Abstract
The purpose of this study was to identify the risk factors associated with low peripheral oxygen saturation (SpO2) and delayed recovery of dental patients with disabilities after intravenous sedation. A total of 1213 patients with disabilities were retrospectively investigated with respect to demographic parameters and sedation conditions. Multivariate logistic analyses were conducted for patients with an SpO2 <90% and a recovery period of >60 minutes to identify the risk factors for poor sedation conditions. A significant odds ratio related to decreased SpO2 was observed for age, sex, midazolam and propofol levels, concurrent use of nitrous oxide, cerebral palsy, Down syndrome, and mental retardation. The most problematic patients were those diagnosed with Down syndrome (odds ratio, 3.003–7.978; 95% confidence interval; P < .001). Decision tree analysis showed an increased risk of decreased SpO2 in males with Down syndrome or after administration of >0.493 mg/kg propofol in combination with midazolam. An increased risk of delayed awakening was seen in patients aged less than 21 years and in males administered >0.032 mg/kg of midazolam. Intravenous sedation for dental patients with disabilities, particularly those with cerebral palsy, Down syndrome, or mental retardation, increases the risk of decreased SpO2. In addition, delayed recovery is expected after midazolam administration.
Key Words: Dental sedation, Low peripheral oxygen saturation, Delayed recovery
Dental practices are currently challenged by the rapidly growing number of patients with intellectual or physical disabilities.1,2 Excessive mental strain during dental treatment can cause systemic complications such as vasovagal reflex, neurogenic shock, pain shock, and hyperventilation. Furthermore, patients with cardiovascular diseases, including cerebrovascular disorders, or decreased vital organ reserve capacity can encounter serious complications. A strategy for relieving mental strain is important for safe dental treatment of such patients, and to this end, intravenous sedation is often used.3,4 However, when using intravenous sedative drugs that have strong systemic actions on the central nervous, respiratory, and circulatory systems, systemic management to ensure patient safety is a prerequisite.5,6
Conscious sedation is generally preferred to maintain independent breathing and biological defense mechanisms such as coughing and swallowing reflexes. However, dental treatment of patients with disabilities may require behavioral control, especially in the case of mentally challenged individuals with strong treatment refusal reactions. In these cases, deeper levels of intravenous sedation are a safer option.
Depending upon the individual case, increased drug doses can cause deep sedation until the patient becomes completely unconscious, which is a deeper degree of sedation compared with conscious sedation.6,7 If this deep sedative state overrides the nervous system, basic defense mechanisms may also be lost. Therefore, careful perioperative management, similar to that for general anesthesia, is necessary.
Therefore, dental treatment of mentally or physically impaired patients using intravenous anesthetics requires careful perioperative management, similar to general anesthesia. Unfortunately, there is little information available on the disabilities and sedation conditions particularly at risk of causing low peripheral oxygenation and delayed recovery.8,9
In this study, we investigated and analyzed the risk factors that may be involved in causing decreased peripheral oxygen saturation (SpO2) and delayed recovery, including age, sex, treatment duration, type of disability or disease, and type and dose of anesthetic, in dental patients with disabilities.
METHODS
Over the past 7 years, a total of 1335 patients with disabilities received dental treatment under deep intravenous sedation at the dental care division of the National Medical and Educational Consulting Center and at the National Welfare Foundation for Disabled Children. The 122 patients who were administered flumazenil, a benzodiazepine antagonist used for reversal of deep sedation, were excluded from the study. Therefore, this retrospective study included 1213 patients.
The electronic records of each patient were analyzed with respect to demographic data, type of disabilities, and sedation conditions. The types of mental disabilities listed in these records were Alzheimer disease, autism, cerebral palsy, Down syndrome, dysautonomia, dementia, dental phobia, and mental retardation. The types of physical disabilities included epilepsy, asthma, hypertension, diabetes mellitus, and abnormal gag reflex.
The sedation conditions listed in these records were nitrous oxide combination, treatment duration, drugs and their levels used in intravenous sedation, and recovery time required from the end of surgery to regaining consciousness.
Each of the above mentioned items was cross-tabulated for patients with intraoperative minimum SpO2 <90% and those with delayed awakening. Consciousness was evaluated depending on its recovery; recovery of vital signs, swallowing function, and the ability to drink and urinate independently; and Romberg test results. Patients who required ≥60 minutes to achieve this state after the end of treatment were included as subjects. Following the chi-square test, multivariate logistic regression and decision tree analyses were performed. To determine the factors involved in decreased SpO2 and those affecting delayed recovery, multivariate logistic regression analysis was performed. Statistical analysis was performed using a stepwise method (step-up procedure: likelihood ratio) for each disease and the following explanatory variables: age, gender, treatment duration (from the start of anesthesia to the end of the treatment), and type and level of drugs. IBM SPSS Statistics 19 (IBM, Tokyo, Japan) was used as the statistical software. All values are expressed as mean ± SD, and differences with P < .05 were considered statistically significant.
RESULTS
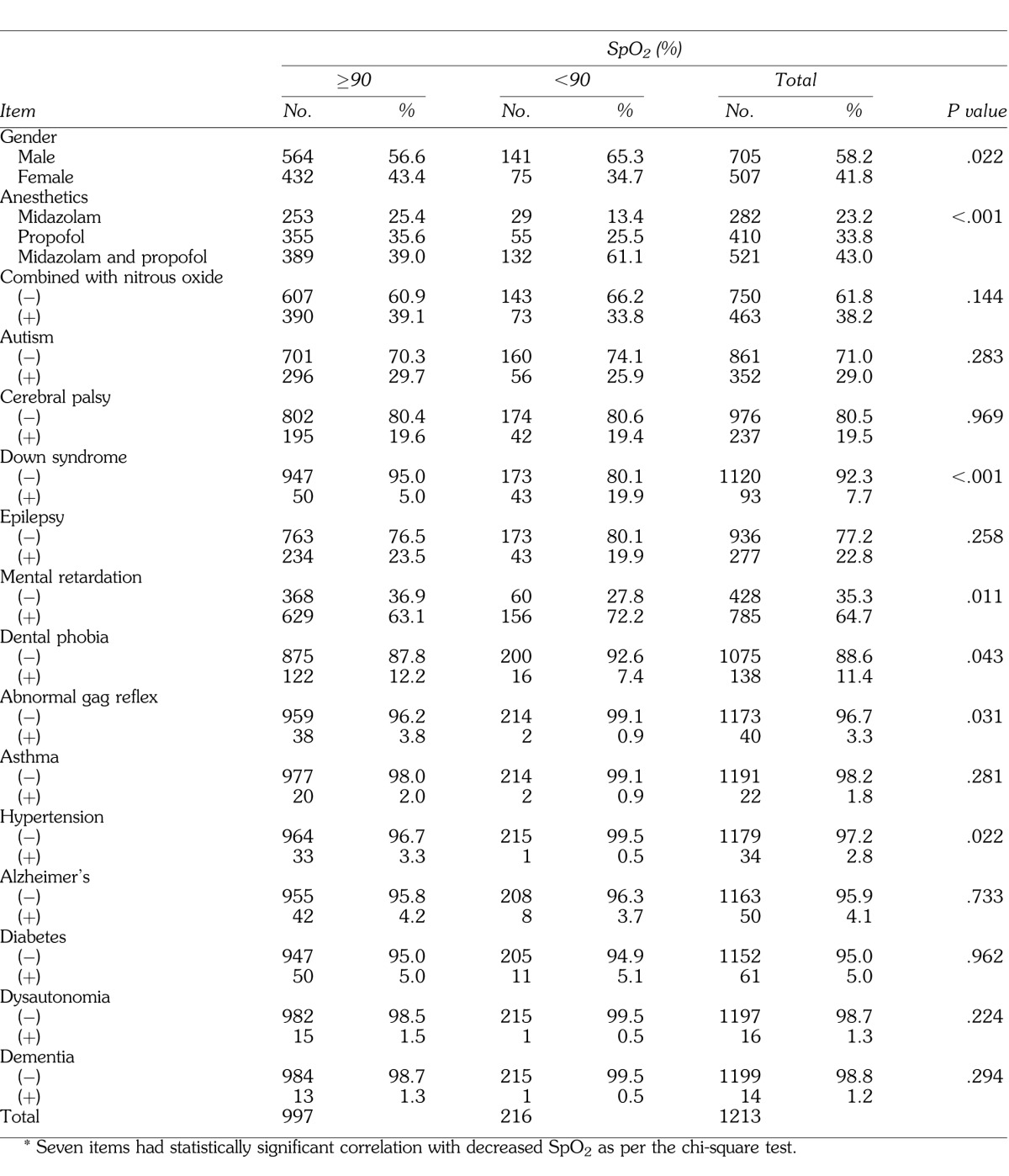
We included 1213 patients (705 male, 508 female) with a mean age of 36 ± 14 years (range, 9–83 years) in our study. Mean time from the start of sedation to the end of treatment was 43.0 ± 19.8 minutes, and the mean time required from the end of treatment to recovery of consciousness was 33.0 ± 18.5 minutes. The drugs used for intravenous sedation were midazolam (23.2%; 0.07 ± 0.36 mg/kg) or propofol (33.8%; 1.69 ± 0.99 mg/kg). When midazolam and propofol were used in combination (43.0%), the mean doses were 0.68 ± 0.04 mg/kg and 1.35 ± 0.85 mg/kg, respectively. Nitrous oxide was also used in 463 patients (38.2%) sedated with each drug used alone or in combination. Table 1 represents the impact of the type of sedation on SpO2 during dental treatment. Among the 1213 patients, 216 (17.8%) exhibited SpO2 <90%. Chi-square analysis revealed a significant effect of sedation type on the regulation of SpO2 (P < .001). Among the mental and physical disabilities listed in the records, Down syndrome, mental retardation, dental phobia, abnormal gag reflex, and hypertension were all associated with lower oxygenation during dental treatment under intravenous sedation (Table 1).
Table 1.
Cross-Tabulation of Decreased Peripheral Oxygen Saturation (SpO2) and Each Item*
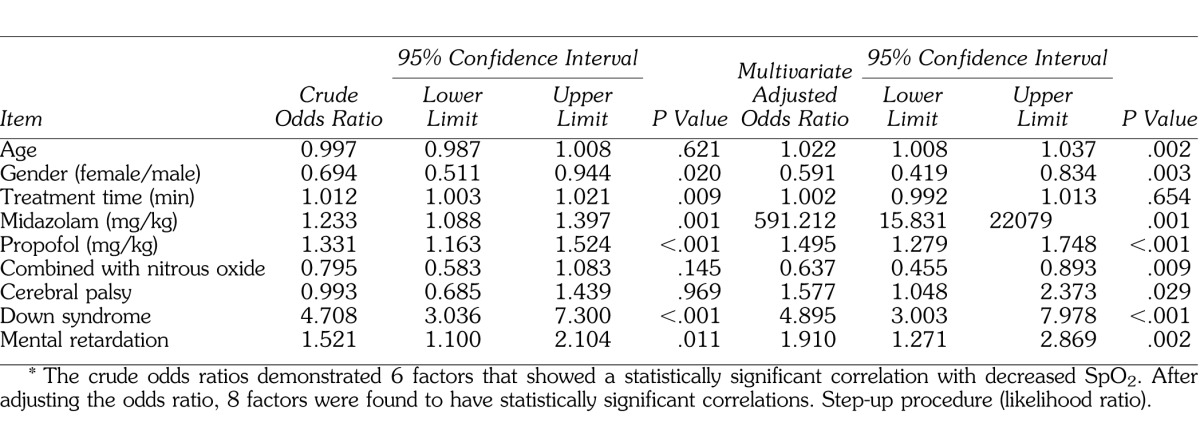
Multivariate logistic regression analyses revealed significant odds ratios for cerebral palsy, Down syndrome, mental retardation, age, sex, and all sedation protocols (Table 2). Again, the most significant findings were observed in patients diagnosed with Down syndrome, with an adjusted odds ratio of 4.895–3.003 (95% confidence interval; P < .001). In total, these analyses suggest that patients with mental disabilities are particularly at risk of oxygen deprivation during dental treatment under intravenous sedation in the following ascending order according to disability type: cerebral palsy < mental retardation < Down syndrome.
Table 2.
Results of Logistic Regression Analysis for Decreased Peripheral Oxygen Saturation (SpO2 < 90%)*
Ninety of the 1213 patients (7.4%) required a longer recovery period, and patients with a delay of ≥60 minutes were cross-tabulated according to the type of drug and disease (Table 3). The chi-square test showed significant differences for sex and the type of sedative.
Table 3.
Cross-Tabulation of the Recovery Time and Each Item*
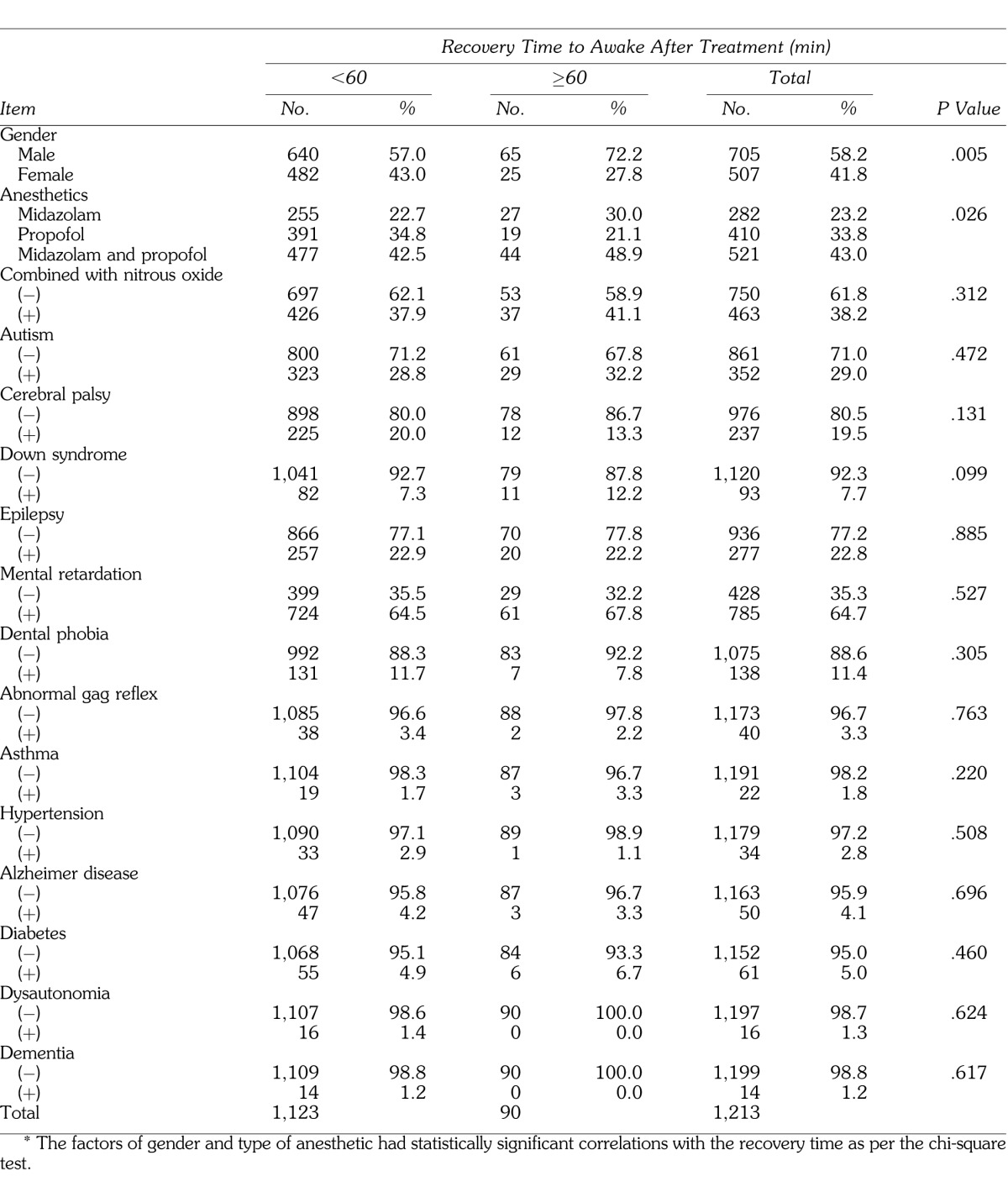
To determine the factors affecting delayed recovery, multivariate logistic regression analysis was performed. A significant difference was found for sex and midazolam levels (Table 4).
Table 4.
Results of Logistic Regression Analysis for Recovery Time (≥60 minutes)*
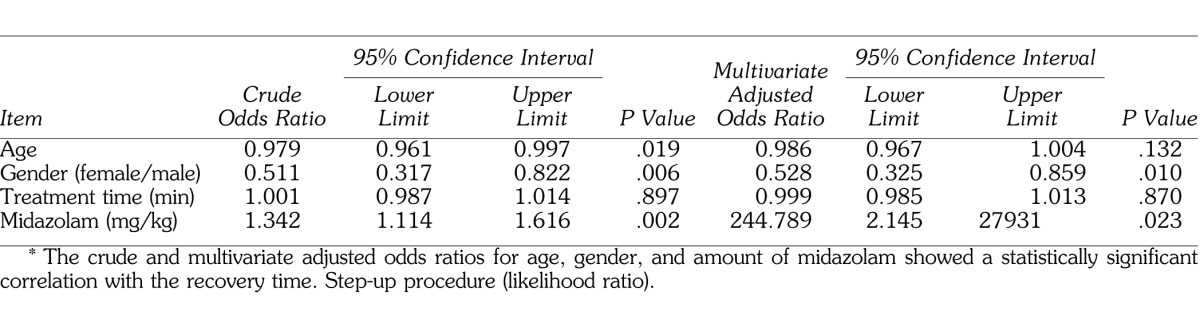
In addition, to verify the possibility of drug interactions among midazolam, propofol, and nitrous oxide, the factor of drug interaction was added as a factor to the results from Tables 2 and 4, and these results are shown in Tables 5 and 6. For the variables included under drug interactions, each mean was converted to 0 and the product was used as the interaction value to prevent the occurrence of multicollinearity. Multivariate logistic regression analysis using the drug interaction value demonstrated that neither decreased SpO2 nor delayed recovery showed a significant odds ratio for the factor of drug interaction (Tables 5 and 6).
Table 5.
Results of Logistic Regression Analysis for Decreased Peripheral Oxygen Saturation (90%), Including Interaction Terms of Anesthetics*
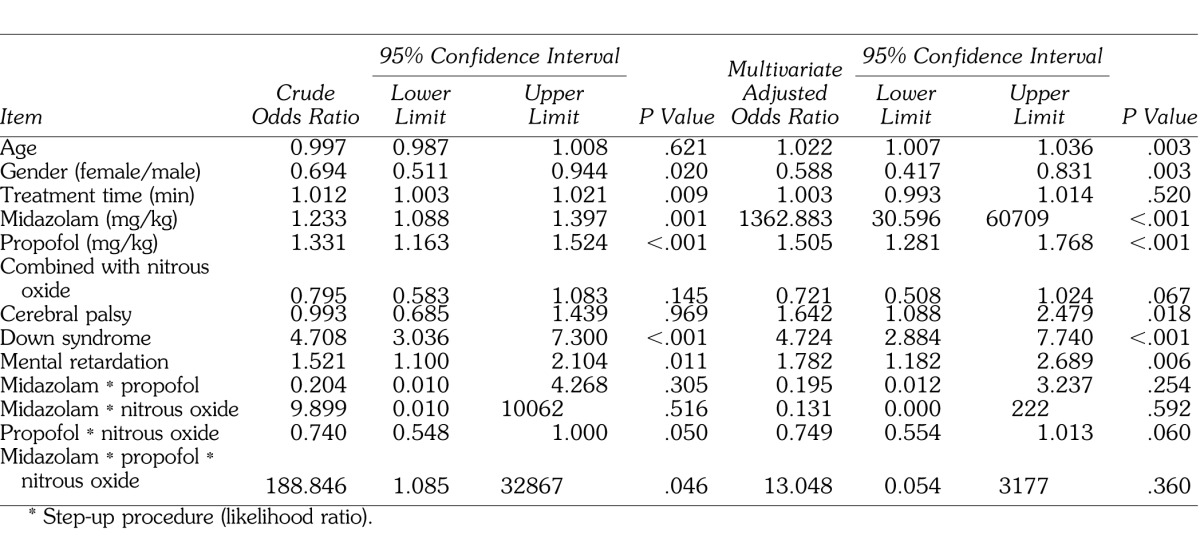
Table 6.
Results of Logistic Regression Analysis for Recovery Time (≥60 Minutes), Including Interaction Terms of Anesthetics*
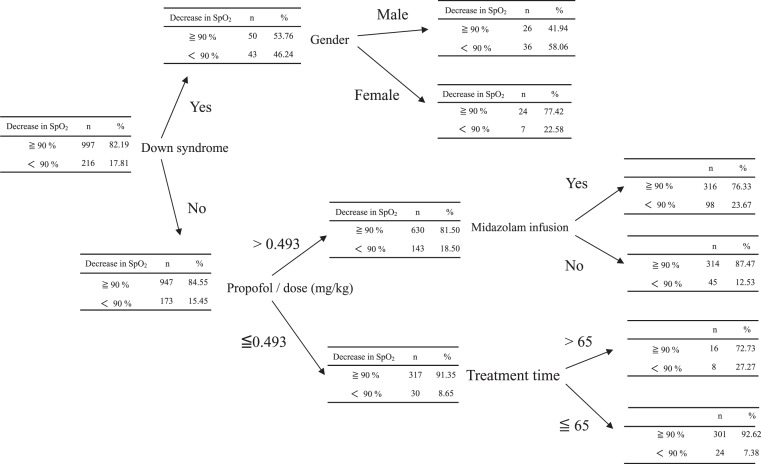
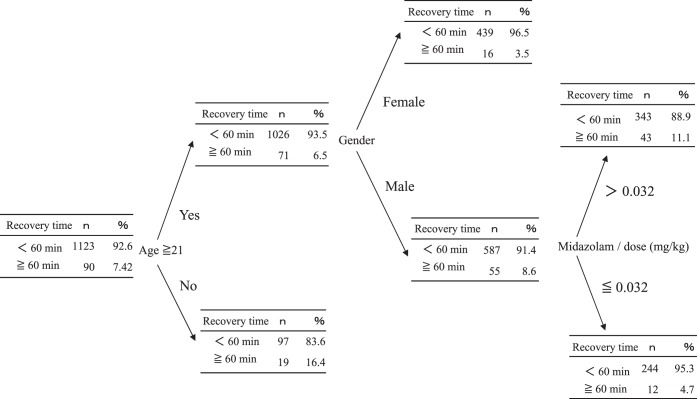
Similarly, decision tree analysis showed a significantly higher risk of decreased SpO2 in males with Down syndrome or males administered >0.493 mg/kg of propofol in combination with midazolam. In addition, even patients administered <0.493 mg/kg of propofol for >65 minutes of treatment had a high risk of decreased SpO2 (Figure 1). A higher probability of delayed recovery was seen in patients aged <21 years and in males administered >0.032 mg/kg of midazolam (Figure 2).
Figure 1.
Decrease in peripheral oxygen saturation (SpO2) was used as an objective variable. Down syndrome was the highest risk factor for decrease in SpO2.
Figure 2.
Results of decision analysis for recovery time (≥60 minutes). Recovery time (≥60 minutes) was used as an objective variable. Age and gender were the highest risk factors for prolongation of recovery time.
DISCUSSION
Patients with mental and physical disabilities often exhibit maladaptive behavior and are not cooperative during dental treatment, which justifies the use of intravenous sedation for their safety.3 Compared with general anesthesia, intravenous sedation is easy to perform and is often used in dental treatment.10,11 However, close monitoring of vital signs is essential because these drugs have strong systemic actions on the nervous, respiratory, and circulatory systems.5,6 The present study identified the safest sedative and patients at the highest risk of experiencing respiratory depression during and delayed recovery after intravenous sedation for dental treatment.
Among the demographic parameters, gender was the most consistent and significant factor affecting respiratory function and recovery period. Female patients were at lower risk of low SpO2 and delayed recovery time. There are several reports on sex-related differences with respect to the effects of anesthesia,12–15 all suggesting that women have lower sensitivity to anesthetics and recover consciousness more rapidly. Some researchers have argued that female hormones such as progesterone may explain these phenomena.15 Accordingly, male patients should be more closely monitored during dental treatment under intravenous sedation.
Decreased SpO2 can be caused by occlusion of the upper respiratory tract following motion suppression, sedative drug overdose, or deep sedation16; transient glossoptosis caused by choking or cough reflex17; or the use of instruments such as those used for maintaining mouth opening during oral manipulations.
This study identified 3 mental disabilities associated with a high risk of poor sedation control during dental treatment in the following descending order: Down syndrome > mental retardation > cerebral palsy. Nearly 50% of patients with Down syndrome exhibit upper-airway obstruction and have congenital heart disease, both risk factors for pulmonary hypertension (review: King et al. 201118). It has been suggested that low SpO2 is caused by factors such as sleep apnea and upper-airway obstruction due to the presence of a large tongue.19,20 Therefore, these patients are particularly at risk of cardiovascular complications and low SpO2 during intravenous sedation. In the case of cerebral palsy and mental retardation, the patients can suffer from upper-airway stenosis.21,22 Accordingly, the perioperative management of breathing functions is vital during dental treatment under sedation.
Patients who required ≥60 minutes for recovery were included in logistic regression analysis and decision tree analysis that demonstrated midazolam levels to be a risk factor for prolonged recovery time. Midazolam is used during dental treatment for disabled patients,23,24 especially for its amnesic effect and behavior control, although higher doses can easily result in deep sedation. It is also known to provide a longer duration of action compared with propofol, suggesting delayed recovery.
During dental treatment of patients with mental retardation who show vigorous treatment-refusal actions, a period of deep sedation is intentionally selected for controlling such behavior. In such a scenario, independent maintenance of airways is physiologically difficult. Although spontaneous respiration is maintained, respiration and circulation are depressed and basic defense mechanisms are partially suppressed, resulting in decreased SpO2.6 To prevent these signs, oxygen and emergency equipment must be kept ready6 and consciousness, ventilation, oxygenation, and circulation statuses should be carefully monitored. Along with maintaining the defense mechanisms, airway management has to be carefully performed. Titrated drug administration and precise perioperative systemic management are also very important.
The present study suggests that patients diagnosed with Down syndrome, mental retardation, and cerebral palsy should be more closely monitored during dental treatment under intravenous sedation.
REFERENCES
- 1.Glassman P. A review of guidelines for sedation, anesthesia, and alternative interventions for people with special needs. Spec Care Dentist. 2009;29:9–16. doi: 10.1111/j.1754-4505.2008.00056.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang YC, Lin IH, Huang CH, Fan SZ. Dental anesthesia for patients with special needs. Acta Anaesthesiol Taiwan. 2012;50:122–125. doi: 10.1016/j.aat.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Ransford NJ, Manley MC, Lewis DA, et al. Intranasal/intravenous sedation for the dental care of adults with severe disabilities: a multicentre prospective audit. Br Dent J. 2010;208:565–569. doi: 10.1038/sj.bdj.2010.501. [DOI] [PubMed] [Google Scholar]
- 4.Chaushu S, Gozal D, Becker A. Intravenous sedation: an adjunct to enable orthodontic treatment for children with disabilities. Eur J Orthod. 2002;24:81–89. doi: 10.1093/ejo/24.1.81. [DOI] [PubMed] [Google Scholar]
- 5.Dionne RA, Yagiela JA, Moore PA, et al. Comparing efficacy and safety of four intravenous sedation regimens in dental outpatients. J Am Dent Assoc. 2001;132:740–751. doi: 10.14219/jada.archive.2001.0271. [DOI] [PubMed] [Google Scholar]
- 6.American Society of Anesthesiologists Task Force on Sedation and Anesthesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1017. [Google Scholar]
- 7.Messieha Z, Cruz-Gonzalez W, Hakim MI. Retrospective outcomes evaluation of 100 parenteral moderate and deep sedations conducted in a general practice dental residency. Anesth Prog. 2008;55:116–120. doi: 10.2344/0003-3006-55.4.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Lima AR, da Costa LR, da Costa PS. A randomized, controlled, crossover trial of oral midazolam and hydroxyzine for pediatric dental sedation. Pesqui Odontol Bras. 2003;17:206–211. doi: 10.1590/s1517-74912003000300002. [DOI] [PubMed] [Google Scholar]
- 10.Boyle CA, Manley MC, Fleming GJ. Oral midazolam for adults with learning disabilities. Dent Update. 2000;27:190–192. doi: 10.12968/denu.2000.27.4.190. [DOI] [PubMed] [Google Scholar]
- 11.Beyer R, Seyde WC. Propofol versus midazolam. Long-term sedation in the intensive care unit. Anaesthesist. 1992;41:335–341. [PubMed] [Google Scholar]
- 12.Gan TJ, Glass PS, Sigl J, et al. Women emerge from general anesthesia with propofol/alfentanil/nitrous oxide faster than men. Anesthesiology. 1999;90:1283–1287. doi: 10.1097/00000542-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan FF, Myles PS, Cicuttini F. Patient sex and its influence on general anaesthesia. Anaesth Intensive Care. 2009;37:207–2118. doi: 10.1177/0310057X0903700201. [DOI] [PubMed] [Google Scholar]
- 14.Pleym H, Spigset O, Kharasch ED, Dale O. Gender differences in drug effects: implications for anaesthetists. Acta Anaesthesiol Scand. 2003;47:241–259. doi: 10.1034/j.1399-6576.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 15.Buchanan FF, Myles PS, Cicuttini F. Effect of patient sex on general anaesthesia and recovery. Br J Anaesth. 2011;106:832–839. doi: 10.1093/bja/aer094. [DOI] [PubMed] [Google Scholar]
- 16.Ayuse T, Inazawa T, Kurata S, et al. The mouth opening increases upper-airway collapsibility without changing resistance during midazolam sedation. J Dent Res. 2004;83:718–722. doi: 10.1177/154405910408300912. [DOI] [PubMed] [Google Scholar]
- 17.Kohjitani A, Egusa M, Shimada M, Miyawaki T. Accumulated oropharyngeal water increases coughing during dental treatment with intravenous sedation. J Oral Rehabil. 2008;35:203–208. doi: 10.1111/j.1365-2842.2007.01752.x. [DOI] [PubMed] [Google Scholar]
- 18.King P, Tulloh R. Management of pulmonary hypertension and Down syndrome. Int J Clin Pract Suppl. 2011;174:8–13. doi: 10.1111/j.1742-1241.2011.02823.x. [DOI] [PubMed] [Google Scholar]
- 19.Southall DP, Stebbens VA, Mirza R, Lang MH, Croft CB, Shinebourne EA. Upper airway obstruction with hypoxaemia and sleep disruption in Down syndrome. Dev Med Child Neurol. 1987;29:734–742. doi: 10.1111/j.1469-8749.1987.tb08818.x. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson JD, Redmond WM. Surgical management of obstructive sleep apnea in children with Down syndrome. J Otolaryngol. 1988;17:398–403. [PubMed] [Google Scholar]
- 21.Preciado DA, Sidman JD, Sampson DE, Rimell FL. Mandibular distraction to relieve airway obstruction in children with cerebral palsy. Arch Otolaryngol Head Neck Surg. 2004;130:741–745. doi: 10.1001/archotol.130.6.741. [DOI] [PubMed] [Google Scholar]
- 22.Kavanagh KT, Beckford NS. Airway obstruction in the mentally handicapped. South Med J. 1992;85:779–781. doi: 10.1097/00007611-199207000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury J, Vargas KG. Comparison of chloral hydrate, meperidine, and hydroxyzine to midazolam regimens for oral sedation of pediatric dental patients. Pediatr Dent. 2005;27:191–197. [PubMed] [Google Scholar]
- 24.Fukuta O, Braham RL, Yanase H, Kurosu K. The sedative effects of intranasal midazolam administration in the dental treatment of patients with mental disabilities. Part 2: optimal concentration of intranasal midazolam. J Clin Pediatr Dent. 1994;18:259–265. [PubMed] [Google Scholar]