Abstract
The purpose of this prospective randomized, single blind study was to determine the anesthetic efficacy of 68.8 mg of lidocaine with 50 μg epinephrine compared to 68.8 mg lidocaine with 50 μg epinephrine plus 0.9 M mannitol in inferior alveolar nerve (IAN) blocks. Forty subjects randomly received 2 IAN blocks consisting of a 1.72-mL formulation of 68.8 mg lidocaine with 50 μg epinephrine and a 5-mL formulation of 68.8 mg lidocaine with 50 μg epinephrine (1.72 mL) plus 0.9 M mannitol (3.28 mL) in 2 separate appointments spaced at least 1 week apart. Mandibular anterior and posterior teeth were blindly electric pulp tested at 4-minute cycles for 60 minutes postinjection. No response from the subject to the maximum output (80 reading) of the pulp tester was used as the criterion for pulpal anesthesia. Total percent pulpal anesthesia was defined as the total of all the times of pulpal anesthesia (80 readings), for each tooth, over the 60 minutes. One hundred percent of the subjects had profound lip numbness with both inferior alveolar nerve blocks. The results demonstrated that the 5 mL-formulation of 68.8 mg lidocaine with 50 μg epinephrine plus 0.9 M mannitol was significantly better than the 1.72-mL formulation of 68.8 mg lidocaine with 50 μg epinephrine for all teeth, except the lateral incisor. We concluded that adding 0.9 M mannitol to a lidocaine with epinephrine formulation was significantly more effective in achieving a greater percentage of total pulpal anesthesia (as defined in this study) than a lidocaine formulation without mannitol. However, the 0.9 M mannitol/lidocaine formulation would not provide 100% pulpal anesthesia for all the mandibular teeth.
Key Words: Inferior alveolar nerve block, Lidocaine, Mannitol
The inferior alveolar nerve (IAN) block is the most frequently used injection technique for achieving local anesthesia for mandibular restorative and surgical procedures. However, the IAN block does not always result in successful pulpal anesthesia.1–6 Failure rates (never achieving 2 consecutive 80 readings with the electric pulp tester) of 10 to 39% have been reported.1 A possible reason for failure is the perineurial barrier around the nerve may not allow complete diffusion of the anesthetic solution into the nerve trunk.
Previous studies by Wolf et al7 and Smith et al8 demonstrated that lidocaine with epinephrine in combination with 0.5 M mannitol significantly improved the success of the IAN block (defined as the total of all the times of pulpal anesthesia [80 readings], for each tooth, over the 60 minutes) in asymptomatic subjects. Kreimer et al9 found, for mandibular posterior teeth in patients with symptomatic irreversible pulpitis, that the addition of 0.5 M mannitol to 1.9 mL of lidocaine with epinephrine resulted in a statistically higher success rate (success was defined as no or mild pain on visual analog scale [VAS] recordings) upon endodontic assess or instrumentation when compared to a lidocaine formulation without mannitol. The proposed mechanism is that mannitol opens the perineurial membrane to allow for enhanced penetrability for lipophilic compounds,10 and it may affect nerve conduction.11
However, 100% pulpal anesthesia was not obtained by Wolf et al,7 Smith et al,8 or Kreimer et al.9 Perhaps increasing the molarity of mannitol from 0.5 M to 0.9 M could potentially allow the anesthetic solution to permeate the nerve trunk in greater amounts, thereby increasing anesthetic efficacy. The purpose of this prospective randomized, single blind study was to determine the anesthetic efficacy of 68.8 mg of lidocaine with 50 μg epinephrine compared to 68.8 mg lidocaine with 50 μg epinephrine plus 0.9 M mannitol in IAN blocks. Pain of injection and postoperative pain were also studied.
MATERIALS AND METHODS
Forty adult subjects participated in this study. The subjects were in good health and were not taking any medications that would alter pain perception. Exclusion criteria were as follows: younger than 18 years of age; allergies to mannitol, local anesthetics, or sulfites; pregnancy; history of significant medical conditions (American Society of Anesthesiologists, ASA, Class II or higher); taking any medications (over-the-counter pain-relieving medications, narcotics, sedatives, antianxiety, or antidepressant medications) that may affect pain assessment; active pathosis at the site of injection; and inability to give informed consent. The Ohio State University Human Subjects Review Committee approved the study, and written informed consent was obtained from each subject.
Using a crossover design, 40 adult subjects received 2 IAN blocks consisting of a 1.72-mL formulation of 68.8 mg lidocaine with 50 μg epinephrine and a 5-mL formulation of 68.8 mg lidocaine with 50 μg epinephrine (1.72 mL) plus 0.9 M mannitol (3.28 mL) in 2 separate appointments spaced at least 1 week apart.
Equal numbers of mandibular right and left sides were tested, with the first and second molars, first and second premolars, and lateral and central incisors chosen as the test teeth.
With the crossover design, 80 IAN blocks were administered, and each subject served as his or her own control. The same side chosen for the first IAN block was used again for the second IAN block. The mandibular contralateral canine was used as the control to ensure that the pulp tester was operating properly and that the subject was responding appropriately. A visual and clinical examination was conducted to ensure that all teeth were free of caries, large restorations, crowns, periodontal disease, and that none had a history of trauma or sensitivity.
Before the injection at each of the 2 appointments, the experimental tooth and the contralateral canine (control) were tested 3 times with the electric pulp tester (Kerr, Analytic Technology Corp, Redmond, Wash) to ensure tooth vitality and obtain baseline information. The teeth were isolated with cotton rolls and dried with an air syringe. Toothpaste was applied to the probe tip, which was placed in the middle third of the buccal or labial surface of the tooth being tested. The value at the initial sensation was recorded. The current rate was set at 25 seconds to increase from no output (0) to the maximum output (80). Trained personnel, who were blinded to the anesthetic formulations, administered all preinjection and postinjection tests.
Before the experiment, the 2 anesthetic formulations were randomly assigned 5-digit numbers from a random number table. Each subject was randomly assigned to each of the 2 anesthetic formulations to determine which formulation was to be administered at each appointment. Only the random numbers were recorded on the data collection sheets to further blind the experiment.
The anesthetic formulations were prepared as follows. Under sterile conditions, 1.72 mL of 4% lidocaine (Abbott Laboratories, North Chicago, Ill) from a 5-mL single-dose ampule was drawn into a 5-mL Luer-Lok disposable syringe (Becton-Dickinson & Co, Rutherford, NJ). Added to this solution was 0.05 mL of 1 : 1000 epinephrine (American Regent Laboratories Inc, Shirley, NY) drawn from a 1-mL ampule using a 1-mL tuberculin syringe (Becton-Dickinson & Co). All solutions used were checked to ensure that they had not expired. The 1.72-mL formulation contained 68.8 mg of lidocaine with 50 μg epinephrine. For the second formulation, 1.72 mL of 2% lidocaine with 50 μg epinephrine was prepared as described above. Using a 1-mL tuberculin syringe (Becton-Dickinson & Co), 3.28 mL of 0.5 M mannitol was added to the 5-mL syringe. The syringe was then inverted 20 times to mix the solution. The 3.28 mL of mannitol was withdrawn from a 50-mL vial of a 25% (12.5 g/50 mL) supersaturated mannitol solution (American Regent Laboratories). Each vial was used only once. Before the mannitol was added to the syringe containing the lidocaine with epinephrine, the 50-mL vial was heated in a water bath (Teledyne Hanau, Buffalo, NY) to 80°C for 15 minutes to remove any crystals present in the supersaturated solution. The vial was then allowed to cool to room temperature before use. The 5-mL formulation contained 68.8 mg of lidocaine with 50 μg of epinephrine (1.72 mL) plus 0.9 M mannitol (3.28 mL). No precipitate formed when the mannitol was combined with the lidocaine. Selected components and selected final anesthetic formulations had their pH values determined using a pH/millivolt meter (Orion Research Inc, Boston, Mass).
A few calculations are necessary to explain the molarity for the final volume of the lidocaine/mannitol formulation. Molarity (M), or molar concentration, is defined as a ratio between the number of moles of a solute per liter of solution. The mole of a compound is the amount of the compound in grams equal to its molecular weight. Therefore, the number of moles in 12.5 grams of mannitol would be: moles of mannitol = 12.5 g (per 50 mL vial) × 1 mol/182.17 g (molecular weight of mannitol) = 0.0686 moles. The molarity of the mannitol solution used in this study would be: molarity = 0.0686 moles mannitol/0.05 L solution = 1.372 M. Because the solutions were diluted by the lidocaine with epinephrine solution, the final molarity must be calculated from the molarity after dilution using the following formula: (Mi) (Vi) = (Mf)(Vf) where Mi is the initial molarity multiplied by Vi (the initial volume) is equal to Mf (the final molarity) multiplied by Vf (the final volume). Therefore, for the mannitol formulation the calculated molarity of 0.9 M mannitol would require the following volumes: (1.372 M)(X) = (0.9 M)(5 mL) = 3.28 mL of mannitol. For the total volume of 5 mL of a 0.9 M mannitol solution, 1.72 mL of lidocaine would be combined with 3.28 mL of mannitol.
A standard IAN block12 was administered with a 27-gauge 1½-inch needle (Monoject; Sherwood Medical, St Louis, Mo) using the 2 anesthetic formulations. Following needle penetration, and as the needle was advanced during placement, 0.2 mL of solution was deposited. After the target area was reached and aspiration was performed, 1 minute was used to deposit the anesthetic solution, and the subject was asked to rate the pain of solution deposition. The pain scale was from 0 to 3. Zero indicated no pain. One indicated mild pain, that is pain that was recognizable but not discomforting. Two indicated moderate pain, pain that was discomforting but bearable. Three indicated severe pain, pain that caused considerable discomfort and was difficult to bear. The principal investigator (H.C.) performed all IAN injections.
At 1 minute after the IAN block, the first and second molars were pulp tested. At 2 minutes, the first and second premolars were tested. At 3 minutes, the central and lateral incisors were tested. At 4 minutes, the control canine was tested. This cycle of testing was repeated every 4 minutes for 60 minutes. At every fourth cycle, the control tooth (the contralateral canine) was tested with a pulp tester without batteries to test the reliability of the subject. If the subject responded positively to an inactivated pulp tester, then they were unreliable and could not be used in the study. Subjects were asked if their lips/tongues were numb every minute for 5 minutes and at every fourth minute during pulp testing. If profound lip numbness was not recorded within 5 minutes, the block was considered unsuccessful and the subject was then reappointed. Seven of 80 (9%) IAN blocks (2 with the lidocaine formulation and 5 with the lidocaine/mannitol formulation) were unsuccessful in this study, and these subjects required an additional appointment. All testing was stopped at 60 minutes postinjection.
All subjects completed postinjection surveys after each IAN block administered. The subjects rated pain in the injection area, using the previous pain scale (none, mild, moderate, severe), immediately after the numbness wore off and again each morning upon arising for 3 days. The subjects were also asked to record subjectively any additional comments or side effects not related to pain.
No response from the subject at the maximum output (80 reading) of the pulp tester was used as the criterion for pulpal anesthesia. Total percent pulpal anesthesia was defined as the total of all the times of pulpal anesthesia (80 readings) over the 60 minutes. With a nondirectional alpha risk of 0.05 and assuming a total percent pulpal anesthesia rate of 10% for the central incisor,8,9 a sample size of 40 subjects was required to demonstrate a difference in pulpal anesthesia of ±28 percentage points with a power of 0.83.
Comparisons between the 2 formulations regarding total percent pulpal anesthesia were assessed using Mantel-Haenszel chi-square test. A separate model was used for each tooth. The dependent variable was the binary response of anesthesia (80 reading or no 80 reading), and the independent variable was formulation. Comparisons between the 2 formulations regarding solution deposition pain and postinjection pain were made using multiple Wilcoxon, matched-pairs, signed-rank tests adjusted using the step-down Bonferroni method of Holm. Comparisons were considered significant at P < .05.
RESULTS
Twenty-four women and 16 men, ranging in age from 18 to 40 years, with an average age of 24 years, participated in this study.
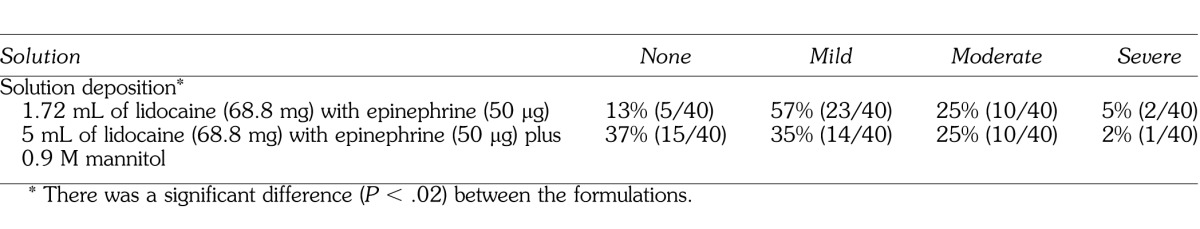
One hundred percent of the subjects used for the data analysis had subjective lip and tongue anesthesia with the IAN blocks. The discomfort ratings of solution deposition for the 2 formulations are presented in Table 1. There was a significant difference (P < .05) between the formulations. The pH of the solutions was: 6.99 for mannitol, 6.68 for the 4% lidocaine, 3.37 for the 1 : 1000 epinephrine solution, 6.64 for the final formulation of lidocaine with epinephrine, and 6.76 for the final formulation of lidocaine/mannitol.
Table 1.
Percentages and Discomfort Ratings of Solution Deposition
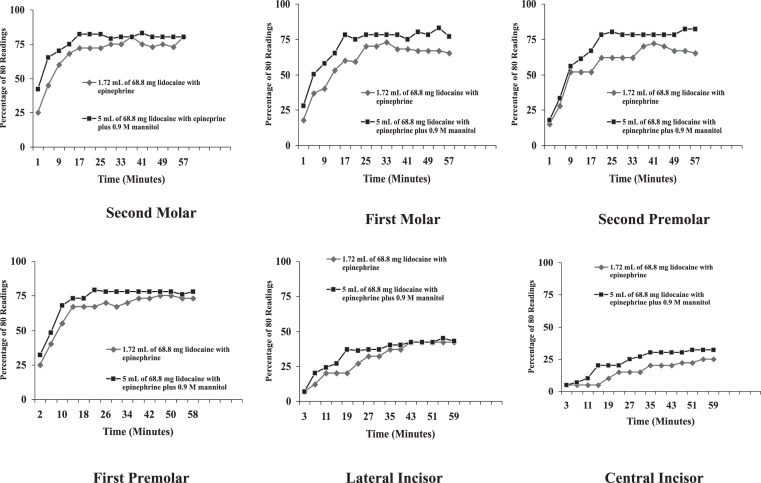
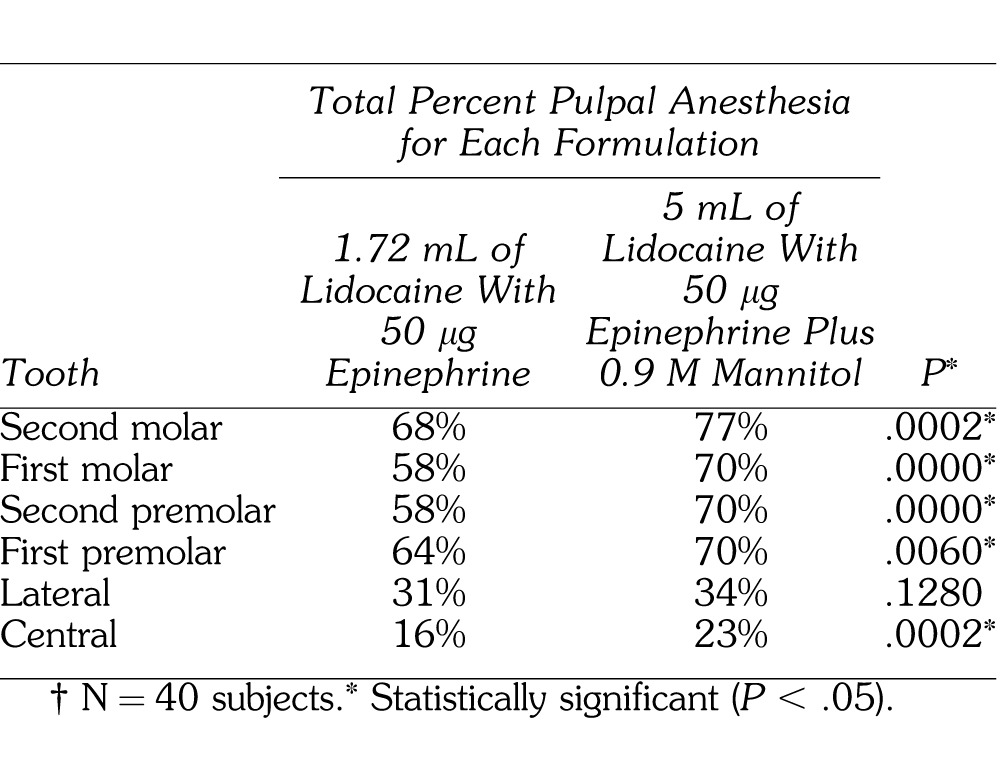
Total percent pulpal anesthesia is presented in Table 2. The posterior teeth had higher values of total pulpal anesthesia than the anterior teeth. The 5-mL formulation of 68.8 mg of lidocaine with 50 μg of epinephrine (1.72 mL) plus 0.9 M mannitol (3.28 mL) was statistically better than the 1.72-mL formulation of 68.8 mg lidocaine with 50 μg epinephrine for all teeth, except the lateral incisor. The incidence of pulpal anesthesia for the 2 formulations is presented in the Figure.
Table 2.
Total Percent Pulpal Anesthesia†
Incidence of pulpal anesthesia for the molars, premolars, lateral and central incisors as determined by lack of response to electrical pulp testing at the maximum setting (percentage of 80/80's), at each postinjection time interval, for the two anesthetic formulations.
The postoperative pain ratings are summarized in Table 3. There was a significant difference (P < .05) between the formulations on Day 0 and Day 3. Table 4 summarizes the number of subjects reporting trismus for the 2 formulations.
Table 3.
Percentages and Discomfort Ratings for Postinjection Survey†
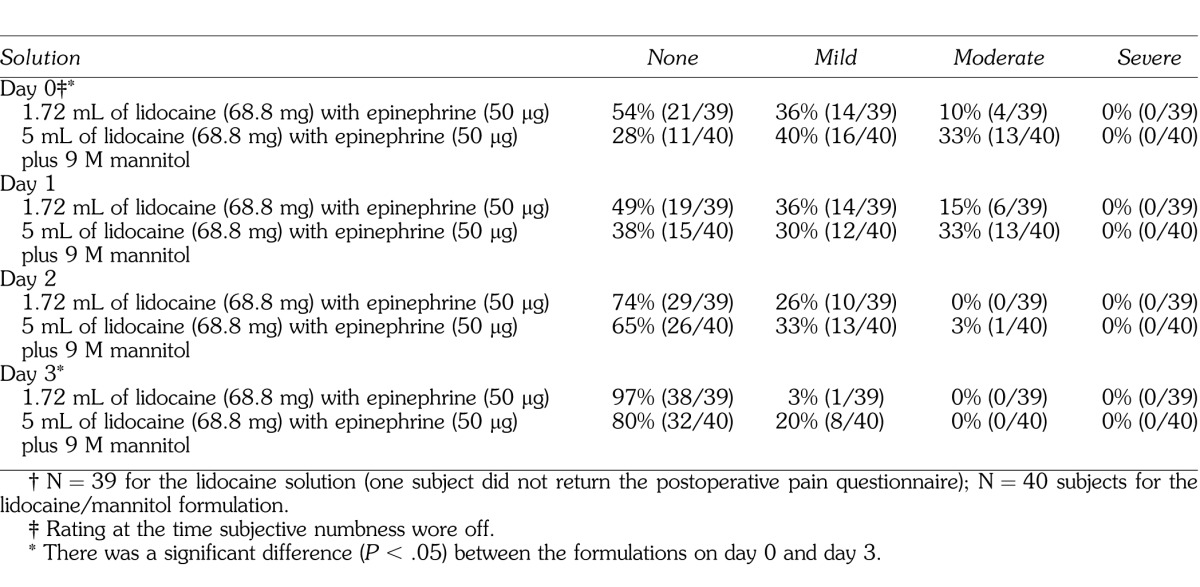
Table 4.
Percentage and Number of Subjects Reporting Postoperative Trismus
DISCUSSION
The use of the 80 reading (signaling maximum output) as a criterion for pulpal anesthesia was based on the studies of Dreven et al13 and Certosimo and Archer.14 These studies13,14 showed that no patient response to an 80 reading with the electric pulp tester ensured pulpal anesthesia in vital asymptomatic teeth. Additionally, Certosimo and Archer14 demonstrated that electric pulp test readings less than 80 resulted in pain during operative procedures in asymptomatic teeth.
Total percent pulpal anesthesia with 1.72 mL of 68.8 mg of lidocaine with 50 μg of epinephrine for the IAN block occurred from 16 to 68% of the time (Table 2) (Figure). Previous studies by Nusstein et al1 and Wali et al6 found that increasing the volume of 2% lidocaine with epinephrine to 3.6 mL or increasing the epinephrine concentration in a lidocaine formulation did not increase the incidence of pulpal anesthesia with the IAN block. In the study by Wolf et al,7 total pulpal anesthesia for 1.8 mL of 36 mg with 18 μg epinephrine ranged from 12 to 75% for the IAN block, which is similar to the current study. Smith et al8 found that 3.18 mL of 127.2 mg with 50 μg epinephrine resulted in total pulpal anesthesia of 25 to 77%. Therefore, there was a small gain by increasing the amount of lidocaine and epinephrine. However, total pulpal anesthesia was not attained in 100% of all teeth tested.
The addition of 0.9 M mannitol to 68.8 mg of lidocaine with 50 μg of epinephrine resulted in a statistically higher percent total pulpal anesthesia for all teeth, except the lateral incisor, when compared to 68.8 mg of lidocaine with 50 μg epinephrine (Table 2). In these teeth, the increase in percent total pulpal anesthesia, over the 68.8 mg of lidocaine with of 50 μg epinephrine, ranged from 6 to 12% (Table 2). This would seem to confirm the study by Antonijevic et al10 who found that mannitol opened the perineurial membrane allowing for an enhanced penetrability of lipophilic compounds or, based on the findings of Matsuka and Spigelman,11 the mannitol possibly delayed or blocked action potential propagation in selective neurons. Wolf et al7 found the percent of total pulpal anesthesia ranged from 20 to 93% with 5 mL of 63.6 mg lidocaine with 36 μg epinephrine plus 0.5 M mannitol. Smith et al8 found the percent total pulpal anesthesia with 3.18 mL of 127.2 mg of lidocaine with 50 μg of epinephrine plus 0.5 M mannitol for the IAN block was 29 to 89%. There was a lower incidence of total pulpal anesthesia for most teeth (23 to 77%) in the current study than recorded by Wolf et al7 and Smith et al.8 It may be the higher concentration of 0.9 M mannitol was not as efficient as a 0.5 M concentration. Antonijevic et al10 investigated the effects of different mannitol concentrations (0.5, 1.0, 2.0 M) on perineurial permeability. They found 0.5 M mannitol was the optimal concentration. Therefore, a higher molar concentration (0.9 M) may be less efficient when combined with lidocaine than the 0.5 M mannitol/lidocaine formulation.
Therefore, either formulation would not provide 100% pulpal anesthesia for all the mandibular teeth (Table 2, Figure), which could present meaningful clinical problems since the teeth may not be anesthetized for procedures requiring complete pulpal anesthesia. Practitioners should consider supplemental techniques, such as intraosseous,15–18 mandibular infiltrations of 4% articaine with 1 : 100,000 epinephrine,19,20 or intraligamentary injections21 when anesthetic formulations fail to provide pulpal anesthesia for a particular tooth. Because we studied a young adult population, the results of this study may not apply to children or the elderly.
In the formulation with mannitol, both the lidocaine and epinephrine concentrations were diluted. While we could have added additional formulations to the study by increasing the total injected volume of lidocaine with epinephrine without mannitol (by adding normal saline or a similar solution), we felt that dilution would not increase the success of the IAN block. Overall, combining mannitol with the lidocaine with epinephrine formulation did statistically increase success. Therefore, diluting the formulations with mannitol increased the efficacy of the lidocaine with epinephrine. Wolf et al7 and Smith et al8 found similar results when diluting mannitol/lidocaine formulations.
We chose a hyperosmolar solution of mannitol since studies9,21 have shown it is inert and it has been used extensively in medicine.22 Antonijevic et al10 demonstrated the injection of 0.5 M mannitol into rat plantar subcutaneous paw tissue was without ill effect. The hypertonic solution of mannitol did not induce an inflammatory cell infiltrate when the tissues were examined histologically.10
In order to keep the mannitol concentration at 0.9 M, the final volume of the solution has to be calculated based on the formula for molarity (see Materials and Methods). The result is a higher volume of solution than would be used if only the lidocaine solution was administered. The 25% (12.5 g/50 mL) solution of mannitol was chosen for this study because it was the highest concentration available commercially. Therefore, when combining the mannitol with lidocaine, the final volume of the solution could still be kept to a clinically acceptable amount. When mannitol was combined with the lidocaine solution, no precipitate formed. Because mannitol is inert,10,22 we would not expect it to chemically combine or react with the lidocaine or epinephrine. The pH values of the lidocaine and lidocaine/mannitol formulations were similar, 6.60 and 6.72, respectively. It is unlikely that pH caused differences in success rates of the IAN blocks.
Moderate solution deposition pain was 25%, with 2 to 5% of patients reporting severe pain (Table 1). Other studies2,4,5,7,8 of the IAN block, using 2% lidocaine with 1 : 100,000 epinephrine, have reported a 20 to 25% incidence of moderate/severe pain. Therefore, the IAN block has the potential to be painful even though the solution was deposited slowly over 1 minute. Previous studies by Wolf et al7 and Smith et al8 found no statistical difference between lidocaine/mannitol formulations and a lidocaine formulation for solution deposition pain during the inferior alveolar nerve block. In the current study, the lidocaine formulation was statistically more painful for solution deposition than the lidocaine/mannitol formulation (Table 1). The lower pain ratings may have been because the lidocaine with epinephrine was diluted with the addition of mannitol.
Moderate postinjection pain, at the time subjective numbness wore off, was 10% for the lidocaine with epinephrine formulation (Table 3). The percentage was similar for the lidocaine with epinephrine solution in other studies,2,4,5,7,8 using 2% lidocaine with 1 : 100,000 epinephrine, in which a 14 to 17% incidence of moderate/severe pain was reported at the time subjective numbness wore off. Judging from this study and others,2–5,7,8 the IAN block has the potential to result in moderate postoperative pain. For the lidocaine formulation, moderate pain increased by day 1 to 15%, with no reports of moderate pain for days 2 and 3 (Table 3). Similar results have been reported in other studies2,4,5,7,8 of postoperative pain for the IAN block.
For the lidocaine/mannitol formulation, 33% had moderate pain at the time subjective numbness wore off and at day 1 (Table 3). The percentages decreased to 3% moderate pain at day 2 and 0% at day 3. Wolf et al7 reported an incidence of 25% moderate pain at the time subjective numbness wore off for both a 2.84-mL formulation of 36 mg lidocaine with 18 μg epinephrine plus 0.5 M mannitol and a 5-mL formulation of 63.6 mg lidocaine with 36 μg epinephrine plus 0.5 M mannitol. Smith et al8 found a 5-mL formulation of 127.2 mg lidocaine with 50 μg epinephrine plus 0.5 M mannitol had a 22% incidence of moderate pain when subjective numbness wore off and a 15% incidence of moderate pain at day 1, which decreased to 2% at day 2, and 0% at day 3. Wolf et al7 also reported decreasing pain (2 to 0% over 3 days) for the lidocaine/mannitol formulations used in their study. The higher ratings in the current study may be related to the higher molarity of mannitol. We postulated that the higher molarity caused fluid to be removed from the cells causing reversible tissue irritation. Previous studies by Wolf et al7 and Smith et al8 did not record higher postoperative pain with 0.5 M mannitol/lidocaine formulations.
No patients experienced trismus postoperatively with the lidocaine formulation (Table 4). Previous studies of the IAN block using 2% lidocaine with 1 : 100,000 epinephrine recorded a 3 to 9% incidence of trismus postoperatively.4,5 Wolf et al7 using 36 mg lidocaine (36 μg of epinephrine) without mannitol and 36 mg lidocaine (36 μg of epinephrine) and 63.6 mg of lidocaine (36 μg of epinephrine) with mannitol found a 2 to 5% incidence of trismus. Smith et al8 using 127.2 mg of lidocaine with 50 μg epinephrine found a 12% incidence at the time subjective numbness wore off; these moderate ratings continued for day 1, decreased to 2% for day 2, and then increased to 17% for day 3. They related this to the increased amount of lidocaine (127.2 mg). The formulation containing 0.9 M mannitol had a 10% incidence of trismus from day 1 through 3 (Table 4). The molarity of 0.9 used in the current study probably caused the higher incidence of trismus in comparison to the lidocaine formulation.
CONCLUSIONS
We concluded that adding 0.9 M mannitol to 68.8 mg lidocaine with 50 μg epinephrine was significantly more effective in achieving a greater percentage of total pulpal anesthesia than a formulation of 68.8 mg lidocaine with 50 μg epinephrine without mannitol. However, the 0.9 M mannitol/lidocaine formulation would not provide 100% pulpal anesthesia for all mandibular teeth.
ACKNOWLEDGMENTS
This study was supported by Graduate Endodontic Research Funds and The Steven Goldberg Memorial Fund.
REFERENCES
- 1.Nusstein J, Reader A, Beck FM. Anesthetic efficacy of different volumes of lidocaine with epinephrine for inferior alveolar nerve blocks. Gen Dent. 2002;50:372–375. [PubMed] [Google Scholar]
- 2.Nist R, Reader A, Beck M, Meyers W. An evaluation of the incisive nerve block and combination inferior alveolar and incisive nerve blocks in mandibular anesthesia. J Endod. 1992;18:455–459. doi: 10.1016/S0099-2399(06)80849-6. [DOI] [PubMed] [Google Scholar]
- 3.Clark S, Reader A, Beck M, Meyers WJ. Anesthetic efficacy of the mylohyoid nerve block and combination inferior alveolar nerve block/mylohyoid nerve block. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:557–563. doi: 10.1016/s1079-2104(99)70133-2. [DOI] [PubMed] [Google Scholar]
- 4.Mikesell P, Nusstein J, Reader A, Beck M, Weaver J. A comparison of articaine and lidocaine for inferior alveolar nerve blocks. J Endod. 2005;31:265–270. doi: 10.1097/01.don.0000140576.36513.cb. [DOI] [PubMed] [Google Scholar]
- 5.Ridenour S, Reader A, Beck M, Weaver J. Anesthetic efficacy of a combination of hyaluronidase and lidocaine with epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2001;48:9–15. [PMC free article] [PubMed] [Google Scholar]
- 6.Wali M, Drum M, Reader A, Nusstein J. Prospective, randomized single-blind study of the anesthetic efficacy of 1.8 and 3.6 milliliters of 2% lidocaine with 1 : 50,000 epinephrine for inferior alveolar nerve blocks. J Endod. 2010;36:1459–1462. doi: 10.1016/j.joen.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Wolf R, Reader A, Drum M, Nusstein J, Beck M. Anesthetic efficacy of combinations of 0.5 M mannitol and lidocaine with epinephrine in inferior alveolar nerve blocks: a prospective randomized, single-blind study. Anesth Prog. 2011;58:157–165. doi: 10.2344/11-30.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith S, Reader A, Drum M, Nusstein J, Beck M. Anesthetic efficacy of a combination of 0.5 M mannitol plus 127.2 mg of lidocaine with 50 μg epinephrine in inferior alveolar nerve blocks: a prospective randomized, single blind study. Anesth Prog. 2012;60:3–10. doi: 10.2344/11-00040.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreimer T, Kiser R, II, Reader A, Nusstein J, Drum M, Beck M. Anesthetic efficacy of combinations of 0.5 mol/L mannitol and lidocaine with epinephrine for inferior alveolar nerve blocks in patients with symptomatic irreversible pulpitis. J Endod. 2012;38:598–603. doi: 10.1016/j.joen.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Antonijevic I, Mousa S, Schafer M, Stein C. Perineural defect and peripheral opioid analgesia in inflammation. J Neurosci. 1995;15:165–172. doi: 10.1523/JNEUROSCI.15-01-00165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuka Y, Spigelman I. Hyperosmolar solutions selectively block action potentials in rat myelinated sensory fibers: Implications for diabetic neuropathy. J Neurophysiol. 2004;91:48–56. doi: 10.1152/jn.00689.2003. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen NB, Hayden J., Jr . Premedication, Local and General Anesthesia in Dentistry. 2nd ed. Philadelphia, Pa: Lea & Febiger;; 1967. [Google Scholar]
- 13.Dreven L, Reader A, Beck M, Meyers W, Weaver J. An evaluation of the electric pulp tester as a measure of analgesia in human vital teeth. J Endod. 1987;13:233–238. doi: 10.1016/s0099-2399(87)80097-3. [DOI] [PubMed] [Google Scholar]
- 14.Certosimo A, Archer R. A clinical evaluation of the electric pulp tester as an indicator of local anesthesia. Oper Dent. 1996;21:25–30. [PubMed] [Google Scholar]
- 15.Dunbar D, Reader A, Nist R, Beck M, Meyers W. Anesthetic efficacy of the intraosseous injection after an inferior alveolar nerve block. J Endod. 1996;22:481–486. doi: 10.1016/S0099-2399(96)80083-5. [DOI] [PubMed] [Google Scholar]
- 16.Guglielmo A, Reader A, Nist R, Beck M, Weaver J. Anesthetic efficacy and heart rate effects of the supplemental intraosseous injection of 2% mepivacaine with 1 : 20,000 levonordefrin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:284–293. doi: 10.1016/s1079-2104(99)70210-6. [DOI] [PubMed] [Google Scholar]
- 17.Gallatin E, Stabile P, Reader A, Nist R, Beck M. Anesthetic efficacy and heart rate effects of the intraosseous injection of 3% mepivacaine after an inferior alveolar nerve block. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:83–87. doi: 10.1016/s1079-2104(00)80019-0. [DOI] [PubMed] [Google Scholar]
- 18.Stabile P, Reader A, Gallatin E, Beck M, Weaver J. Anesthetic efficacy and heart rate effects of the intraosseous injection of 1.5% etidocaine (1 : 200,000 epinephrine) after an inferior alveolar nerve block. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:407–411. doi: 10.1016/s1079-2104(00)70120-x. [DOI] [PubMed] [Google Scholar]
- 19.Haase A, Reader A, Nusstein J, Beck M, Drum M. Comparing anesthetic efficacy of articaine versus lidocaine as a supplemental buccal infiltration of the mandibular first molar after an inferior alveolar nerve block. J Am Dent Assoc. 2008;139:1228–1235. doi: 10.14219/jada.archive.2008.0338. [DOI] [PubMed] [Google Scholar]
- 20.Kanaa MD, Whitworth JM, Corbett IP, Meechan JG. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J. 2009;42:238–246. doi: 10.1111/j.1365-2591.2008.01507.x. [DOI] [PubMed] [Google Scholar]
- 21.Childers M, Reader A, Nist R, Beck M, Meyers W. The anesthetic efficacy of the periodontal ligament injection after an inferior alveolar nerve block. J Endod. 1996;22:317–320. doi: 10.1016/S0099-2399(96)80267-6. [DOI] [PubMed] [Google Scholar]
- 22.Neuwelt EA, Dahlborg SA. Blood-brain barrier disruption in the treatment of brain tumors. Clinical implications. In: Neuwelt EA, editor. Implications of the Blood-Brain Barrier and Its Manipulation. Vol 2. Plenum; New York, NY: 1989. pp. 195–261. In. ed. [Google Scholar]