Abstract
Since 2008, three new analgesic entities, tapentadol immediate release (Nucynta) diclofenac potassium soft gelatin capsules (Zipsor), and bupivacaine liposome injectable suspension (EXPAREL) were granted US Food and Drug Administration (FDA) approval to treat acute pain. Tapentadol immediate-release is a both a mu-opioid agonist and a norepinephrine reuptake inhibitor, and is indicated for the treatment of moderate to severe pain. Diclofenac potassium soft gelatin capsules are a novel formulation of diclofenac potassium, which is a nonsteroidal anti-inflammatory drug (NSAID), and its putative mechanism of action is through inhibition of cyclooxygenase enzymes. This novel formulation of diclofenac allows for improved absorption at lower doses. Liposomal bupivacaine is a new formulation of bupivacaine intended for single-dose infiltration at the surgical site for postoperative analgesia. Bupivacaine is slowly released from this liposomal vehicle and can provide prolonged analgesia at the surgical site. By utilizing NSAIDs and local anesthetics to decrease the transmission of afferent pain signals, less opioid analgesics are needed to achieve analgesia. Since drug-related adverse events are frequently dose related, lower doses from different drug classes may be employed to reduce the incidence of adverse effects, while producing synergistic analgesia as part of a multimodal analgesic approach to acute pain.
Key Words: Liposomal bupivacaine, Tapentadol, Diclofenac potassium soft gelatin capsules, Analgesics
Pain, as defined by the International Association for the Study of Pain, is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 Although there is a subjective and perhaps emotional component to pain, acute pain is usually proportional to the degree of tissue damage.2
Pain may serve a protective mechanism to the individual by signaling tissue injury or organ dysfunction that may otherwise result in illness if left unnoticed and untreated. However, pain that is secondary to a surgical intervention is undesirable. Postoperative pain can result in increased sympathetic nervous system activity, guarded breathing, increased likelihood of thrombi formation, and delayed recovery.3 The benefits of adequate postoperative analgesia include improved respiratory, cardiovascular, and gastrointestinal function, improved arterial graft survival, and decreased incidence of thrombotic or septic complications. Not only does adequate postoperative analgesia result in earlier return to function, more rapid patient mobilization, improved healing, reduced health care costs, and improved patient satisfaction, but it may play a role in preventing the progression of chronic pain.4–6
Opioids have traditionally been the most common analgesic for treating moderate to severe postoperative pain.2 However, their utility is hampered by undesirable side effects, which are sometimes intolerable to the patient. These side effects include central nervous depression, respiratory depression, pruritus, nausea, vomiting, ileus, tolerance, and opioid-induced hyperalgesia.4–6 By treating pain with several modalities, including local anesthetics and nonsteroidal anti-inflammatory drugs (NSAIDs,) lower doses of opioids are needed to achieve analgesia while reducing or eliminating opioid-mediated adverse effects.4,5
The multimodal approach to analgesia intervenes pharmacologically at many different inputs along the pain pathway. The synergistic analgesia achieved by affecting pain transmission at multiple points also results in lower doses of each drug administered. Adverse drug reactions or drug toxicities are dose-dependent, so there are fewer side effects from each drug class. This article presents and reviews 3 new analgesic agents that may be useful in treating postoperative dental and maxillofacial pain: tapentadol immediate release (Nucynta) diclofenac potassium soft gelatin capsules (Zipsor) and bupivacaine liposome injectable suspension (EXPAREL).
TAPENTADOL IMMEDIATE-RELEASE
Tapentadol immediate-release was granted approval by the US Food and Drug Administration (FDA) in November 2008 and is indicated for the treatment of moderate to severe pain.7,8 Tapentadol is a both a mu-opioid agonist and a norepinephrine reuptake inhibitor.9,10 Unlike tramadol, this opioid agonist predominantly inhibits norepinephrine reuptake with minimal serotonin effects.9
In rodent models, tapentadol has one fiftieth the affinity to the murine mu-opioid receptor but one third the analgesic potency when compared to morphine11 because norepinephrine reuptake also contributes to analgesia.8 Tapentadol's lower affinity for the opioid receptor results in decreased potential for opioid receptor mediated side effects such as sedation, nausea, emesis, and ileus, when compared to relatively high-doses of immediate-release opioids.8 Whether this property holds true for doses of opioids (hydrocodone 5–10 mg, oxycodone 5–10 mg, or codeine 30–60 mg) that are routinely employed in combination with aspirin, acetaminophen, or ibuprofen in treating postoperative dental pain has not been proven.8 Tapentadol 50 mg to 100 mg provided analgesia while having lower incidence of nausea and constipation as compared to immediate-release single-entity oxycodone 10 mg to 15 mg.12,13 Since tapentadol mediates both mu-opioid agonism and norepinephrine reuptake, there is multimodal analgesia with a single compound.9
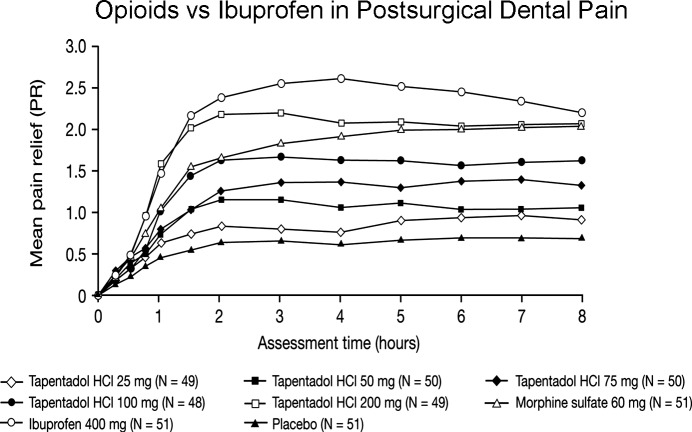
Clinical trials have demonstrated safety and efficacy for the management of acute postoperative pain for third molar extraction and bunionectomy as well as for acute exacerbations in patients with degenerative joint disease.9,14 However, in acute postsurgical dental pain, even the highest approved dose of tapentadol IR (100 mg) was inferior to ibuprofen 400 mg,15 demonstrating the key role prostaglandins play in the acute pain phenomena following the surgical removal of impacted third molars16 (Figure 1). Tapentadol 100 mg also produced a much greater incidence of dizziness and nausea compared to ibuprofen 400 mg and placebo: 38 and 10% for tapentadol 100 mg, 12 and 2% for ibuprofen 400 mg, and 14 and 2% for placebo, respectively.15
Figure 1.
Reprinted with permission from Kleinert R, Lange C, Steup A, Black P, Goldberg J, and Desjardins P.16
Tapentadol IR is available in 50-mg, 75-mg, and 100-mg doses and is dosed in a 4- to 6-hour interval.9,10 The redosing interval is a range in order to maintain balance between analgesia and tolerability of adverse drug reactions such as nausea and vomiting.9 The maximum daily dose is 700 mg on the first day and no more than 600 mg on the following days; if the patient is still in pain after the first dose on the first day, the second dose may be administered 1 hour later. Higher doses cannot be recommended as they have not been studied.7
Tapentadol is primarily metabolized by the liver, where it is glucuronidated to an inactive metabolite.7 Tapentadol is not a microsomal enzyme inducer or inhibitor.10 The manufacturer does not recommend dose reductions for patients with mild hepatic disease or mild to moderate renal disease. Recommendations for patients with moderate hepatic disease are to initiate treatment at 50 mg and to redose every 8 hours. The effects of tapentadol in patients with severe renal or hepatic disease have not been studied and therefore the drug is not recommended in this patient population.7
Tapentadol is contraindicated in patients with limited pulmonary function, paralytic ileus, or concomitant monoamine oxidase inhibitor use.7 Although tapentadol has less potential for respiratory depression as compared to other opioids, it is contraindicated for unmonitored use in patients with significant pulmonary disease or acute or severe asthma in the absence of resuscitative equipment.7 Therapeutic doses may be problematic in the following: those with preexisting respiratory conditions such as asthma, chronic obstructive pulmonary disease, obstructive sleep apnea, severe obesity, kyphoscoliosis, upper airway obstructions; concomitant central nervous (CNS) depressant use; and the elderly or debilitated.7 The administration of any mu-opioid agonist, including tapentadol, is contraindicated if the patient has or is suspected to have paralytic ileus.7 Tapentadol is also contraindicated in patients taking monoamine oxidase inhibitors in the past 14 days as both drugs increase synaptic levels of norepinephrine that may precipitate a hypertensive crisis.7
Tapentadol is a CNS depressant and caution should be taken in patients receiving other opioid analgesics, anesthetics, sedatives, and hypnotics. Dose reduction should be contemplated as synergistic cardiovascular, respiratory, and central nervous system depression may result in coma or death.7 As a CNS depressant, care should be cautioned to not drive or operate heavy machinery. Additionally, patients should be warned of the cumulative sedative effects of taking other tranquilizers, illicit or legitimate, as well as alcohol concomitantly with tapentadol.7 Although tapentadol's dual mechanism of action was hoped to limit its potential for abuse, the drug has high abuse potential, and the FDA assigned it to the Drug Enforcement Administration Schedule II drug category.8–10
Opioids can raise intracranial pressure so tapentadol should be used with caution in patients with known raised intracranial pressure, intracranial lesions, or head injury. Tapentadol has not been studied in patients with seizures. As with other mu-opioids, tapentadol may cause sphincter of Oddi spasm and should be used with caution in patient with pancreatitis and biliary tract disease.7
While tapentadol's mechanism of action includes that of minimal serotonin reuptake, concomitant use of other serotonergic drugs, such as selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, and triptans, may result in a potentially life-threatening condition called serotonin syndrome.9 Serotonin syndrome presents with altered mental status, agitation, hallucinations, seizures, coma, hyperthermia, myoclonus, tachycardia, hypertension, diarrhea, nausea, or vomiting. Drugs that increase synaptic levels of serotonin taken in concert with tapentadol can lead to dangerously high levels of serotonin to precipitate serotonin syndrome.
Tapentadol has not been studied for safety and efficacy in pregnancy, labor and delivery, nursing mothers, and in patients under 18 years of age. Tapentadol is not recommended for use in any of these patient populations. Tapentadol is a Pregnancy Category C drug and should only be used if the benefit outweighs the risk to the mother and fetus. If used during labor and delivery, the neonate may be at risk for respiratory depression: neonatal monitoring and an opioid antagonist naloxone should be available.7
For postsurgical dental pain, NSAIDs should be used as a first line agent.17 However, for severe pain, tapentadol can be used in combination with NSAIDs and/or acetaminophen where it should provide an additive analgesic effect.18
Diclofenac Potassium Soft Gelatin Capsules
Diclofenac potassium soft gelatin capsules (DPSGC) were FDA approved in 2009 and are indicated for the relief of mild to moderate pain.19 This novel formulation of diclofenac potassium, an NSAID, has analgesic, anti-inflammatory, and antipyretic properties.20 It is formulated as a liquid-filled capsule using a proprietary Prosorb technology and a gelatin-containing capsule shell.20 The mechanism of action of DPSGC as well as other NSAIDs is not fully elucidated, but it may involve inhibition of cyclooxygenase enzymes, both COX-1 and COX-2, with approximately threefold selectivity for inhibiting COX-2 over COX-1.21
DPSGC is indicated to treat mild to moderate acute pain. The drug is indicated for use only in adults, age 18 or older; use in children is not recommended. DPSGC is only available as a 25-mg liquid filled capsule intended for oral administration. The dosing regimen is 25 mg, up to 4 times a day, although higher doses have been studied.20,22,23
DPSGC is well absorbed after oral administration. In fact, studies with fasting patients demonstrate 100% absorption and the first pass effect results in approximately 50% bioavailability of the drug.20 Compared to immediate-release diclofenac potassium tablets, DPSGC can reach double the maximal plasma concentration in half the time.22 When taking DPSGC with food, the maximal plasma concentration is nearly halved and requires double the time as compared to taking DPSGC while fasting.20 DPSGC is highly protein bound, especially to albumin (at least 99%), which at least could theoretically increase blood levels and subsequent toxicity of other highly protein bound drugs such as sulfonylurea hypoglycemic drugs, warfarin, and phenytoin.20,24
Liver microsomal enzymes, including CYP2C9, 3A4, and 2C8, are responsible for metabolism of DPSGC. CYP2C9 forms the major metabolite, 4′-hydroxy-diclofenac, which has very weak activity.20 The parent drug and its metabolites also undergo sulfation and glucuronidation reactions followed by urinary and biliary excretion. Reduced dosage may need to be considered for patients with hepatic impairment.20 Since DPSGC is a CYP 450 2C9 substrate, concomitant administration of known inducers or inhibitors of the enzyme may alter metabolism and result in unpredictable decreases or increases in plasma levels, respectively.20 Two thirds of the excretion is through the kidneys and the other third through the bile. Since little to none of the dose is excreted unchanged, the dose does not need to be changed in mild to moderate renal impairment.20
Like other NSAIDs, an acute diclofenac overdose should be treated with supportive care. Patients may present with nausea, vomiting, epigastric pain, drowsiness, and lethargy. Overdose may also result in acute kidney failure, gastrointestinal bleeds, hypertension, respiratory depression, and coma. Although there is no specific treatment for an overdose, an osmotic cathartic or activated charcoal may be administered within 4 hours of ingestion in order to prevent absorption. Since DPSGC is highly protein bound, alkalinization of the urine, an established treatment for overdoses of weak acids, or diuresis may not be helpful.20
The FDA black box warning for all NSAIDs, including DPSGC, describes gastrointestinal events. NSAIDs may cause potentially fatal gastrointestinal ulcers, perforations, and/or bleeds that may occur without warning with elderly individuals at a higher risk. In fact, of the patients who experience severe gastrointestinal events, only 1 in 5 have gastrointestinal symptoms.20 DPSGC should be avoided or used cautiously in patients with a history of gastrointestinal ulcers and bleeds. The 2 most significant risk factors for a gastrointestinal bleed are a history of gastrointestinal bleeds with NSAID use and/or a history of peptic ulcer disease.20 Other risk factors to consider are poor overall health, old age, smoking tobacco, alcohol consumption, simultaneous administration of oral anticoagulants or corticosteroids, and long-term NSAID therapy.20 By utilizing the Prosorb technology, it is possible to administer a lower dose while retaining analgesic efficacy due to improved absorption. This does not mitigate the risk for serious gastrointestinal events in high-risk patients; DPSGC should be discontinued if there is any suspicion of serious gastrointestinal events. DPSGC may not be the analgesic of choice in the highest risk patients if the potential benefit is not outweighed by risk of gastrointestinal events.
The FDA black box warning also states that NSAIDs may increase the risk of potentially fatal cardiovascular adverse events such as myocardial infarction, thrombosis, and stroke. Patients with cardiovascular disease, cardiovascular disease risk factors, and prolonged NSAID use are at higher risk for cardiovascular adverse events. Administering aspirin does not offset the risk of thrombotic events, but it will increase the risk of gastrointestinal events.
There are several absolute contraindications to DPSGC. A known diclofenac allergy is a contraindication to DPSGC. An allergy to bovine protein is also a contraindication as the capsule shell contains gelatin.20 Patients with a history of allergic-type reactions such as asthma or urticaria when taking NSAIDs or aspirin should also avoid DPSGC.20 The aspirin triad is a contraindication for DPSGC use; it consists of patients with asthma exacerbations when taking aspirin and/or NSAIDs and who may or may not have nasal polyps.20
Other adverse effects of DPSGC and other NSAIDs include renal complications. NSAID treatment may result in fluid retention, edema, and hypertension. Prolonged NSAID use may cause renal injury such as renal papillary necrosis. Therefore, DPSGC should be used cautiously in elderly patients as well as those with renal dysfunction, liver dysfunction, heart failure, or those on angiotensin-converting-enzyme inhibitors or diuretics. Liver dysfunction may occur at any time with DPSGC and without warning. Although DPSGC does not have a chronic pain indication, chronic diclofenac use has been known to raise liver enzymes, and monitoring for liver enzyme elevations is recommended with chronic use. DPSGC should be prescribed cautiously when patients are receiving hepatotoxic medications such as antiepileptics or acetaminophen,20 although diclofenac's single-dose use in combination with acetaminophen in treating acute postsurgical dental pain has been well tolerated.25
Anaphylactic reactions may occur in patients who may or may not have prior exposure to DPSGC. DPSGC may cause potentially fatal skin reactions such as toxic epidermal necrolysis, exfoliative dermatitis, and Steven-Johnson syndrome. Patients may experience such potentially fatal dermatologic reactions without warning; DPSGC should be stopped with any sign of skin hypersensitivity.20
There are notable drug interactions while taking DPSGC. Simultaneous use of anticoagulants, such as aspirin or warfarin, is not recommended due to increased risk of gastrointestinal bleeding. NSAIDs, including DPSGC, inhibit the synthesis of renal prostaglandins, which can inhibit the antihypertensive effects of angiotensin-converting-enzyme inhibitors and diuretics; patients should be observed for signs of nephrotoxicity. By interfering with renal prostaglandin synthesis, DPSGC may increase the nephrotoxicity of other drugs such as cyclosporine when taken at the same time. DPSGC may also increase the toxicity of lithium and high-dose methotrexate by competing with the renal excretion of both.24
DPSGC has not been well studied in pediatric, pregnant, or geriatric patients or patients who are in labor and delivery or nursing. NSAIDs close the ductus arteriosus by inhibiting prostaglandin synthesis. DPSGC is considered a Pregnancy Category C drug before 30 weeks gestational age, meaning that the drug should be used only if the therapeutic benefit outweighs any potential harm to the fetus. At 30 weeks, DPSGC is considered a Pregnancy Category D drug and should be avoided. DPSGC has not been studied in parturients and is thus not recommended in that patient population. Additionally, the presence or amount of DPSGC in human milk has not been studied; nursing while taking DPSGC is not recommended.20
There is insufficient data regarding DPSGC in patients 65 years of age and older; it is not possible to conclude that this patient population will respond differently to DPSGC when compared to younger patients. Elderly patients often have reduced renal function and increased risk for gastrointestinal bleeding. Elderly patients also have a higher likelihood for other comorbidities, which may include reduced cardiac, hepatic, or renal function and are more likely to be taking other medications so a reduction in DPSGC dosing should be considered based on the patient's history.20
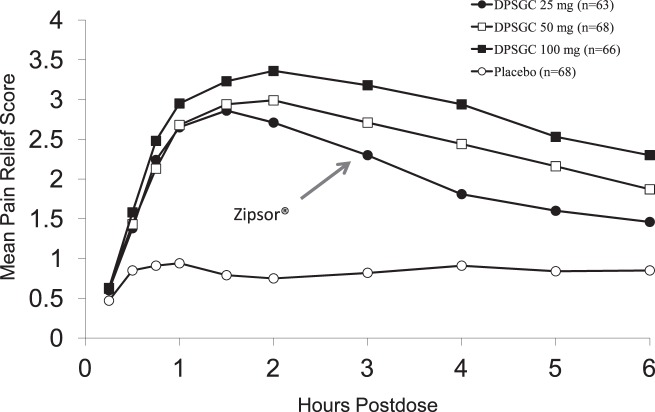
The 25-mg dose of DPSGC has been demonstrated to be highly effective in postsurgical dental pain, though supratherapeutic doses increase its peak effect and duration of action22 (Figure 2). An onset of confirmed perceptible pain relief occurs in less than 30 minutes with DPSGC.26
Figure 2.
Adapted with permission from Hersh EV, Levin LM, Adamson D, et al.22
DPSGC is an option in postsurgical dental patients who require an NSAID but refuse a prescription for ibuprofen or naproxen because they believe their over-the-counter availability equates to poor pain relief. In young healthy adults experiencing moderately severe to severe dental pain, combining DPSGC with acetaminophen and/or an opioid should provide enhanced analgesic activity.17,25
BUPIVACAINE LIPOSOME INJECTABLE SUSPENSION
Liposomal bupivacaine was granted FDA approval in 2011 and is indicated for postsurgical analgesia by way of a single-dose local administration to the surgical site.27 It is a new multivesicular liposome formulation of bupivacaine using DepoFoam technology (Pacira Pharmaceuticals Inc, San Diego, Calif) (Figure 3). Liposomes usually consist of a phospholipid bilayer with an aqueous core. Liposomal bupivacaine consists of multivesicular liposomes, which have multiple lipid bilayers that are arranged like a honeycomb with bupivacaine in the aqueous cores. The bupivacaine concentration is 1.3% (or 13.3 mg/mL.) This multivesicular liposome structure accounts for liposomal bupivacaine's increased stability and extended drug release.5,28
Figure 3.
Diagram of liposomal bupivacaine particle in DepoFoam vehicle. Image supplied courtesy of Pacira Pharmaceuticals Inc.
Liposomal bupivacaine is available as a single-dose vial at a 1.3% concentration (266 mg/20 mL).28 It should be administered as a single dose by infiltration only, aspirating frequently to avoid inadvertent intravascular injection, using a 25-gauge needle or larger, and the maximum dose should not exceed 266 mg (or 20 mL of 1.3% bupivacaine).28 It is also advised to invert the vial several times before withdrawing the drug because the particles may have settled.28 The vials may be refrigerated but not frozen. Once removed from the refrigerator, the vials may remain at room temperature for 30 days. Once the liposomal bupivacaine is drawn into a syringe, it must be administered within 4 hours.28 There are currently no specific guidelines for liposomal bupivacaine dosing for postoperative analgesia in the oral cavity, other than the labeled statement that the recommended dose is based on the surgical site and volume required to cover the area.
Liposomal bupivacaine is not indicated as a substitute for preincisional local anesthesia. Liposomal bupivacaine is only indicated for local administration (specifically, infiltration and instillation) and has not yet been approved for other routes of administration including nerve blocks, intravascular, or intra-articular use, though pivotal nerve block trials are currently ongoing. It is currently not advised or indicated to use any bupivacaine-containing products (including immediate-release and liposomal formulations) for intra-articular infusions. Postmarketing surveillance data has reported chondrolysis or loss of cartilage in a joint following the degradation of the cartilage matrix and cells.28 There is no current treatment for chondrolysis and some patients have required arthroplasty or joint replacement.28
Studies show that there is no effect on wound healing when liposomal bupivacaine is infiltrated into the surgical site for postoperative analgesia.4,5 Studies demonstrate that common implantable materials do neither affect liposomal bupivacaine nor does liposomal bupivacaine affect these materials. These materials include stainless steel, titanium, silicone, polypropylene, and polytetrafluoroethylene.28
Liposomal bupivacaine possesses some differences in its pharmacokinetic profile as compared to other bupivacaine formulations. Once in the blood, systemic levels of bupivacaine may persist for 96 hours.28,29 Therefore, it is advised to avoid administering immediate-release bupivacaine and other local anesthetics for 96 hours after liposomal bupivacaine is infiltrated to avoid unintentional overdose.28 Although liposomal bupivacaine has longer local anesthetic efficacy at the infiltration site compared to bupivacaine HCl, systemic levels of liposomal bupivacaine last beyond the longer local anesthetic effect of liposomal bupivacaine; blood levels are correlated with systemic toxicity, not local efficacy.28,29
Once released from the liposome, the pharmacokinetics of bupivacaine are similar to the bupivacaine HCl formulation with respect to absorption, distribution, metabolism, and excretion. Systemic absorption depends on route of administration, the vascularity of the injection site, and the total drug dose.28 Once in the systemic circulation, liposomal bupivacaine is distributed to highly perfused organs (such as the brain, heart, liver, and lungs) in a fashion similar to bupivacaine HCl. Bupivacaine undergoes hepatic metabolism, mainly by glucuronic acid conjugation and N-dealkylation into pipecolylxylidine, the major inactive metabolite (approximately 5% of bupivacaine).5,28 Pipecolylxylidine is hydroxylated and glucuronidated as well.5 There are no recommended dose adjustments for patients with moderate hepatic impairment.5,28 Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations.28 Most local anesthetics, including bupivacaine, are excreted by the kidneys. Approximately 6% of bupivacaine is excreted in the urine unchanged.5,28 There are no recommended dose adjustments for patients with renal impairment.
Bupivacaine can produce a use-dependent block on the myocardium even at regular heart rates and should be used carefully in patients with known impaired cardiovascular function.28 Allergic reactions to amide local anesthetics are rare, but cross allergenicity with other amide local anesthetics has been reported.28,30 A true allergic reaction can present with pruritus, erythema, urticaria, angioedema, possible dizziness and/or syncope, tachycardia, hypotension, nausea, vomiting, elevated temperature, and increased perspiration.28
There are several drug-drug interactions that may occur involving the liposomal carrier. Disruption of the liposomal carrier may result in uncontrolled, instantaneous release of bupivacaine, which may lead to overdose and systemic toxicity. The liposomal vesicles will be disrupted if hypotonic solutions are used or if the drug is admixed with any other local anesthetics, including bupivacaine HCl. Liposomal bupivacaine does not need to be diluted for use, but dilutions are permitted with sterile 0.9% normal saline, the maximum dilution is 0.89 mg/mL (a 1 : 14 dilution).28 Due to the liposomal formulation, liposomal bupivacaine dosing is not interchangeable with other formulations of bupivacaine even if the drugs are in the same concentration. Also, it is not advisable to administer traditional bupivacaine HCl before liposomal bupivacaine; there is potential for increased exposure to the drug when the bupivacaine HCl dose is more than half of the liposomal bupivacaine dose.28 However, if a local anesthetic must be administered immediately before liposomal bupivacaine, bupivacaine HCl is the only local anesthetic recommended. It is recommended to wait at least 20 minutes after administering a nonbupivacaine local anesthetic such as lidocaine because coadministration may cause an immediate release of bupivacaine from the liposomes. In order to prevent disruption of the liposomes and inadvertent uncontrolled drug release, topical skin antiseptics such as chlorhexidine or povidone iodide must dry.5,28 Since there are no data on liposomal bupivacaine on intraoral procedures and any interactions with oral antimicrobials such as chlorhexidine rinses, recommendations cannot be made.28
Like bupivacaine HCl, the systemic toxicity of local anesthetic overdose is dose-dependent and primarily affects the CNS and cardiovascular system.28,31 Anesthetic toxicity is additive if other local anesthetics are administered.31 Overdosage can be avoided by being mindful of the total amount administered and using aspirating technique to avoid intravascular injection. Other causes for increased plasma levels of local anesthetic include decreased hepatic function and metabolism, decreased plasma protein production, or decreased plasma protein binding due to acidosis or competition for protein binding sites from other highly protein-bound drugs. Rarely, some individuals may be more sensitive to the effects of local anesthetics and can experience toxicity as lower doses.28
Traditional local anesthetics are limited by dose-dependent risk of systemic toxicity and short duration of action. Liposomal formulations keep the local anesthetic at the infiltration site and slowly release bupivacaine, which contributes to a longer duration of action while reducing and delaying systemic drug absorption. A clinical trial compared subcutaneous injection of 0.5% bupivacaine HCl plain (20 mL or 100 mg) to 2% liposomal bupivacaine (20 mL or 400 mg).32 The maximum recommended dose for immediate-release bupivacaine is 2.5 mg/kg, or approximately 175 mg for a 70-kg adult.32 The dose of liposomal bupivacaine exceeded the MRD by more than a factor of 2; however, none of the patients presented with any signs or symptoms of local anesthetic toxicity, likely the result of its slower absorption and lower peak blood levels (Cmax) than immediate-release bupivacaine solutions. The same maximum blood concentration was detected as the immediate-release bupivacaine solution, meaning that the Cmax remained in the safe range when the dose was raised by a factor of 4 in the liposomal formulations.32 The Cmax was in the range of 0.4–1.4 μg/mL for the liposomal bupivacaine, which is below the blood concentration of bupivacaine associated with central nervous system toxicity (2–4 μg/mL).32 The time to Cmax (Tmax) was 262 ± 149 minutes (mean ± SD) for liposomal bupivacaine as compared to 37.5 ± 16 minutes (mean + SD) for bupivacaine plain; it took 7 times longer to reach the Cmax which confers additional safety.32 However, this also implies that there is a longer window for which Cmax can peak, so supplemental doses of local anesthetic cannot be given as it is uncertain how this would affect drug release from the liposomes and subsequent peaks in plasma levels of local anesthetic.32 Clinical trials showed no difference between liposomal bupivacaine and bupivacaine HCl with epinephrine with respect to heart rate and electrocardiographic changes in PR or QT interval.4,5
Liposomal bupivacaine has not been studied in pregnant or nursing patients or patients under 18 years of age and cannot be recommended for use in these groups at this time. Liposomal bupivacaine is a Pregnancy Category C drug. There have been no safety or efficacy data demonstrating any differences in geriatric patients; however, some older individuals may be more sensitive to the effects of liposomal bupivacaine.28
Liposomal bupivacaine has not been studied as an intraoral analgesic infiltrated around surgical wound sites. Its cost of approximately $250 per single-use 20-mL vial may render it cost-prohibitive for most outpatient dental surgical procedures, unless concomitant benefits such as a decrease in postsurgical opioids requirements and their sequelae offset this cost.33 Liposomal bupivacaine may be a useful analgesic for inpatients with facial trauma or elective orthognathic surgery because it provides prolonged analgesia and may possibly reduce opioid consumption, reduce the incidence of opioid-related adverse events, and reduce the costs associated with managing them. In an open-label phase IV health economic trial comparing patient-controlled analgesic with opioids to single-dose liposomal bupivacaine following open colectomy, the results demonstrated significant reductions in opioid consumption, cost of hospitalization, and reduced length of hospital stay when patients received liposomal bupivacaine.34 Following open colectomy, patients either received patient-controlled analgesic opioid analgesia, per their institution's patient-controlled analgesic regimen, or were given a single dose of liposomal bupivacaine 266 mg at the end of the procedure.34 The rescue medication was oxycodone 5 mg with acetaminophen 325 mg every 6 hours as needed.34 The group receiving liposomal bupivacaine received a multimodal analgesia regimen that consisted of ketorolac 30 mg intravenously at the end of the procedure followed by oral administration of ibuprofen 600 mg and acetaminophen 1 g every 6 hours.34 A maximum of acetaminophen 4 g was allowed for a 24-hour period in both groups.34 Both groups were studied for 72 hours.34 Multimodal analgesia with liposomal bupivacaine as compared to patient-controlled analgesic opioids resulted in statistically significant reductions in the study endpoints such as a reduction in mean opioid consumption (57 mg morphine equivalents vs 115 mg, respectively), mean total cost of hospitalization ($8,766 vs $11,850), and median length of stay (2 days vs 4.9 days).34 In a double-blind study comparing infiltration of bupivacaine HCl 75 mg to liposomal bupivacaine 366 mg at the end of hemorrhoidectomy procedures, liposomal bupivacaine demonstrated 47% reduction in cumulative pain scores, 66% reduction in opioid consumption, and 89% reduction in opioid-related adverse events.35
DISCUSSION AND CONCLUSIONS
The goal of multimodal analgesia is to utilize different analgesics with different mechanisms of action to synergistically produce analgesia while reducing the dose of each agent. Since adverse reactions are dose related, there may be fewer and less severe side effects when using smaller doses of several different agents as compared to using a larger dose, single agent analgesic. EXPAREL is a formulation of bupivacaine using a liposomal vehicle, resulting in prolonged local anesthesia. DPSGC is a liquid-filled capsule formulation of diclofenac, which confers better absorption and requires lower doses of diclofenac to provide analgesia, possibly reducing gastrointestinal toxicity. Lastly, tapentadol is both a mu-opioid agonist and a norepinephrine reuptake inhibitor. Tapentadol's dual mechanism of action provides analgesia but less gastrointestinal side effects such as constipation when compared to other relatively high-dose opioid agonists.
Using 2 or 3 of these drugs in a multimodal approach may enhance analgesia while lowering dosing requirements and subsequent toxicity of employing equianalgesic doses of individual agents. For example, the combination of enteric-coated diclofenac 100 mg and acetaminophen 1000 mg has produced an analgesic effect beyond that of either drug alone or the combination of acetaminophen 1000 mg plus codeine 60 mg.25 Combining NSAIDs other than diclofenac with opioids has also demonstrated enhanced analgesia beyond that of the opioid or NSAID alone.36 A review of 10 randomized, double-blind, clinical studies demonstrated that patients receiving liposomal bupivacaine reported lower pain scores and consumed less opioids during the first 72 hours postoperatively than those who did not receive the drug.29 Future clinical trials of analgesic agents should focus on their efficacy and safety as part of multimodal analgesic regimens.
REFERENCES
- 1.Bonica JJ. The need of a taxonomy. Pain. 1979;6:247–248. [Google Scholar]
- 2.Daniels SE, Riff D, Diamond E, Clark F, Boesing SE. An assessment of the efficacy and safety of diclofenac potassium liquid-filled capsules in patients with various levels of baseline pain intensity. Curr Med Res Opin. 2012;28:953–961. doi: 10.1185/03007995.2012.694363. [DOI] [PubMed] [Google Scholar]
- 3.Barash PG, Cullen BF, Stoelting RK, Calahan MK, Stock MC. Clinical Anesthesia. Philadelphia, Pa: Lippincott Williams & Wilkins;; 2009. [Google Scholar]
- 4.Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesia in total knee arthroplasty. Knee. 2012;19:530–536. doi: 10.1016/j.knee.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Chahar P, Cummings KC., 3rd Liposomal bupivacaine: a review of a new bupivacaine formulation. J Pain Res. 2012;5:257–264. doi: 10.2147/JPR.S27894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manvelian G, Daniels S, Gibofsky A. A phase 2 study evaluating the efficacy and safety of a novel, proprietary, nano-formulated, lower dose oral diclofenac. Pain Med. 2012;13:1491–1498. doi: 10.1111/j.1526-4637.2012.01479.x. [DOI] [PubMed] [Google Scholar]
- 7.PriCara Pharmaceuticals Inc. Nucynta (Tapentadol) Prescribing Information. 2013 Available at: http://www.nucynta.com/nucynta. Accessed October 20. [Google Scholar]
- 8.Hersh EV, Golubic S, Moore PA. Analgesic update: tapentadol hydrochloride. Compend Contin Educ Dent. 2010;31:594–599. quiz 600, 603. [PubMed] [Google Scholar]
- 9.Hartrick CT. Rodríguez Hernandez JR. Tapentadol for pain: a treatment evaluation. Expert Opin Pharmacother. 2012;13:283–286. doi: 10.1517/14656566.2012.648616. [DOI] [PubMed] [Google Scholar]
- 10.Young A, Buvanendran A. Recent advances in multimodal analgesia. Anesthesiol Clin. 2012;30:91–100. doi: 10.1016/j.anclin.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Tzschentke TM, de Vry J, Terlinden R, et al. Tapentadol HCl. Drugs Future. 2006;31:1053–1061. [Google Scholar]
- 12.Stegmann JU, Weber H, Steup A, Okamoto A, Upmalis D, Daniels S. The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery. Curr Med Res Opin. 2008;24:3185–3196. doi: 10.1185/03007990802448056. [DOI] [PubMed] [Google Scholar]
- 13.Daniels SE, Upmalis D, Okamoto A, Lange C, Häeussler J. A randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) pain. Curr Med Res Opin. 2009;25:765–776. doi: 10.1185/03007990902728183. [DOI] [PubMed] [Google Scholar]
- 14.Kavanagh S, Kwong WJ, Hammond GC, Nelson W, Upmalis D, Yang M. Pain relief and tolerability balance of immediate release tapentadol or oxycodone treatment for patients with moderate to severe osteoarthritis or low back pain. Pain Med. 2012;13:1110–1120. doi: 10.1111/j.1526-4637.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 15.Gordon SM, Brahim JS, Rowan J, Kent A, Dionne RA. Peripheral prostanoid levels and nonsteroidal anti-inflammatory drug analgesia: replicate clinical trials in a tissue injury model. Clin Pharmacol Ther. 2002;72:175–183. doi: 10.1067/mcp.2002.126501. [DOI] [PubMed] [Google Scholar]
- 16.Kleinert R, Lange C, Steup A, Black P, Goldberg J, Desjardins P. Single dose analgesic efficacy of tapentadol in postsurgical dental pain: the results of a randomized, double-blind, placebo-controlled study. Anesth Analg. 2008;107:2048–2055. doi: 10.1213/ane.0b013e31818881ca. [DOI] [PubMed] [Google Scholar]
- 17.Hersh EV, Kane WT, O'Neil MG, et al. Prescribing recommendations for the treatment of acute pain in dentistry. Compend Contin Educ Dent. 2011;32 22, 24–30; quiz 31–32. [PubMed] [Google Scholar]
- 18.Beaver WT. Combination analgesics. Am J Med. 1984;77:38–53. doi: 10.1016/s0002-9343(84)80101-1. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Zipsor NDA 22–202 Approval Letter, June 16, 2009. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022202_zipsor_toc.cfm. Accessed January 20, 2013. [Google Scholar]
- 20.Depomed Inc. Zipsor (Diclofenac Potassium) Liquid Filled Capsules Prescribing Information. 2013 Available at: http://www.zipsor.com/files/PI.pdf. Accessed October 20, [Google Scholar]
- 21.Hersh EV, Lally ET, Moore PA. Update on cyclooxygenase inhibitors: has a third COX isoform entered the fray? Curr Med Res Opin. 2005;21:1217–1226. doi: 10.1185/030079905X56367. [DOI] [PubMed] [Google Scholar]
- 22.Hersh EV, Levin LM, Adamson D, et al. Dose-ranging analgesic study of Prosorb diclofenac potassium in postsurgical dental pain. Clin Ther. 2004;26:1215–1227. doi: 10.1016/s0149-2918(04)80033-x. [DOI] [PubMed] [Google Scholar]
- 23.Zuniga JR, Malmström H, Noveck RJ, et al. Controlled phase III clinical trial of diclofenac potassium liquid-filled soft gelatin capsule for treatment of postoperative dental pain. J Oral Maxillofac Surg. 2010;68:2735–2742. doi: 10.1016/j.joms.2010.05.075. [DOI] [PubMed] [Google Scholar]
- 24.Hersh EV, Moore PA. Adverse drug interactions in dentistry. Periodontol 2000. 2008;46:109–42. doi: 10.1111/j.1600-0757.2008.00224.x. [DOI] [PubMed] [Google Scholar]
- 25.Breivik EK, Barkvoll P, Skovlund E. Combining diclofenac with acetaminophen or acetaminophen-codeine after oral surgery: a randomized, double-blind single-dose study. Clin Pharmacol Ther. 1999;66:625–635. doi: 10.1053/cp.1999.v66.103629001. [DOI] [PubMed] [Google Scholar]
- 26.Zuniga JR, Noveck RJ, Schmidt WK, Boesing SE, Hersh EV. Onset of action of diclofenac potassium liquid-filled capsules in dental surgery patients. Curr Med Res Opin. 2011;27:1733–1739. doi: 10.1185/03007995.2011.600300. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration, Center for Drug Evaluation and Research. EXPAREL NDA 022496 Approval letter. 2013 October 28, 2011. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2011/022496s000ltr.pdf. Accessed January 20, [Google Scholar]
- 28.Pacira Pharmaceuticals Inc. EXPAREL (Bupivacaine Liposome Injectable Suspension) Prescribing Information. 2013 Available at: http://www.exparel.com/pdf/EXPAREL_Prescribing_Information.pdf. Accessed January 20, [Google Scholar]
- 29.Bergese SD, Ramamoorthy S, Patou G, Bramlett K, Gorfine SR, Candiotti KA. Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. J Pain Res. 2012;5:107–116. doi: 10.2147/JPR.S30861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hersh EV. Local anesthetics. In: Fonseca RJ, editor. Oral and Maxillofacial Surgery. Vol 1. 1st ed. Philadelphia, Pa: WB Saunders Company;; 2000. pp. 58–78. In. ed. [Google Scholar]
- 31.Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010;54:587–599. doi: 10.1016/j.cden.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Davidson EM, Barenholz Y, Cohen R, Haroutiunian S, Kagan L, Ginosar Y. High-dose bupivacaine remotely loaded into multivesicular liposomes demonstrates slow drug release without systemic toxic plasma concentrations after subcutaneous administration in humans. Anesth Analg. 2010;110:1018–1023. doi: 10.1213/ANE.0b013e3181d26d2a. [DOI] [PubMed] [Google Scholar]
- 33.International Business Times. FDA Approves Pacira's Pain Drug Exparel. 2011 Available at: http://www.ibtimes.com/fda-approves-paciras-pain-drug-exparel-363830. Accessed November 1, [Google Scholar]
- 34.Cohen SM. Extended pain relief trial utilizing infiltration of Exparel(®), a long-acting multivesicular liposome formulation of bupivacaine: a Phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567–572. doi: 10.2147/JPR.S38621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas E, Onel E, Miller H, Ragupathi M, White PF. A double-blind, randomized, active-controlled study for post-hemorrhoidectomy pain management with liposome bupivacaine, a novel local analgesic formulation. Am Surg. 2012;78:574–581. doi: 10.1177/000313481207800540. [DOI] [PubMed] [Google Scholar]
- 36.Van Dyke T, Litkowski LJ, Kiersch TA, Zarringhalam NM, Zheng H, Newman K. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: a double-blind, placebo- and active-controlled parallel-group study. Clin Ther. 2004;26:2003–2014. doi: 10.1016/j.clinthera.2004.12.002. [DOI] [PubMed] [Google Scholar]