Abstract
Recent studies and our current data demonstrated the deficits in the numbers and/or functions of the CD4+CD25+Foxp3+ Treg cells in the patients with autoimmune diseases, indicating that restoration of Treg cells in these patients could be a potential therapeutic approach. Here, we demonstrated that CD4+CD25+Foxp3+ Treg cells can be purified, activated and expanded from peripheral blood of patients with immune-mediated diseases, to a similar degree to those from healthy donors. Within 3 weeks, Treg cells from most patients could be expanded ex vivo 100–2000 fold and maintained their phenotypic characteristics. Furthermore, ex vivo expanded Treg cells displayed potent and enhanced in vitro suppressive activities inhibiting T effector cell proliferation compared to Treg cells freshly purified from the same patients. The expanded Treg cells with enhanced biological function may provide an opportunity to restore the proper balance of immunity and tolerance, suggesting the potential of using Treg cell therapy for treatment of immune-mediated diseases.
Keywords: Human, Treg cells, Expansion, Suppression, Immune-mediated disease, Severe asthma, SLE, RA, MS, CD
Introduction
Regulatory T (Treg) cells are critical for the development and maintenance of immune tolerance to self-antigens [1–6]. Naturally occurring CD4+CD25+Foxp3+ Treg cells, in particular, play an indispensable role in self-tolerance by actively suppressing the activation and expansion of auto-reactive T cells [1,2]. Disruption in the development and/or the function of Treg cells causes severe autoimmunity and inflammatory diseases in both humans and animals [1,2]. The Foxp3 gene defect was discovered in Scurfy mice, an X-linked recessive mutant with lethality in males [7]. Mutations of human Foxp3 gene induce the genetic disease IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome) with clinical autoimmune presentations [8,9]. In multiple animal models of immune-mediated diseases, regulatory T cell studies clearly demonstrated that (1) the depletion of Foxp3+ Treg cells accelerated diseases such as graft versus host disease (GVHD), experimental autoimmune encephalomyelitis (EAE), collagen-induced arthritis (CIA), inflammatory bowel disease (IBD), systemic lupus erythematosus (SLE, NZB×NZW mice), and type 1 diabetes (T1D); (2) defects of Treg cell functions were associated with autoimmune disease onset and progression; and (3) adoptive transfer of polyclonal or antigen-specific Foxp3+ Treg cells could prevent, treat, or even reverse the diseases in mice [10–19].
In clinical study, Viglietta et al first demonstrated the significant defect of the functions of the CD4+CD25+ Treg cells from peripheral blood of patients with multiple sclerosis (MS) [20]. Subsequently, numerous studies have found the deficits in numbers and/or functions of human CD4+CD25+ Treg cells from peripheral blood of patients with systemic lupus erythmatosus (SLE), rheumatoid arthritis (RA), type 1 diabetes (T1D), IBD, GVHD and asthma [21–31]. Recently, Longhi et al reported that functionally enhanced Treg cells can be expanded from patients with autoimmune hepatitis [32]. Putnam et al also demonstrated that expanded human Treg cells from type 1 diabetic patients and normal donors were equally capable of suppressing T-cell proliferation [33]. We and others have reported that expanded human Treg cells from healthy donors successfully prevented lethal GVHD in hu-PBL-NOD/SCID mice [34,35]. However, functional studies of expanded human Treg cells from other commonly seen autoimmune and inflammatory diseases have yet to be reported. In this study, we evaluated the feasibility of ex vivo expansion of Treg cells from patients with autoimmune and inflammatory diseases including SLE, IBD, MS, RA, and asthma. We have demonstrated that human Treg cells from those patients could be successfully enriched and expanded ex vivo to 100–2000 fold. In addition, expanded human Treg cells displayed enhanced in vitro suppressive function in inhibiting effector T cell proliferation compared to that of freshly purified human Treg cells from the same patients. Our study supported the potential use of expanded human Treg cells for therapy in autoimmune and inflammatory diseases such as IBD, SLE, MS, RA and asthma.
Materials and methods
Subjects
All enrolled subjects were 18 years or older, and excluded from study with known pregnancy, cancer, or HIV, HBV and HCV infections. Ten patients with diagnosis of Crohn’s disease and 5 patients with ulcerative colitis as determined by the Global Physician’s Index [36], 9 patients with relapsing remitting MS according to the 2001 Guidelines from the International Panel on the Diagnosis of MS [37], 10 patients with severe refractory asthma according to the 2000 criteria published by the American Thoracic Society Workshop [38], 10 patients with active SLE diagnosis and 10 patients with active RA according to the American College of Rheumatology criteria [39,40] were included in the study. All SLE, RA and MS patient blood samples were purchased from Asterand (Detroit, MI). Peripheral blood samples from severe asthmatics were collected from University of Pittsburg Medical Center (Pittsburg, PA). The blood samples of refractory Crohns’s disease and ulcerative colitis patients were provided by Mayo Clinic (Rochester, MN). Human peripheral blood units from healthy donors were purchased from Interstate Blood Bank (Memphis, TN) and used as controls. All human subject studies were approved by local institutional review boards, and all patients have signed the consent form.
Purification of CD4+CD25+ Treg cells from human peripheral blood
50 ml of heparinized whole human blood was obtained from healthy donors and patients with autoimmune and inflammatory diseases via standard procedure. Human peripheral blood mononuclear cells (PBMCs) were isolated from blood samples by density gradient centrifugation with Ficoll Hypaque (Amersham). The CD4+CD25+ Treg cells were purified from PBMC using autoMACS and human CD4+CD25+ regulatory T cell isolation kits (Miltenyi Biotec, Auburn, CA) according to manufacturer instructions. Briefly, CD4+ T cells were first negatively isolated from PBMC by depleting non-CD4 cells with the mixture of monoclonal antibodies against human CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ and CD235a. Human CD4+CD25+ Treg cells were then positively isolated with anti-human CD25 antibody-conjugated microbeads from the enriched CD4+ T cell population. The purity of the isolated cells was analyzed with flow cytometry after purification.
Ex vivo activation and expansion of human CD4+CD25+ Treg cells
The purified human CD4+CD25+Treg cells were activated and expanded ex vivo in cell culture plates with CD3/CD28 T cell expander beads (Dynal, Invitrogen) in the presence of recombinant human IL-2 (rhIL-2, 1000 U/ml, R&D systems). The CD4+CD25+ Treg cells were cultured in X-VIVO™ 15 medium supplemented with 10% heat inactivated human AB serum (Lonza, MD), L-glutamine, hepes, sodium pyruvate, penicillin, streptomycin (Gibco). Fresh medium with rhIL-2 were added 2–3 times per week. After 2–3 weeks, the CD3/CD28 beads were removed from the Treg cells, and the expanded Treg cells were then rested for 1–2 days in low IL-2 (50 U/ml) containing medium before in vitro characterization and function analysis.
In vitro suppression assay
Human dendritic cells (DCs) were generated from adherent cells or CD14 bead-purified monocytes from PBMC and cultured with RPMI 1640 medium in the presence of 10% FCS, recombinant human GM-CSF (50 ng/ml, R&D systems) and IL-4 (25 ng/ml, R&D systems). Cytokines and medium were changed every other day. On day 5 to 6, DCs were harvested and cryopreserved for in vitro suppression assays.
Human CD4+CD25− T responder cells were also purified from PBMC of normal donors using Miltenyi kit and autoMACS. The purified human CD4+CD25− T cells were frozen in aliquots for in vitro suppression assays.
The in vitro suppressive activity of the ex vivo expanded human Treg cells was measured in anti-CD3 antibody induced T cell proliferation assays. Human CD4+CD25− T effector cells (1 ×105 cells/well) and allogeneic human dendritic cells (1×104 cells/well) were co-cultured in the 96-well U-bottom plates in the presence of anti-human CD3 antibody (1 μg/ml, OKT3, Ebioscience). Expanded human Treg cells or freshly purified human Treg cells were serially diluted and added into the cultures at different Treg/T effector ratios and cells were cultured for 4 days. At the last 16 h of culture, 3H-thymidine (1 μCi/well) was added. The plates were harvested and 3H-thymidine incorporation was counted with Topcount (PerkinElmer). Mean counts per minute (cpm) of triplicate cultures and standard deviation were calculated. Percent inhibition of proliferation was calculated as: % inhibition =[(cpm responder cells – cpm responder/Treg)/(cpm responder cells)]×100.
Flow cytometry analysis
For immunofluorescence staining, cells were stained for 30 min at 4 °C with different fluorescence-conjugated antibodies as indicated. CD4-FITC, CD4-allophycocyanin (APC), and CD25-PE were purchased from BD Biosciences. For intracellular staining of Foxp3, the cells were fixed, permeabilized and further blocked with normal mouse serum according to the manufacturer’s protocol using anti-human Foxp3 staining set (Ebioscience). The Foxp3 was stained with either Clone 236A/E7 PE (Ebioscience) or isotype control. The cells were acquired with FACSCanto (BD Pharmingen) and the data were analyzed using FlowJo software (Tree Star, Inc.).
Statistical analysis
The data were analyzed by ANOVA or Mann Whitney test (nonparametric test) with Prism software. Values of p≤0.05 were considered statistically significant.
Results
CD4+CD25+ Treg cells can be purified from the peripheral blood of the patients with autoimmune and inflammatory diseases
As reported by several groups [20–31], the Treg cell deficiency might be an underlying cause of certain autoimmune and inflammatory diseases, so we first measured the frequency of Treg cells in the CD4+ T cell population of the patients. The average percentage of Treg cells in CD4 population for normal donor was 9.18%, and for SLE, MS, RA, asthma, CD and UC patients were 7.89%, 5.99%, 6.07%, 7.83%, 7.02% and 6.92%, respectively. There was no significant difference for Foxp3+ Treg cell frequency in CD4+ T cell population among all patients (Fig. 1). The average percentage of Treg cells in SLE, severe asthma and Crohn’s patients were similar to that of the normal donors, but the average percentage of the Treg cells in RA, MS and UC patients were significantly lower compared to that of normal donors (p=0.0076, 0.0043 and 0.035, respectively, Mann Whitney test). Overall, in our study the Treg cell frequencies in patients with immune mediated diseases displayed lower trend compared to normal donors and there were considerable subpopulations of patients bearing lower frequencies of Treg cells (Fig. 1). These results may indicate Treg cell number defects in these patients; however, additional studies with a larger patient sample size are needed to confirm the results.
Figure 1.
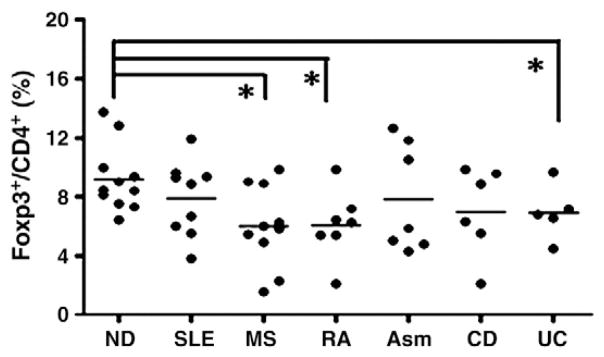
The frequency of Foxp3+ Treg cells in the CD4+ T cell population of patients and normal donors. CD4+ T cells were purified from the peripheral blood of patients with SLE, RA, MS, CD, UC, asthma and normal donors (ND) as described. The frequencies of Foxp3+ Treg cells in the peripheral blood CD4+ population of patients and normal donors were determined with intracellular Foxp3 staining. The percentage of Foxp3+ Treg cells in CD4+ T cell population from each patient was shown. Each dot represents one subject and the bar represents the average percentage of Foxp3+ Treg cells in each group (*p<0.05, Mann Whitney test for two group comparison).
Human CD4+CD25+ Treg cells were purified from PBMC of the patients with autoimmune and inflammatory diseases, as well as from normal donors using the autoMACS system and human CD4+CD25+ regulatory T cell isolation kits as described in materials and methods. After purification, the purity of human CD4+CD25+ Treg cells determined by Foxp3 expression ranged from 40% to 80% of the total cells. As shown in Fig. 2, the average percentage of purified CD4+Foxp3+ Treg cells from normal donors and the patients with SLE, MS, RA, asthma, CD or UC were 53.1%, 56.5%, 49.1%, 55.7%, 59.4% and 66.6%, respectively. Interestingly, the purity of the purified CD4+CD25+ Treg cells from the patients was similar to that of the normal donors (Fig. 2). These results demonstrated the successful enrichment of human CD4+Foxp3+ Treg cells from the peripheral blood PBMC of the patients with variety of immune-mediated diseases.
Figure 2.
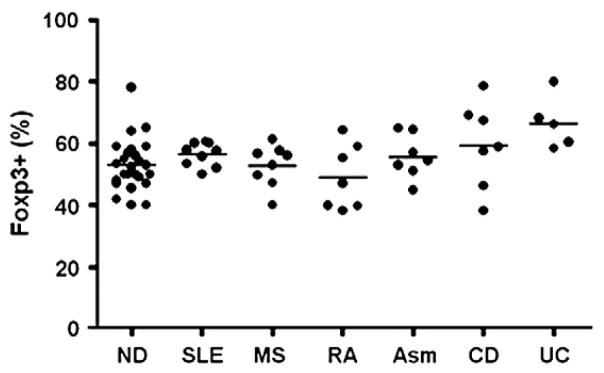
Successful enrichment of CD4+CD25+Foxp3+ Treg cells from the peripheral blood of patients with SLE, RA, MS, UC, CD, severe asthma and normal donors (ND). Human CD4+CD25+ regulatory T cells were purified from the peripheral blood of patients and normal donors using the autoMACS system and the CD4+CD25+ human regulatory T cell isolation kit as described. The purity of purified CD4+CD25+ Treg cells was determined by intracellular Foxp3 staining and was expressed as the percentage of the Foxp3+ cells in the total purified cell population. Each dot represents one subject, and the average purity in each group was represented by the bar.
Ex vivo expansion of human Treg cells from the patients with autoimmune and inflammatory diseases
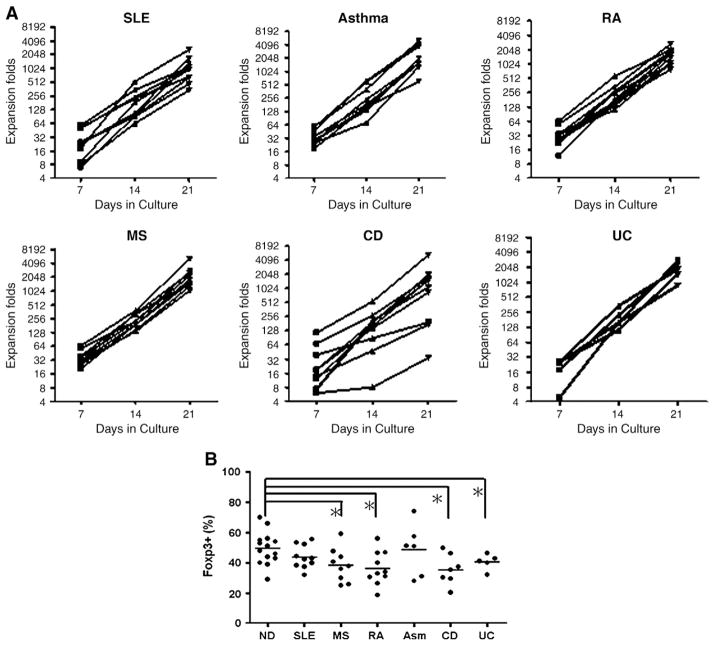
The enriched human CD4+CD25+Foxp3+ Treg cells were then activated and expanded with CD3/28 T cell expander beads at a 1/3 cell to bead ratio in the X-VIVO 15 medium with rhIL2 and 10% of heat-inactivated human male AB serum. After 2 to 3 weeks of culture, the human Treg cells were expanded approximately 1000 fold for the majority of the patients (Fig. 3A). These results demonstrated that a clinically relevant number of human Treg cells could be obtained from patients with certain immune-mediated diseases by ex vivo expansion.
Figure 3.
CD4+CD25+ Treg cells isolated from the peripheral blood of patients with immune-mediated diseases can be expanded 100–2000 fold ex vivo with sustained purity. Human CD4+CD25+ Treg cells enriched from the peripheral blood of patients with immune-mediated diseases were activated and expanded ex vivo with Dynal CD3/28 T cell expander beads and recombinant IL-2 as described. Cell numbers on day 7, 14 and 21 cultures were counted for each individual culture. The ex vivo Treg cell growth curves were shown in A. Each line represents a single subject. The purity of ex vivo expanded human Treg cells at week 2 was determined by intracellular Foxp3 staining, and was shown in B. Each dot represents one subject and the bar represents the average purity of expanded Treg cells (Fig. 3B, *p<0.05, Mann Whitney test for two group comparison).
The purity of week 2 expanded human Treg cells was evaluated using intracellular Foxp3 staining as shown in Fig. 3B. The average purity of expanded human Treg cells from normal donors and patients with SLE, MS, RA, Asthma, CD, and UC were 49.6%, 43.9%, 38.5%, 36.4%, 48.7%, 35.6% and 40.5%, respectively. Treg cell purity in most cultures was slightly lower compared to the starting cells (Fig. 2, post purification). The average Treg cell purity in the culture of cells from MS, RA, CD and UC patients was significantly lower than that of normal control subjects. Reduction of the Treg purity seemed to be independent of the initial purity, as the average Treg purity from patients with CD and UC was higher than that of normal donor (Fig. 2). Due to the small sample size, additional studies with more patient samples are needed to confirm the significance of the Treg cell purity difference in different patient populations.
Ex vivo expanded Treg cells from the patients with autoimmune and inflammatory diseases acquired enhanced in vitro suppressive function
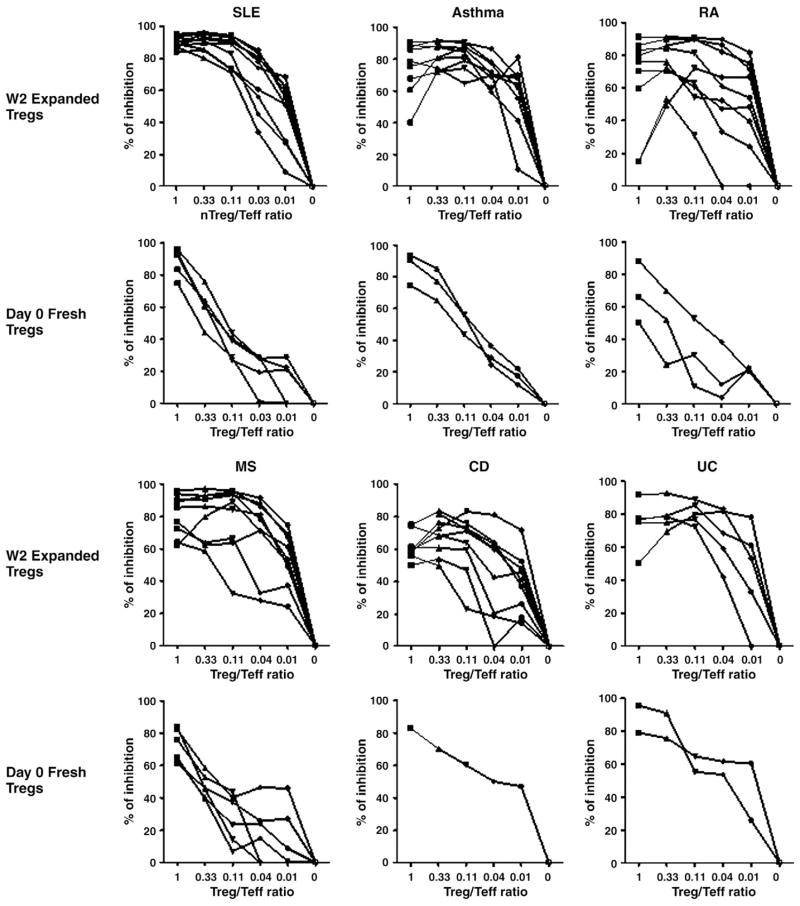
In vitro function of expanded human Treg cells was evaluated in an anti-CD3 antibody induced T effector cell proliferation assay as described in the materials and methods. In order to standardize the assay and compare in vitro function of Treg cells among patients as well as between freshly purified and expanded Treg cells from the same patient, we used the same lots of CD4+CD25− T responder cells, allogeneic DCs, and anti-CD3 antibody in the in vitro suppressive assays, so the only variants in the assay were the Treg cells. As shown in Fig. 4, almost all the ex vivo expanded CD4+CD25+Foxp3+ Treg cells from patients demonstrated potent dose-dependent in vitro suppression of anti-CD3-induced T cell proliferation. Most of the ex vivo expanded CD4+CD25+Foxp3+ Treg cells from patients with immune-mediated diseases exhibited more than 50% inhibition of T cell proliferation at a Treg/Teffector ratio of 1/10 in the suppression assays (Fig. 4), suggesting expanded human Treg cells from patients retained strong suppressive activities. The potency of expanded Treg cells from patients with inflammatory diseases was similar to that of expanded Treg cells from normal donors (previous publication) [34].
Figure 4.
Ex vivo expanded CD4+Foxp3+ Treg cells from patients with immune-mediated diseases acquired enhanced inhibitory activities. In vitro suppressive function of expanded human Treg cells at week 2 and freshly purified Treg cells from some of the patients were measured with a standardized T cell proliferation inhibition assay. Expanded or freshly purified human Treg cells were serially diluted and added into the cultures at different Treg/T effector ratios as indicated. The percentage of inhibition of T effector cell proliferation by either expanded or fresh Treg cells was shown in Fig. 4. Each line represents a single patient.
In addition, we also evaluated the in vitro function of freshly purified Treg cells from some, but not all patients due to the limited availability of freshly purified Treg cells. Most of freshly purified Treg cells from patients with immune-mediated diseases still displayed 50% inhibition to anti-CD3 antibody induced T cell proliferation at a Treg/Teffector ratio of 1/1. Although the freshly purified Treg cells exhibited dose-dependent inhibition of T effector cell proliferation, the potency was about 3–10 fold lower comparing to that of the expanded Treg cells from the same patients. These results demonstrated that ex vivo expansion not only increased Treg cell number but also enhanced the biological function of these cells. Our study further supports the potential use of ex vivo expanded human Treg cells for the treatment of autoimmune and inflammatory diseases.
Discussion
Naturally occurring CD4+CD25+Foxp3+ regulatory T cells play an indispensable role in the induction and maintenance of self-tolerance. Defect in the development and function of Treg cells is a primary cause of autoimmunity and certain inflammatory diseases in humans and animals [1,2]. Recent clinical studies and our current data demonstrated deficits in the numbers and/or functions in the CD4+CD25+Foxp3+ Treg cells from patients with certain autoimmune diseases [20–31], suggesting that correcting these defects of Treg cells might be a feasible therapeutic approach for these diseases. Our study demonstrated for the first time the feasibility of ex vivo expanding Treg cells from patients with SLE, RA, CD, UC, MS and severe asthma. In addition, our results suggested that ex vivo expansion not only increased the number but also enhanced the in vitro inhibitory function of Treg cells from these patients. Our result is consistent with the recent reports that functional Treg cells can be expanded from patients with autoimmune hepatitis, type 1 diabetes and healthy normal donors [32–35]. Therefore, providing additional support for the potential use of adoptive transfer of expanded human Treg cells as a therapeutic approach for autoimmune and certain inflammatory diseases [48,49].
Our study indicated that there were considerable subpopulations of patients with immune-mediated diseases bearing lower frequencies of Treg cells (Fig. 1), which may result from a shift of blood Treg cells to inflamed tissues in certain diseases, further characterization of the subpopulation may help Treg cell therapy to target the right populations of patients. The technical challenge of using ex vivo expanded Treg cells as a cell therapy is generating the billions of human Treg cells to fulfill the clinical requirements [41]. The paucity of human Treg cells in peripheral blood becomes a major hurdle for the clinical use [41], especially for patients with certain autoimmune diseases. In this study, we showed that human CD4+CD25+Foxp3+ Treg cells can be efficiently enriched from peripheral blood of patients with autoimmune diseases, interestingly, with the similar purity to normal donors, and can be successfully activated and expanded ex vivo to 100–2000 fold within 3 weeks, suggesting that multiple clinical administrations may be feasible. In addition, the enhanced Treg function may offset the deficiency in numbers.
Regulatory T cells inhibit T effector cell proliferation by cell-contact-dependent suppression, suppressive cytokines, modification or killing of antigen-presenting cells and T effector cells [1,47]. Expanded human Treg cells with enhanced suppressive function may help to restore the proper balance of immunity and tolerance, reverse or repair the Treg functional deficits in patients with certain autoimmune diseases, and eventually achieve a sustained immune tolerance to self-antigens. Our results suggest that adoptive transfer of functional Treg cells may be beneficial to patients and support the potential clinical use of Treg cell therapy for autoimmune diseases [48,49]. We and others recently demonstrated expanded human Treg cells prevented lethal GVHD in vivo, suggesting that the expanded Treg cells could carry their potent in vitro functions into in vivo environments and effectively suppress pathogenic T effector cell activation, proliferation, and the disease progression [34,35].
A recent murine study demonstrated a substantial percentage of Treg cells had transient or unstable expression of Foxp3 in vivo, especially higher in the inflamed tissues [50], which raises potential concern for the stability of the expanded human Treg cells in vivo once infused back to the patients. Therefore, strict phenotype characterizations such as CTLA-4, GITR, CD27, CD62L, CD127 expressions, cytokine profiles and DNA methylation at the Foxp3 locus of expanded human Treg cells from patients with each disease are important. To mitigate the challenge, several other approaches could be applied to enhance the efficacy and safety in future clinical studies: (1) Additional purification of Treg cells based on their lack of expression of CD127 could enhance the purity of the therapeutic Treg cells [42,43]; (2) expanding human Treg cells with rapamycin could strengthen the purity as well as the in vitro suppressive function of the expanded Treg cells, however, cell expansion was suppressed in the presence of rapamycin [44–46]; and (3) administration of low dose of IL-2 could help to stabilize Treg cells in vivo.
In conclusion, ex vivo expanded CD4+CD25+Foxp3+ Treg cells from peripheral blood of the patients with SLE, MS, RA, IBD and severe asthma acquired potent and enhanced in vitro suppressive function by inhibiting T effector cell proliferation. Within 3 weeks, human CD4+CD25+Foxp3+ Treg cells from the diseased patients could be expanded ex vivo 100–2000 fold. By exploiting the enhanced suppressive activity, these expanded Treg cells could restore the proper balance of the immune system and reverse or repair the Treg functional deficits in the patients. Our data support the feasibility of using the expanded Treg for the treatment of autoimmune and inflammatory diseases [48,49]. Although there are still technical and clinical challenges, we believe that with a better understanding of Treg cell biology and the pathology of different immune-mediated diseases, Treg cell-based therapy could provide a unique therapeutic benefit to patients with autoimmune or inflammatory diseases, such as SLE, MS, RA, CD, UC and severe asthma.
Acknowledgments
We thank Mindi Walker for reviewing manuscript, and also thank Steve Eck, Allis Soto, Malinda Aitken, Rich Gore, Weihong Wang and Sandy Hackman for their invaluable support in these studies.
Abbreviations
- Foxp3
forkhead box P3
- Treg cell
regulatory T cell
- DC
dendritic cell
- APC
allophycocyanin
- rhIL-2
recombinant human IL-2
- SLE
systemic lupus erythmatosus
- RA
rheumatoid arthritis
- MS
multiple sclerosis
- CD
Crohn’s disease
- UC
ulcerative colitis
- IBD
inflammatory bowel disease
Footnotes
Disclosure of conflict of interest
A US patent was filed to the present work. T.C. and G.H. were employee of Therakos Inc. L.L. is employee of Therakos Inc. S.E.W. received funding from Therakos to recruit and study the asthma subjects. W.A.F. received funding from Therakos to recruit and study the patients with Crohn’s disease and ulcerative colitis.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 3.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 4.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 5.Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, Bacchetta R, Levings MK. CD4+ T-regulatory cells: toward therapy for human diseases. Immunol Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 6.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 7.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 8.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 9.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 10.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;96:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 13.Kohm AP, Carpentier PA, Anger HA, Miller SD. CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 14.Olivares-Villagómez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–1460. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 16.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, Toes RE. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 17.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 18.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J Immunol. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 20.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 22.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, Nochy D, Debré P, Piette JC, Gorochov G. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 23.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 25.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 26.Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M, Nakamura K, Honda K, Kitamura Y, Mizutani T, Araki Y, Kabemura T, Chijiiwa Y, Harada N, Nawata H. An inverse correlation of human peripheral blood regulatory T cell frequency with the disease activity of ulcerative colitis. Dig Dis Sci. 2006;51:677–686. doi: 10.1007/s10620-006-3191-2. [DOI] [PubMed] [Google Scholar]
- 28.Miura Y, Thoburn CJ, Bright EC, Phelps ML, Shin T, Matsui EC, Matsui WH, Arai S, Fuchs EJ, Vogelsang GB, Jones RJ, Hess AD. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 29.Rieger K, Loddenkemper C, Mau J, Fietz T, Wolff D, Terpe H, Steiner B, Berg E, Mielhlke S, Bornhäuser M, Schneider T, Zeitz M, Stein H, Thiel E, Duchmann R, Uharek L. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 30.Lin YL, Shieh CC, Wang JY. The functional insufficiency of human CD4+ CD25 high T-regulatory cells in allergic asthma is subjected to TNF-alpha modulation. Allergy. 2008;63:67–74. doi: 10.1111/j.1398-9995.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 31.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+ CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 32.Longhi MS, Meda F, Wang P, Samyn M, Mieli-Vergani G, Vergani D, Ma Y. Expansion and de novo generation of potentially therapeutic regulatory T cells in patients with autoimmune hepatitis. Hepatology. 2008;47:581–591. doi: 10.1002/hep.22071. [DOI] [PubMed] [Google Scholar]
- 33.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao T, Soto A, Zhou W, Wang W, Eck S, Walker M, Harriman G, Li L. Ex vivo expanded human CD4+ CD25+ Foxp3+ regulatory T cells prevent lethal xenogenic graft versus host disease (GVHD) Cell Immunol. 2009;258:65–71. doi: 10.1016/j.cellimm.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Golovina TN, Mikheeva T, Suhoski MM, Aqui NA, Tai VC, Shan X, Liu R, Balcarcel RR, Fisher N, Levine BL, Carroll RG, Warner N, Blazar BR, June CH, Riley JL. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181:2855–2868. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winship DH, Summers RW, Singleton JW, Best WR, Becktel JM, Lenk LF, Kern F., Jr National Cooperative Crohn’s Disease Study: study design and conduct of the study. Gastroenterology. 1979;77:829–842. [PubMed] [Google Scholar]
- 37.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 38.This Official American Thoracic Society Workshop Report, Proceedings of the ATS Workshop on Refractory Asthma current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 39.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 40.Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992;35:498–502. doi: 10.1002/art.1780350502. [DOI] [PubMed] [Google Scholar]
- 41.June CH, Blazae BR. Clinical application of expanded CD4+25+ cells. Semin Immunol. 2006;18:78–88. doi: 10.1016/j.smim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Liu W, Putnam AL, Zhou X-Y, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas Test B. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 45.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 46.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 47.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esensten JH, Wofsy D, Bluestone JA. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:560–565. doi: 10.1038/nrrheum.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]