over time, cycling through a greater number of states that correspond to variable cognitive operations. This might occur when there is no explicit experimental task (i.e. the ‘rest’ state), when spontaneously recalling memories of different types, when maintaining a broad vigilant state, or when switching between tasks.
To understand how PCC activity might relate to these time-dependent changes in attentional state and network activity, a way of describing its dynamic interactions is required. One approach is to view the brain as a complex dynamic network (Deco et al., 2009; Kitzbichler et al., 2009; Chialvo, 2010; Shanahan, 2010b; Cabral et al., 2011). This is likely to be particularly useful for understanding network hubs, as the approach provides a framework within which to summarize complex interactions across the whole brain, as well as allowing the investigation of the influence of single regions such as the PCC. An important observation from the field of complex network theory is that the brain appears to exhibit features of self-organized criticality (for review see Chialvo, 2010; Expert et al., 2011). This type of organization has been described in other complex networks, and allows a system to naturally exist in a stable yet adaptable state, poised at the point of second-order phase transitions. As the brain needs to be capable of responding flexibly but have stable global interactions, this configuration may be the common feature of networks capable of giving rise to the type of emergent complexity found in neural activity and cognition (Chialvo, 2010). Two ways of describing a critical system are with measures of network synchrony and network metastability (Shanahan, 2010a). Network synchrony provides a summary of the extent to which clusters of nodes or the whole network shows coherent activity over time. A network where all nodes follow the same pattern has a very high synchrony, whereas a network where nodes are completely unrelated will have very low synchrony. Network metastability quantifies the extent to which synchrony fluctuates over time (Kitzbichler et al., 2009; Shanahan, 2010a). A high metastability indicates that the network shifts through multiple short-lived yet stable states (in the brain these might be thought of as different cognitive states).
The PCC’s structural and functional connectivity are suggestive of an important role in regulating global brain dynamics (Leech et al., 2012; van den Heuvel et al., 2012). We propose that through its extensive connectivity the dorsal PCC influences whole-brain metastability, providing a mechanism by which attentional focus is altered. In a broad attentional state, network activity is relatively unconstrained, with reduced synchrony and high metastability. The ‘echoes’ of intrinsic connectivity networks within the PCC reflect or perhaps permit activity across the rest of the brain to traverse through numerous different neural states (Fig. 5). Activity in the PCC is relatively high in the unfocused state (left panel), and over time PCC activity ‘echoes’ the dynamic changes of activity within distinct intrinsic connectivity networks (middle panel). The pattern of activity in distinct intrinsic connectivity networks is plotted in the right panel, which would be associated with rapidly changing cognitive states.
Figure 5.
An illustration of a dynamic systems approach to understanding PCC function. (A) Activity within the PCC is relatively high in the broad attentional state and low in the narrow attentional state. (B) In the broad attentional state, the system transitions through a number of different network configurations, which accompany different cognitive processes. These are shown for the purposes of illustration as the FPCN (green), the DMN (blue) and the dorsal attention network (red/yellow), In the narrow attentional state, one particular pattern of neural synchrony persists over time (here the dorsal attentional network), allowing stable cognitive processing. (C) Activity in distinct intrinsic connectivity networks plotted over time. Activity in the dorsal PCC ‘echoes’ these dynamic intrinsic connectivity network changes. Large fluctuations in activity in the broad attentional state are associated with low overall synchrony and high metastability, whereas relatively stable activity in the narrow attentional state is associated with high synchrony and low metastability.
In contrast, we propose that a narrow attentional focus is associated with a more constrained neural state, reflecting the cognitive processing demands of a single task. Variability in the system as a whole reduces as the brain settles into a single neural state, which facilitates stable task performance. Synchrony within the intrinsic connectivity networks involved in the task is high, and overall metastability low. The bottom panel of Fig. 5 illustrates performance of a task such as the N-back, where a narrow external focus is required. Here PCC activity is relatively reduced, synchrony within the dorsal attentional network persists over time, and activity within the DMN and dorsal attentional network are anti-correlated.
The observation of complex ‘echoes’ of other intrinsic connectivity activity in the dorsal PCC is in keeping with this theory (Leech et al., 2012). In addition, we have observed that high global metastability and low synchrony is associated with high PCC activity in empirical functional MRI analysis and complementary computational simulations (Hellyer et al., 2012). We speculate that the dorsal PCC is involved in ‘tuning’ the current level of metastability in the system as a whole. Increased activity in the dorsal PCC would allow increased whole-brain metastability, and rapid transitions between different neural states. This would result in rapid changes of activity in intrinsic connectivity networks. Reduced activity within the dorsal PCC would be associated with a reduction in metastability, as the rest of the system settles into a stable state to allow consistent performance of a specific task. It remains to be seen how the ventral PCC and dorsal PCC interact in modulating global brain dynamics, and whether the ventral PCC, for example, has a specific role in modulating the metastability of networks involved in internally directed attention (e.g. hippocampus and ventromedial prefrontal cortex). PCC dysfunction in some disorders may produce abnormal brain metastability. For example, in traumatic brain injury the perseverative behaviour might be explained by reduced metastability, resulting in an inability to flexibly change between different cognitive states. Other disorders, such as schizophrenia, may involve abnormally high metastability, resulting in too many rapid state transitions. Future empirical and computational work is needed to understand metastability in different diseases and the PCC’s role in its control.
Relationship to existing theories of attention
Our proposals are largely complementary to existing theories about the neuroanatomical basis of attention. Our account emphasizes the role of the PCC as a transmodal thalamo-cortical hub, which interacts with other fronto-parietal and subcortical networks more traditionally thought of as attentional in nature. The PCC is absent from the dominant theory of attention in the brain (Posner and Petersen, 1990; Petersen and Posner 2012). This emphasizes neural networks involved in alerting, orienting and exerting executive control over attention (Posner and Petersen, 1990; Petersen and Posner 2012). We extend this by considering the role of the PCC, and propose how the internal/external focus and the breadth of attention may be controlled.
Input from the frontal lobes is likely to be particularly important in determining the level of dorsal PCC activity, with evidence for both top-down and bottom-up attentional signals influencing the PCC through the prefrontal cortex. Task goals encoded in the prefrontal cortex have been shown to influence activity within the PCC, and our own work demonstrates that the salience network is particularly important in the control of task-dependent deactivation within the PCC in response to unexpected bottom-up cues to change behaviour (Bonnelle et al., 2012). The right anterior insula appears to play a particularly important role in co-ordinating the activity of other brain networks in response to unexpected events. Menon and colleagues propose a model whereby sensory and limbic inputs are processed by the anterior insula, which then interacts with other brain networks to regulate their activity. In support of this theory, activation of this structure may cause deactivation of the PCC in response to unexpected salient events (Sridharan et al., 2008), and damage to the white matter tract connecting the anterior insula to the dorsal anterior cingulate cortex/pre-supplementary motor area results in uncontrolled PCC activity after brain injury (Bonnelle et al., 2012). We extend this model by proposing that the right anterior insula and the dorsal PCC together form a system that regulates the attentional focus. We propose that the anterior insula signals the need for attention to be focused externally, and its interaction with the dorsal PCC leads to a reduction in metastability that facilitates the consistent activation of networks appropriate for the current behavioural state. The dorsal PCC and right anterior insula can be viewed as providing opposing signals in terms of attentional focus, but may also operate over different temporal scales, with the right anterior insula providing phasic cues and electrophysiology studies suggesting that the PCC integrates information tonically over a slower time scale (Pearson et al., 2009).
Future directions
Detailed computational simulations will provide one important avenue for future research. Recently, biologically plausible network simulations have begun to be implemented, which are constrained by our increasing understanding of the white matter connectome. For example, Deco et al. (2009) have used knowledge of the connections of the PCC derived from diffusion spectrum imaging to constrain the interactions of a computational model of the DMN and its interactions with other intrinsic connectivity networks. This has shown how anti-correlations can spontaneously arise from the interactions between networks. One strength of this type of approach is that predictions of the model can be tested against empirical functional MRI data, providing a way to evaluate the models validity. As we gain greater knowledge of the human connectome and more detailed anatomical descriptions of the distributions of neurotransmitters and regional receptor densities, more neurobiologically constrained simulations of the brain and the PCC’s role within it will become possible. These simulations can be tested alongside increasingly detailed neuroimaging data at a range of temporal and spatial resolutions by fusing different neuroimaging modalities, allowing an increasingly comprehensive description of the region’s function.
Studying the PCC in disease states will continue to be important in future work. This will allow an increased understanding of disease pathophysiology, but will also provide insights to the region’s function. The interactions between intrinsic connectivity networks is likely to be a fruitful avenue for future research, and our work in traumatic brain injury illustrates how work in patient populations informs how the PCC interacts with other brain regions (Bonnelle et al., 2012). Work in humans will need to be combined with animal studies to obtain a definitive understanding of PCC function. The basic organization of the PCC has been preserved across primates, and lesion studies in these species may be required in combination with animal neuroimaging. This is particularly important given the reports of altered PCC function in a range of neurological and psychiatric disorders. The proposed large-scale integrative role across arousal and the balance and breadth of attention, provides an explanation why multiple types of remote or diffuse pathology accumulate as dysfunction within the PCC. However, future work is needed to unpick precisely how different patterns of pathology, both local and remote to the PCC, lead to impairment of different aspects of arousal, balance and breadth of attention. Future clinical work should be mindful of the anatomical heterogeneity within the PCC and consider whether dorsal or ventral regions or both are affected. Understanding the cause and consequences of this dysfunction is likely to be an important part of developing biomarkers for altered cognitive states and the development of novel treatments.
Summary
The PCC is highly connected and metabolically active, although there is no consensus about its function in cognition. We discuss the prevalence of PCC dysfunction across a range of psychiatric and neurological conditions. We synthesize key findings about the PCC into a novel model of the region’s function (the ‘Arousal, Balance and Breadth of Attention’ model, ABBA). In this model the distinction between dorsal and ventral PCC can be understood by considering: (i) whether attention is focused internally or externally; (ii) the breadth of this attentional focus; and (iii) the state of arousal. The ventral PCC appears to be highly integrated within the DMN, and is involved in internally directed cognition such as memory retrieval and planning. In contrast, the dorsal PCC shows a highly complex pattern of connectivity, with prominent connections to the frontal lobes. We propose that through its interactions with the prefrontal cortex the dorsal PCC is involved in controlling attentional focus, perhaps through influencing the metastability of the brain as a whole. Hence, interactions of these PCC subregions with other intrinsic connectivity networks are then involved in shifting the balance of attention along an internal/external and broad/narrow dimension.
Funding
This work was supported by grants from The Medical Research Council (UK) and the National Institute of Health Research (NIHR) to D.J.S., The Imperial College Healthcare Charity to D.J.S. and Research Councils UK to R.L.
Glossary
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- DMN
default mode network
- FPCN
fronto-parietal control network
- PCC
posterior cingulate cortex
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–77. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaadi T, Binder JR, Lazar RM, Doorani T, Mohr JP. Pure topographic disorientation: a distinctive syndrome with varied localization. Neurology. 2000;54:1864–6. doi: 10.1212/wnl.54.9.1864. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, et al. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–35. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol. 2010;104:322–35. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci. 2011;6:548–55. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. 2009;63:754–61. doi: 10.1111/j.1440-1819.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, et al. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–29. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Moran R, Murphy M, Boveroux P, Bruno MA, Noirhomme Q, et al. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–90. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31:13442–51. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci USA. 2012;109:4690–5. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci USA. 2011;108:20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: J. A. Barth; 1909. [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–63. doi: 10.1016/s0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Cabral J, Hugues E, Sporns O, Deco G. Role of local network oscillations in resting-state functional connectivity. Neuroimage. 2011;57:130–9. doi: 10.1016/j.neuroimage.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci USA. 2012a;109:2138–43. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Erritzoe D, Williams TM, Stone JM, Evans J, et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull. 2012b doi: 10.1093/schbul/sbs117. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Williams TM, Erritzoe D, Abbasi N, Bargiotas T, et al. Implications for psychedelic-assisted psychotherapy: functional magnetic resonance imaging study with psilocybin. Br J Psychiatry. 2012c;200:238–44. doi: 10.1192/bjp.bp.111.103309. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–7. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky VL, Kana RK, Keller TA, Just MA. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17:1687–90. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Chialvo DR. Emergent complex neural dynamics. Nat Phys. 2010;6:744–50. [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, et al. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron. 2008;57:463–73. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. Am J Neuroradiol. 2005;26:2267–74. [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone JS, Ladurner G, Höller Y, Golaszewski S, Trinka E, Kronbichler M. Deactivation of the default mode network as a marker of impaired consciousness: an fMRI study. PLoS One. 2011;6:e26373. doi: 10.1371/journal.pone.0026373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the ‘default network’ in normal aging. Cereb Cortex. 2008;18:1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Dastjerdi M, Foster BL, Nasrullah S, Rauschecker AM, Dougherty RF, Townsend JD, et al. Differential electrophysiological response during rest, self-referential, and non-self-referential tasks in human posteromedial cortex. Proc Natl Acad Sci USA. 2011;108:3023–8. doi: 10.1073/pnas.1017098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa V, McIntosh A, Sporns O, Kötter R. Key role of coupling, delay, and noise in resting brain fluctuations. Proc Natl Acad Sci USA. 2009;106:10302–7. doi: 10.1073/pnas.0901831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen CW, Yeh YY. Allocation of attention in the visual field. J Exp Psychol Hum Percept Perform. 1985;11:583–97. doi: 10.1037//0096-1523.11.5.583. [DOI] [PubMed] [Google Scholar]
- Expert P, Lambiotte R, Chialvo DR, Christensen K, Jensen HJ, Sharp DJ, et al. Self-similar correlation function in brain resting-state functional magnetic resonance imaging. J R Soc Interface. 2011;8:472–9. doi: 10.1098/rsif.2010.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106:7209–14. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset P, Paus T, Daloze T, Plourde G, Meuret P, Bonhomme V, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci. 1999;19:5506–13. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher T, Aharon-Peretz J, Pratt H. Dis-regulation of response inhibition in adult Attention Deficit Hyperactivity Disorder (ADHD): an ERP study. Clin Neurophysiol. 2011;122:2390–9. doi: 10.1016/j.clinph.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–9. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: default network and executive activity associated with goal-directed mental simulations. Neuroimage. 2011;55:1816–24. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on ‘Wandering minds: the default network and stimulus-independent thought’. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. author reply 43. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb Cortex. 2007;17:1664–71. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–43. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Yücel M, Pujol J, Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophrenia research. 2007;91(1):82–86. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Nair AC, McCoy AN, Platt ML. Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008;60:19–25. doi: 10.1016/j.neuron.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA. 2009;106:5948–53. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, Shihabuddin L, New A, Siever LJ. Cingulate gyrus volume and metabolism in the schizophrenia spectrum. Schizophr Res. 2004;71:249–62. doi: 10.1016/j.schres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–94. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine L, Soddu A, Gómez F, Vanhaudenhuyse A, Tshibanda L, Thonnard M, et al. Resting state networks and consciousness: alterations of multiple resting state network connectivity in physiological, pharmacological, and pathological consciousness states. Front Psychol. 2012;3:295. doi: 10.3389/fpsyg.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer P, Scott G, Shanahan M, Sharp D, Leech R. Local network dynamics and structural connectivity during task based activity in the brain. Hum Brain Mapp. 2012 OHBM 2012. [Google Scholar]
- Hillary FG, Slocomb J, Hills EC, Fitzpatrick NM, Medaglia JD, Wang J, et al. Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol. 2011;82(1):115–123. doi: 10.1016/j.ijpsycho.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Ho AP, Gillin JC, Buchsbaum MS, Wu JC, Abel L, Bunney WE., Jr Brain glucose metabolism during non-rapid eye movement sleep in major depression. A positron emission tomography study. Arch Gen Psychiatry. 1996;53:645–52. doi: 10.1001/archpsyc.1996.01830070095014. [DOI] [PubMed] [Google Scholar]
- Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, et al. Decoupling of the brain's default mode network during deep sleep. Proc Natl Acad Sci USA. 2009;106:11376–81. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, De Zwart JA, Van Gelderen P, Fulton SC, Balkin TJ, et al. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–82. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Grady CL, Rapoport SI. The cerebral metabolic landscape in autism. Intercorrelations of regional glucose utilization. Arch Neurol. 1988;45:749–55. doi: 10.1001/archneur.1988.00520310055018. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, et al. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58:1118–25. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Jones K, Holman BL, Becker JA, Spiers PA, Satlin A, et al. Preclinical prediction of Alzheimer's disease using SPECT. Neurology. 1998;50:1563–71. doi: 10.1212/wnl.50.6.1563. [DOI] [PubMed] [Google Scholar]
- Katayama K, Takahashi N, Ogawara K, Hattori T. Pure topographical disorientation due to right posterior cingulate lesion. Cortex. 1999;35:279–82. doi: 10.1016/s0010-9452(08)70801-3. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–37. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci USA. 2006;103:8275–80. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Whyte J, Patel S, Avants B, Europa E, Wang J, et al. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J Neurotrauma. 2010;27:1399–411. doi: 10.1089/neu.2009.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(Pt 2):449–63. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzbichler MG, Smith ML, Christensen SR, Bullmore E. Broadband criticality of human brain network synchronization. PLoS Comput Biol. 2009;5:e1000314. doi: 10.1371/journal.pcbi.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502:810–33. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Green AL, Aziz TZ. Balancing the brain: resting state networks and deep brain stimulation. Front Integr Neurosci. 2011;5:8. doi: 10.3389/fnint.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry. 2003;54:1171–80. doi: 10.1016/s0006-3223(03)00419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26:1109–18. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350:337–56. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- Larson-Prior LJ, Zempel JM, Nolan TS, Prior FW, Snyder AZ, Raichle ME. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106:4489. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Goldman S, Phillips C, Van Bogaert P, Aerts J, Luxen A, et al. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. Neuroimage. 1999;9:377–82. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–46. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32:215–22. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–24. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–13. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Liddle PF. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br J Psychiatry. 1987;151:145–51. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
- Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, et al. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry. 2011;52:761–71. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–7. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9:54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106:20069–74. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32:1825–35. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AN, Crowley JC, Haghighian G, Dean HL, Platt ML. Saccade reward signals in posterior cingulate cortex. Neuron. 2003;40:1031–40. doi: 10.1016/s0896-6273(03)00719-0. [DOI] [PubMed] [Google Scholar]
- Meguro K, Blaizot X, Kondoh Y, Le Mestric C, Baron JC, Chavoix C. Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non-human primate as shown by PET. Implications for Alzheimer's disease. Brain. 1999;122(Pt 8):1519–31. doi: 10.1093/brain/122.8.1519. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Byne W, Kemether EM, Hazlett EA, Buchsbaum MS. Metabolic disconnection between the mediodorsal nucleus of the thalamus and cortical Brodmann's areas of the left hemisphere in schizophrenia. Am J Psychiatry. 2005a;162:1733–5. doi: 10.1176/appi.ajp.162.9.1733. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. Volume of the cingulate and outcome in schizophrenia. Schizophr Res. 2005b;72:91–108. doi: 10.1016/j.schres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, et al. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–72. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168:1154–63. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Nakayama N, Miwa K, Okumura A, Soeda A, Iwama T. Focal brain glucose hypometabolism in patients with neuropsychologic deficits after diffuse axonal injury. AJNR. 2007;28:236–42. [PMC free article] [PubMed] [Google Scholar]
- Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp Brain Res. 2006;172:556–60. doi: 10.1007/s00221-006-0503-x. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Huang XF. Ionotropic glutamate receptor binding in the posterior cingulate cortex in schizophrenia patients. Neuroreport. 2005;16:1363–7. doi: 10.1097/01.wnr.0000174056.11403.71. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Jew SK, Huang XF. Alterations of muscarinic and GABA receptor binding in the posterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:225–33. doi: 10.1016/j.pnpbp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Oblak AL, Rosene DL, Kemper TL, Bauman ML, Blatt GJ. Altered posterior cingulate cortical cyctoarchitecture, but normal density of neurons and interneurons in the posterior cingulate cortex and fusiform gyrus in autism. Autism Res. 2011;4:200–11. doi: 10.1002/aur.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–33. [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci USA. 2006;103:1563–8. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JM, Hayden BY, Raghavachari S, Platt ML. Neurons in posterior cingulate cortex signal exploratory decisions in a dynamic multioption choice task. Curr Biol. 2009;19:1532–7. doi: 10.1016/j.cub.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn Sci. 2011;15:143–51. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annual review of neuroscience. 2012;35:73. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, et al. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–94. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Muller-Oehring E, Shankaranarayanan A, Alsop DC, et al. Cerebral blood flow in posterior cortical nodes of the default mode network decreases with task engagement but remains higher than in most brain regions. Cereb Cortex. 2011;21:233–44. doi: 10.1093/cercor/bhq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127(Pt 12):2703–16. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Posner ML, Petersen SE. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Heo S, Voss MW, Patterson B, Kramer AF. Age-related differences in cortical recruitment and suppression: implications for cognitive performance. Behav Brain Res. 2012;230:192–200. doi: 10.1016/j.bbr.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, Kuceyeski A, Weiner M. A network diffusion model of disease progression in dementia. Neuron. 2012;73:1204–15. doi: 10.1016/j.neuron.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1997;379:313–32. [PubMed] [Google Scholar]
- Rudge P, Warrington EK. Selective impairment of memory and visual perception in splenial tumours. Brain. 1991;114(Pt 1B):349–60. doi: 10.1093/brain/114.1.349. [DOI] [PubMed] [Google Scholar]
- Samann PG, Wehrle R, Hoehn D, Spoormaker VI, Peters H, Tully C, et al. Development of the brain's default mode network from wakefulness to slow wave sleep. Cereb Cortex. 2011;21:2082–93. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2013 doi: 10.1111/j.1552-6569.2012.00779.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, et al. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31:839–52. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Bedard AC, Clerkin SM, Ivanov I, Tang CY, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69:952–61. doi: 10.1001/archgenpsychiatry.2011.2053. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan M. Metastable chimera states in community-structured oscillator networks. Chaos. 2010a;20:013108. doi: 10.1063/1.3305451. [DOI] [PubMed] [Google Scholar]
- Shanahan M. Embodiment and the inner life: Cognition and Consciousness in the Space of Possible Minds. Oxford: Oxford University Press; 2010b. [Google Scholar]
- Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134(Pt 8):2233–47. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–12. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderqvist S, McNab F, Peyrard-Janvid M, Matsson H, Humphreys K, Kere J, et al. The SNAP25 gene is linked to working memory capacity and maturation of the posterior cingulate cortex during childhood. Biol Psychiatry. 2010;68:1120–5. doi: 10.1016/j.biopsych.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–86. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Levitt J, Thompson PM, Holmes CJ, Blanton RE, Kornsand DS, et al. Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. Am J Psychiatry. 2000;157:1475–84. doi: 10.1176/appi.ajp.157.9.1475. [DOI] [PubMed] [Google Scholar]
- Spreng RN. The fallacy of a ‘task-negative’ network. Front Psychol. 2012;3:145. doi: 10.3389/fpsyg.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci. 2010;22:1112–23. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 2012;22:2610–21. doi: 10.1093/cercor/bhr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–74. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Lovejoy D, Kim J, Oakes H, Kureshi I, Witt ST. Multiple resting state network functional connectivity abnormalities in mild traumatic brain injury. Brain imaging and behavior. 2012;6(2):293–318. doi: 10.1007/s11682-012-9157-4. [DOI] [PubMed] [Google Scholar]
- Sun L, Cao Q, Long X, Sui M, Cao X, Zhu C, et al. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naive boys with attention deficit hyperactivity disorder. Psychiatry Res. 2012;201:120–7. doi: 10.1016/j.pscychresns.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Sun Z, Wang F, Cui L, Breeze J, Du X, Wang X, et al. Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. Neuroreport. 2003;14:1833–6. doi: 10.1097/00001756-200310060-00015. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 1997;49:464–9. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–37. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–46. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Kahn RS, Goñi J, Sporns O. High-cost, high-capacity backbone for global brain communication. Proc Natl Acad Sci USA. 2012;109:11372–7. doi: 10.1073/pnas.1203593109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Groen T, Vogt BA, Wyss JM. Interconnection between the thalamus and retrosplenial cortex in the rodent brain. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser; 1993. [Google Scholar]
- Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–71. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–31. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Structural organization of the cingulate cortex: areas, neurons, and somatodendritic transmitter receptors. In: Vogt BA, Gaybriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser; 1993. pp. 19–70. [Google Scholar]
- Vogt BA. Cingulate neurobiology and disease. Oxford: Oxford University Press; 2009. [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–17. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–89. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–70. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–66. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt LJ, Perl DP, Hof PR. Cytology of human caudomedial cingulate, retrosplenial, and caudal parahippocampal cortices. J Comp Neurol. 2001;438:353–76. doi: 10.1002/cne.1320. [DOI] [PubMed] [Google Scholar]
- Wachtel PL. Conceptions of broad and narrow attention. Psychol Bull. 1967;68:417–29. doi: 10.1037/h0025186. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Horti AG, Raymont V, Brasic J, Guevara M, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage. 2010;52:1505–13. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Corticothalamic connections of paralimbic regions in the rhesus monkey. J Comp Neurol. 1988;269:130–46. doi: 10.1002/cne.902690111. [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, et al. Increased neural resources recruitment in the intrinsic organization in major depression. J Affect Disord. 2010;121:220–30. doi: 10.1016/j.jad.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Zang YF, Cao QJ, Yan CG, He Y, Jiang TZ, et al. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. Neuroimage. 2008;40:110–20. doi: 10.1016/j.neuroimage.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, et al. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–7. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]