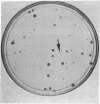
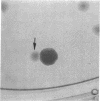
Abstract
The bacteriophage P1 infects and functions as a generalized transducing phage for nitrogen-fixing strains of the coliform bacterium Klebsiella pneumoniae. Bacterial mutants (nif-) unable to grow on molecular nitrogen as a nitrogen source were found to be deficient in nitrogenase activity as assayed by the conversion of acetylene to ethylene. These mutants regained normal nitrogenase activity and the ability to grow on N2 after transduction with lysates of P1 phage prepared from wild-type bacteria. Transductional analysis with P1 revealed that several nif genes are located on the genetic linkage map of Klebsiella near the histidine operon.
Keywords: mutants, P1 phage, histidine operon, nitrogenase
Full text
PDF
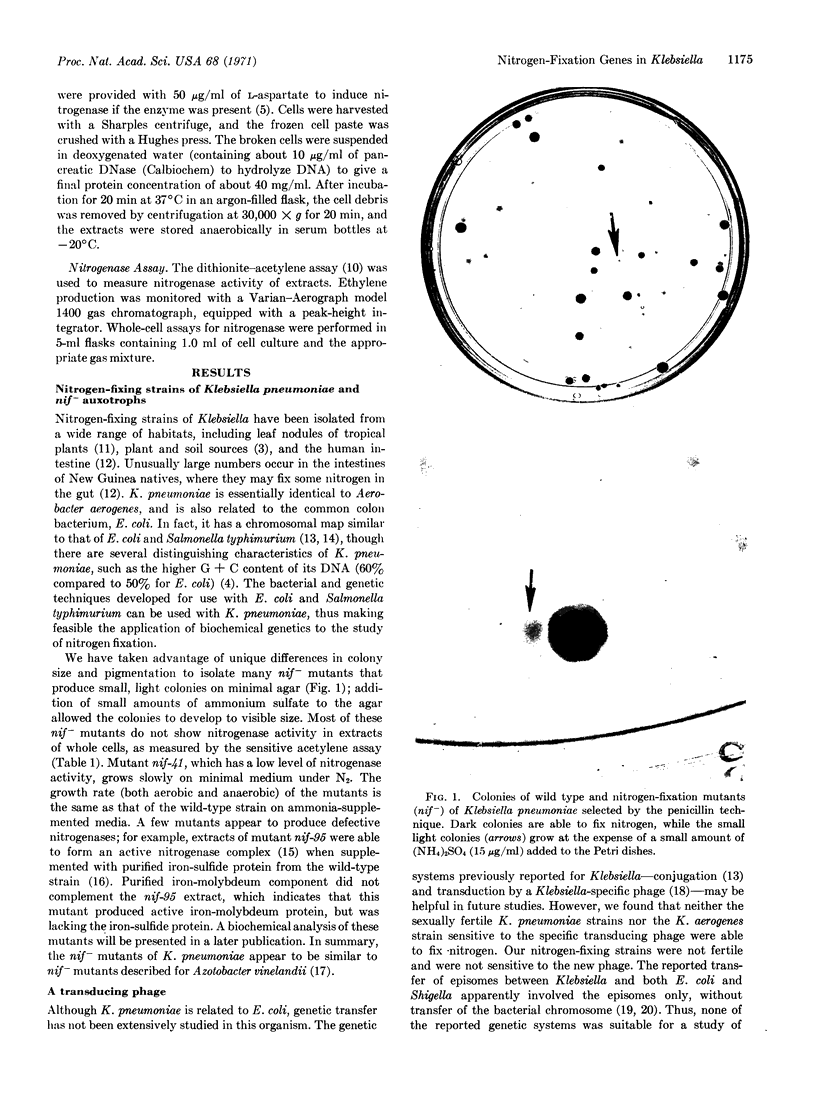
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Hipsley E. H. The presence of N2-fixing bacteria in the intestines of man and animals. J Gen Microbiol. 1970 Jan;60(1):61–65. doi: 10.1099/00221287-60-1-61. [DOI] [PubMed] [Google Scholar]
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris R. H. Progress in the biochemistry of nitrogen fixation. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):339–354. doi: 10.1098/rspb.1969.0025. [DOI] [PubMed] [Google Scholar]
- Fisher R. J., Brill W. J. Mutants of Azotobacter vinelandii unable to fix nitrogen. Biochim Biophys Acta. 1969 Jun 17;184(1):99–105. doi: 10.1016/0304-4165(69)90103-2. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R. V., Iyer V. N. Genetic and molecular properties of an infectious antibiotic resistance (R) factor isolated from Klebsiella. J Bacteriol. 1969 Nov;100(2):605–616. doi: 10.1128/jb.100.2.605-616.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. Comparisons and cross reactions of nitrogenase from Klebsiella pneumoniae, Azotobacter chroococcum and Bacillus polymyxa. Biochim Biophys Acta. 1969;191(3):527–540. doi: 10.1016/0005-2744(69)90346-5. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- MAHL M. C., WILSON P. W., FIFE M. A., EWING W. H. NITROGEN FIXATION BY MEMBERS OF THE TRIBE KLEBSIELLEAE. J Bacteriol. 1965 Jun;89:1482–1487. doi: 10.1128/jb.89.6.1482-1487.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee D. G., Sutherland I. W., Wilkinson J. F. Transduction in Klebsiella. Nature. 1969 Feb 1;221(5179):475–476. doi: 10.1038/221475a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T. Genetic recombination in Klebsiella pneumoniae. An approach to genetic linkage mapping. Jpn J Microbiol. 1970 Mar;14(2):129–141. doi: 10.1111/j.1348-0421.1970.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Ouellette C. A., Burris R. H., Wilson P. W. Deoxyribonucleic acid base composition of species of Klebsiella, Azotobacter and Bacillus. Antonie Van Leeuwenhoek. 1969;35(3):275–286. doi: 10.1007/BF02219149. [DOI] [PubMed] [Google Scholar]
- Roth J. R. UGA nonsense mutations in Salmonella typhimurium. J Bacteriol. 1970 May;102(2):467–475. doi: 10.1128/jb.102.2.467-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver W. S. Biology and ecology of nitrogen fixation by symbiotic associations of non-leguminous plants. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):389–400. doi: 10.1098/rspb.1969.0028. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Pietersma K. Deletion-mapping of resistance against chlorate in Klebsiella aerogenes. Mol Gen Genet. 1970;106(2):174–179. doi: 10.1007/BF00323836. [DOI] [PubMed] [Google Scholar]
- Wolf B., Newman A., Glaser D. A. On the origin and direction of replication of the Escherichia coli K12 chromosome. J Mol Biol. 1968 Mar 28;32(3):611–629. doi: 10.1016/0022-2836(68)90346-x. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Pengra R. M. Effect of amino acids on the nitrogenase system of Klebsiella pneumoniae. J Bacteriol. 1966 Sep;92(3):618–622. doi: 10.1128/jb.92.3.618-622.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]