Abstract
The dense glycan coat that surrounds every cell is essential for cellular development and physiological function1, and it is becoming appreciated that its composition is highly dynamic. Post-translational addition of the polysaccharide repeating unit [-3-xylose-α1,3-glucuronic acid-β1-]n by like-acetylglucosaminyltransferase (LARGE) is required for the glycoprotein dystroglycan to function as a receptor for proteins in the extracellular matrix2,3. Reductions in the amount of [-3-xylose-α1,3-glucuronic acid-β1-]n (hereafter referred to as LARGE-glycan) on dystroglycan result in heterogeneous forms of muscular dystrophy4. However, neither patient nor mouse studies has revealed a clear correlation between glycosylation status and phenotype5,6. This disparity can be attributed to our lack of knowledge of the cellular function of the LARGE-glycan repeat. Here we show that coordinated upregulation of Large and dystroglycan in differentiating mouse muscle facilitates rapid extension of LARGE-glycan repeat chains. Using synthesized LARGE-glycan repeats we show a direct correlation between LARGE-glycan extension and its binding capacity for extracellular matrix ligands. Blocking Large upregulation during muscle regeneration results in the synthesis of dystroglycan with minimal LARGE-glycan repeats in association with a less compact basement membrane, immature neuromuscular junctions and dysfunctional muscle predisposed to dystrophy. This was consistent with the finding that patients with increased clinical severity of disease have fewer LARGE-glycan repeats. Our results reveal that the LARGE-glycan of dystroglycan serves as a tunable extracellular matrix protein scaffold, the extension of which is required for normal skeletal muscle function.
LARGE is a dual-function glycosyltransferase that adds a glycan repeat to the basement membrane receptor dystroglycan (DG). DG is comprised of a transmembrane β-subunit and a cell-surface-associated α-subunit—the LARGE-glycan is bound to the latter through a rare structure at the amino terminus of its mucin-like domain, a phosphorylated O-mannosyl glycan7,8. The LARGE-glycan binds, with high affinity, to laminin-G-domain-containing matrix proteins (including laminin, agrin, perlecan and neurexin9-12). These interactions can be directly competed by the antibody IIH6, which specifically recognizes the LARGE-glycan. The amount of LARGE-glycan repeat present on α-DG has remarkable variability—both developmental13,14 and tissue-specific15, as shown by marked differences in α-DG apparent molecular mass. Mutations in LARGE, the DG-encoding gene (DAG1), and several other genes involved in synthesizing the O-mannosyl glycan16-22 result in a group of muscular dystrophies termed dystroglycanopathies. These disorders encompass a broad spectrum of severity, ranging from late adult onset with predominant weakness of proximal muscle groups to more severe forms that present at birth and sometimes include defects in brain and eye development. Features common to these disorders are reduced ligand binding by α-DG and reduced to absent IIH6 immuno-reactivity. However, when a cohort of dystroglycanopathy biopsies was assessed for IIH6 immunoreactivity by microscopy a correlation between staining intensity and clinical severity was not found5.
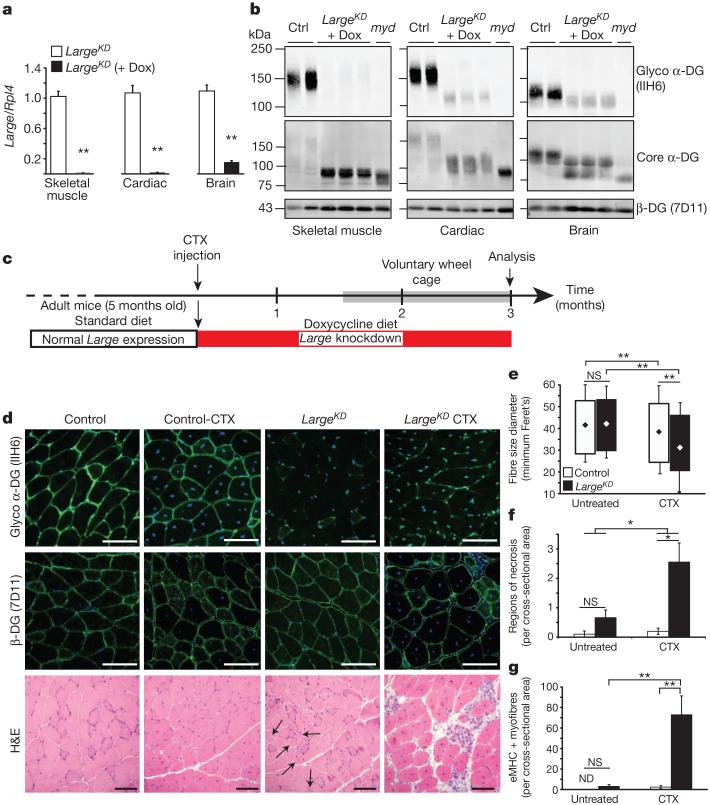
It has been proposed that α-DG has separable, currently unknown roles in developing and fully differentiated skeletal muscle23,24; this could explain some of the observed clinical heterogeneity among dystroglycanopathy patients. To address this possibility without interfering with α-DG during embryogenesis, we developed the GT(ROSA)26Sor tm407(H1/tetO-RNAi:Large) (hereafter called LargeKD) inducible mouse model, in which Large is knocked down systemically through RNA interference (RNAi) (Extended Data Fig. 1a, b). On a standard diet, heterozygotes are phenotypically indistinguishable from littermates and α-DG glycosylation is normal (Extended Data Fig. 1c, d). When doxycycline is introduced through the diet, however, Large transcription is downregulated and IIH6 immuno-reactivity in skeletal muscle and elsewhere is lost (Fig. 1a, b). Skeletal muscle has an extraordinary capacity for regeneration, a process that involves triggering satellite cell differentiation into myoblasts, which then proliferate and undergo myogenesis. We designed an experiment to test the impact of disrupted α-DG on regeneration, comparing muscle formed with normal versus defective α-DG. Specifically, we used Naja nigricollis cardiotoxin snake venom (CTX) to induce regeneration in select muscles of otherwise healthy adult LargeKD mice, and simultaneously began Large knockdown (Fig. 1c). Although β-DG was preserved in LargeKD muscle through 3 months after induction, LARGE-glycan was nearly eliminated (Fig. 1d). Gross analysis and quantification of multiple parameters in muscle from CTX-injected LargeKD mice and uninjected LargeKD and littermate controls (both CTX and uninjected) revealed that the test mice were profoundly dystrophic (Fig. 1d–g). Some uninjected LargeKD muscle myofibres featured centrally localized nuclei, indicating that the myofibre had previously regenerated, but evidence of active necrosis or regeneration (eMHC+ myofibres) was minimal despite the absence of LARGE-glycosylated α-DG. From these results we proposed that the degree to which a patient mutation affects α-DG LARGE glycosylation during muscle formation is a major determinant of disease severity.
Figure 1. Muscle generated during Large knockdown is predisposed to aggressive dystrophy.
a, b, Quantification of Large mRNA expression in LargeKD mice on doxycycline (dox) induction (n=3 animals per group, 2 experimental replicates) (a), and western blot-based determination of LARGE glycosylation 3 months after shRNA induction, and in littermate controls (ctrl) and LARGE-null negative control (myd) (b). Each lane is a sample from an individual animal. c, Experimental outline. Six weeks after CTX injury and simultaneous induction of Large knockdown, the mice were housed in cages with exercise wheels to circumvent the typical sedentary behaviour of laboratory mice (grey bar). Two experimental replicates for all analysis. d, Representative images of IIH6 and β-DG reactivity in immunofluorescence- and haematoxylin-and-eosin (H&E)-stained sections (black arrows, centrally nucleated myofibres). e–g, Tibialis anterior muscle sections (4 sections/muscle) of control and LargeKD mice (white and black bars, respectively, LargeKD mouse, n=3; control, n=4 biological replicates) were assessed for: e, fibre diameter (interaction P<0.001, ~2,000 fibres per group; diamonds represent mean fibre diameter); f, average regions of necrosis (interaction P=0.057); and g, average number of recently regenerated, embryonic myosin-positive fibres (interaction P=0.007). Error bars represent s.d. in e, and s.e.m. elsewhere. Post-hoc comparisons *P<0.05, ** P<0.0001; NS, not significant; ND, not detected. Scale bars, 100 μm.
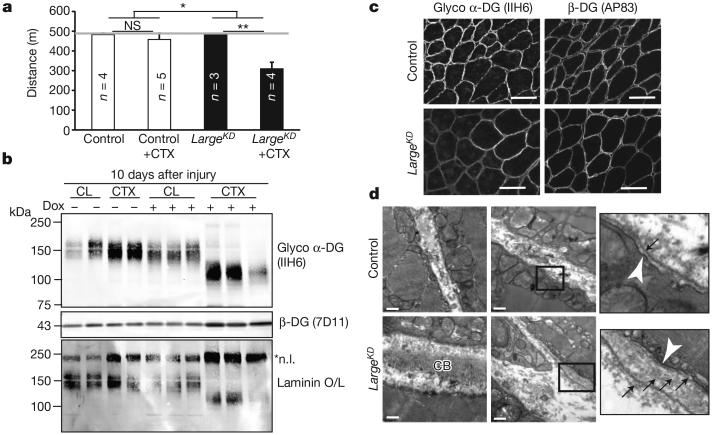
Physiological assessment at earlier time points confirmed that LARGE-glycan is critical for muscle regeneration. Bilaterally CTX-injected LargeKD mice had significant deficits in downhill running within 3 weeks (Fig. 2a), whereas uninjected mice maintained running capacity in spite of a significant reduction in normally glycosylated α-DG (Extended Data Fig. 2a). Notably, LargeKD muscles recovering from CTX injury had abundant IIH6-positive α-DG, although of a reduced molecular mass (Fig. 2b and Extended Data Fig. 2a, b). Despite its low molecular mass, this α-DG was competent to bind laminin (Fig. 2b) and localized to the sarcolemma correctly (Fig. 2c). These results indicated that the number of α-DG LARGE-glycan repeats is critical to the cellular function of α-DG during muscle regeneration.
Figure 2. Regenerating LargeKD muscle has α-DG of reduced molecular mass and basement membrane defects.
a, Quantification of muscle performance in downhill treadmill assay. Mice were injected bilaterally in the tibialis anterior and gastrocnemius muscles, and were given a 21-day recovery period. Grey line indicates predetermined assay end point (performance averages, interaction between genotype and CTX injury P=0.015, post-hoc comparisons *P<0.05, ** P<0.0001, error bars indicate s.e.m., n=biological replicate number for trial depicted, 3 experimental replicates). b, Western blot analysis and laminin overlay assay prepared from regenerating muscle, each lane representing an individual animal. CL, contralateral (un-injured) muscle; *n.l., native laminin. c, IIH6 and β-DG immunoreactivity of muscle sections. d, Muscle ultrastructure as assessed by transmission electron microscopy. White arrowhead, sarcolemma; black arrows, basement membrane; CB, collagen bundles. Samples in b–d were taken 10 days after CTX injury; scale bars, 50 μm (c) and 0.5 μm (d).
Histological examination of regenerating LargeKD muscles early after CTX treatment revealed that myofibres regenerate without a delay, but adipose cells, a feature commonly seen in severe muscular dystrophies, were observed (Extended Data Fig. 2c, d). Staining for perlecan, which normally co-localizes with laminin in the basement membrane, was reduced in intensity at the basement membrane but was also found mislocalized to the collagen-rich, extracellular matrix layer known as the endomysium (Extended Data Fig. 3a–e). Laminin α2 was similarly mislocalized to the endomysium (Extended Data Fig. 3f). Given that these findings were indicative of basement membrane abnormalities, we used transmission electron microscopy to probe for defects at the ultrastructural level. The presence of centrally localized nuclei, an increase in the presence of fibroblasts and macrophages, and an increase in the number of mitochondria at the myofibre periphery confirmed that the regions imaged had been damaged by CTX. In CTX-injected LargeKD muscle, the basement membrane was significantly thickened and, unlike that in CTX control muscles, was often composed of multiple layers (Fig. 2d and Extended Data Fig. 3g). In addition to these abnormalities, collagen fibrils in the endomysial space were unusually abundant and often abnormally oriented with respect to the myofibres, consistent with the fibrous appearance of collagen structures by immunofluorescence (Extended Data Fig. 3a). The expression of collagen VI, perlecan and agrin was unaltered in CTX-injected LargeKD muscles, although laminin isoforms were found to be elevated at both the mRNA and protein levels (Extended Data Fig. 4 and Fig. 2b). The observed layering of the basement membrane and the increase in thickness is consistent with reduced compaction of laminin and the collagen superstructures during basement membrane formation.
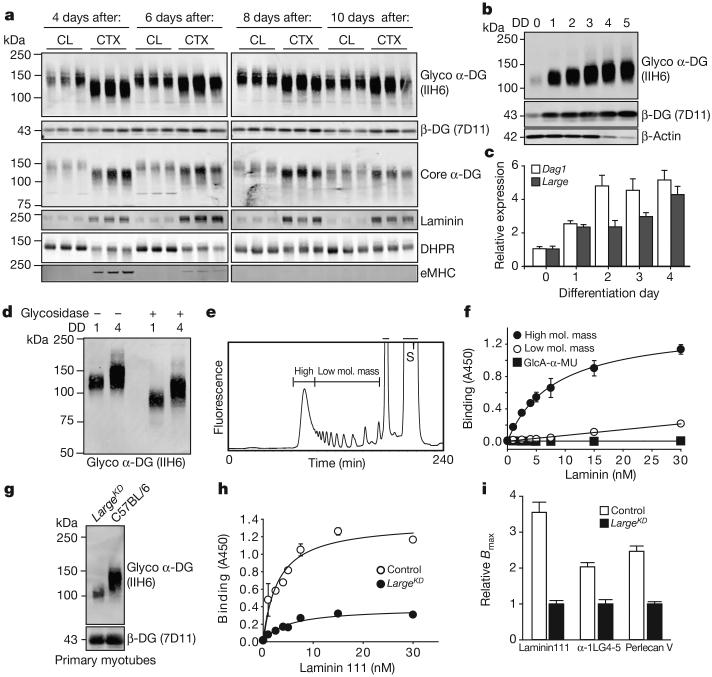
We next assessed the mechanism that underlies the variability in the molecular mass of α-DG. In regenerating control mice, α-DG shifts from approximately 100 kDa to 156 kDa (mode value) within 10 days of injury (Fig. 3a). This shift is closely paralleled by increases in the expression of basement membrane components laminin and collagen VI (Extended Data Fig. 5a), and by other biochemical hallmarks of regeneration such as a gradual loss of embryonic myosin and restoration of dihydropyridine receptor expression (Fig. 3a). To investigate such changes in greater detail we used the C2C12 mouse myoblast cell line, a classical model of myogenesis, which shows a marked increase in DG protein levels upon serum restriction25. The transition to larger glycoforms occurred rapidly after myoblasts are induced to differentiate, preceding myoblast fusion into myotubes on differentiation day (DD) 2 by 24 h (Fig. 3b and Extended Data Fig. 5b). The modal α-DG glycoform increased by 18 kDa over the course of the experiment (Extended Data Fig. 5c). This change correlated with rapid and sustained increases in Large and Dag1 expression (Fig. 3c). Other modifiers of α-DG also showed increased expression levels but later and/or less acutely (Extended Data Fig. 6). We confirmed that other forms of α-DG glycosylation were not responsible for the increase in molecular mass, through enzymatic de-glycosylation removing the N-glycans, certain α-DG mucin O-glycans and terminal trisaccharides of O-mannosyl tetrasaccharides (a treatment that spares the phosphorylated O-mannosyl linked LARGE-glycan7). Notably, although both DD0 and DD4 α-DG migrated faster after de-glycosylation, the difference in molecular mass between the samples remained, as did the glycoform variation (band smearing) within each sample (Fig. 3d). We conclude that both inter- and intra-α-DG molecular mass variability during myogenesis is due to differences in the quantity of LARGE-glycan repeats. Next we measured inorganic phosphate chemically released from α-DG isolated from C2C12 DD1 (myoblasts) and DD5 (myotubes) after acid hydrolysis in conjunction with the malachite green phosphate assay. We found no significant difference (6.15 ± 2.37 μM versus 6.83 ± 2.19 μM phosphate released per unit DG for DD1 and DD5, respectively, n=3 trials). Thus, the increase in α-DG LARGE-glycan content during myogenesis is not attributable to the addition of new LARGE-glycan chains, but rather a consequence of extension of the LARGE-glycan chains present.
Figure 3. Increase in α-DG LARGE-glycan chain length during myogenesis enhances ligand-binding capacity.
a, Western blot analysis of WGA-enriched samples for expression of DG and other proteins at multiple times after CTX-mediated injury to C57BL6/J mice, each lane representing an individual animal. b, Western blot analysis of WGA-enriched lysates from C2C12 myoblasts undergoing myogenesis for functional α-DG (DD, differentiation day). At DD4–5 the loading control, β-actin, is downregulated in myotubes. c, qPCR analysis of Dag1 and Large during C2C12 myogenesis (averages reported, P<0.001, n=3 biological replicates, n≥7 technical replicates, relative to Rpl4 expression, error bars indicate s.e.m.). d, Western blot demonstrating effects of enzymatic deglycosylation of DD0 and DD4 C2C12 protein samples. e, f, Elution profile of LARGE-glycan repeats on a gel filtration column. Bars indicate the fractions that were collected as the high- and low-molecular-mass LARGE repeats (S, substrate) used for solid-phase laminin 111 (f, high molecular mass Kd=8.2) binding. g, DG western blot in DD4 myotubes from LargeKD (+1 μgml−1 doxycycline) and control cultures. h, Solid-phase laminin 111 binding demonstrating that binding capacity is dependent on extension of the LARGE-glycan (control Kd=2.93 ± 0.776 nM; LargeKD Kd=4.75 ± 1.24 nM). i, Comparison of solid-phase determined relative Bmax values for several α-DG ligands (averages represented, Bmax values for LargeKD were set to 1 to allow for direct comparisons; error bars indicate s.e.m.). For solid-phase assays (f and h), error bars indicate s.e.m., n=3 technical replicates, curve fitting to equation f=Bmax*abs(x)/(Kd + abs(x)).
We proposed that a reduction in the LARGE-glycan degree of polymerization results in α-DG with reduced ligand-binding capacity. Using synthesized LARGE-glycan chains of either low (up to 13 repeats) or high molecular mass (>13 repeats) immobilized on ELISA assay plates, we performed binding assays for laminin, IIH6 and the perlecan V domain (Fig. 3e, f and Extended Data Fig. 7a–c). Binding to the low-molecular-mass LARGE-glycan was limited compared to that for the high-molecular-mass LARGE-glycan. Next, myotubes generated from primary cultures of isolated control and LargeKD satellite cells were collected for α-DG solid-phase binding assays (Fig. 3g). The binding capacity (relative Bmax) of α-DG from the LargeKD myotubes was reduced for multiple ligands, including recombinant α1LG4-5 (2.04-fold), the perlecan V domain (2.47-fold), and laminin 111 (3.55-fold); the latter also displayed a small reduction in dissociation constant (Kd) (Fig. 3h and Extended Data Fig. 7d–f, summarized in Fig. 3i). These data demonstrate that the degree of LARGE-glycan polymerization, and thus the molecular mass of α-DG, correlates directly with the ligand-binding capacity of α-DG.
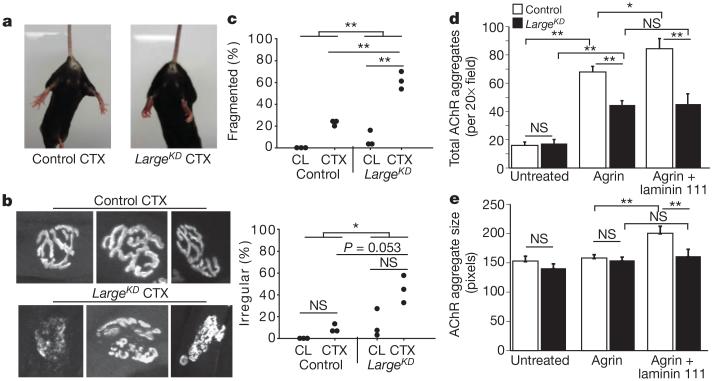
We reasoned that extension of the LARGE-glycan repeat chain during regeneration increased ligand-binding capacity by providing a larger scaffold for basement membrane components. The neuromuscular junction (NMJ) contains a basement membrane especially rich in α-DG ligands. According to our hypothesis, this structure should be disrupted in LargeKD mice. Analysis of mice by tail suspension was consistent with such NMJ abnormalities in regenerated muscles; LargeKD mice subjected to CTX injections into the tibialis anterior and gastrocnemius muscles clasped their hind paws instead of splaying them outward as was typical for control mice (Fig. 4a). Furthermore, analysis of acetylcholine receptor (AChR) labelling in these mice 21 days after CTX damage revealed that the NMJs were fragmented and irregular in shape (Fig. 4b, c), consistent with impaired maturation at the ultra-structural level. Finally, in AChR aggregation assays in primary myotube cultures, the addition of agrin or agrin plus laminin 111 to the medium of control myotubes led to a stepwise increase in AChR aggregate number and size, as previously demonstrated26, but these increases were absent in LargeKD myotubes (Fig. 4d, e). These findings demonstrate that normal NMJ maturation requires a high degree of LARGE-glycan polymerization, and that in the absence of such extension ligand saturation occurs.
Figure 4. Reduction in α-DG LARGE-glycan chain length during myogenesis results in NMJ defects.
a, Hind-paw clasping phenotype during tail suspension in LargeKD mice, 21 days after injury to the tibialis anterior and gastrocnemius muscles. b, Representative confocal images of AChR-labelled NMJs. c, Scoring of NMJ defects by blinded observers (scoring criteria in Methods, interaction P=0.011 for fragmentation). d, e, Average AChR aggregation after ligand addition to control (white) or LargeKD (black) DD4 myotubes (interaction P<0.001 for total number and P=0.007 for size; error bars indicate s.e.m.; ×20 magnification images, n=16 per group). Post-hoc comparisons for c–e *P<0.05, **P<0.0001.
Our data demonstrate that LARGE-glycan extension is required for reformation of both normal NMJs and the basement membrane after injury. Moreover, they indicate that the degree of LARGE-glycan polymerization is temporally coordinated with muscle regeneration. We thus tested whether the presence of excess LARGE-glycan early during regeneration would be detrimental. The overexpression of Large (in CAG-Large transgenic mice) resulted in hyper-glycosylated muscle α-DG, both at steady state (muscles not subjected to CTX injury) and shortly after CTX-induced muscle injury (Extended Data Fig. 8a, b). Glycosidase treatment of wheat-germ-agglutinin-enriched samples indicated that off-target activity owing to Large overexpression was unlikely (Extended Data Fig. 8c, d), although we cannot entirely exclude a small degree of nonspecific LARGE activity. Regenerated CAG-Large myofibres were reduced in diameter and cross-sectional area, and myofibre density was increased, indicative of a defect in myoblast fusion (Extended Data Fig. 8e–i). These data support the notion that the pace of increased LARGE-glycan polymerization is an important aspect of α-DG function during muscle regeneration.
We next assessed human muscle biopsies from several forms of muscular dystrophy. Samples from two broad classes of muscular dystrophy were examined: more severe congenital forms (early-childhood onset, impact on non-muscle organs; congenital muscular dystrophy (CMD)), and a less severe form (adult-onset, impacting proximal muscles to a greater extent; limb girdle muscular dystrophy 2I (LGMD2I)). We observed a reduction in the molecular mass of IIH6-positive α-DG in the congenital muscular dystrophy muscle only (Extended Data Fig. 9a); whereas very little IIH6-positive α-DG was observed in the LGMD2I samples, the residual protein present was of typical molecular mass and restricted (as assessed by immunofluorescence) to muscle regions undergoing regeneration (Extended Data Fig. 9b). These results are in accordance with a previous study13. Consistent with our findings from LargeKD mice, mislocalization of perlecan to the endomysial space was observed only in biopsies where α-DG was of reduced molecular mass (Extended Data Fig. 9c, d). Despite the fact that endomysial fibrosis in the LGMD2I biopsy was extensive, perlecan localization was normal, that is, restricted to the basement membrane. These data suggest that the typical late onset of LGMD2I is caused by loss of functional α-DG in the context of normal basement membrane formation. A previous analysis demonstrated that IIH6-positive α-DG could be recovered with LARGE overexpression in dystroglycanopathy cell lines27. In light of our results, we propose that in some dystroglycanopathies the native increase in LARGE function during myogenesis provides sufficient LARGE-glycan during muscle regeneration to lessen the impact of certain disease-causing mutations. If true, this would mean that the degree to which LARGE-glycan polymerization is reduced during muscle regeneration is a critical determinant of clinical severity.
Our findings demonstrate that the LARGE-glycan repeat of α-DG is a tunable scaffold for extracellular matrix proteins, the restriction of which through Large knockdown during muscle regeneration both reduces muscle physiological function and predisposes it to dystrophy. Whereas the lower molecular mass α-DG retains the ability to bind laminin, our data are consistent with it binding fewer ligands per α-DG protein. In addition to binding the laminin network of the basement membrane, the LARGE-glycan also binds agrin and perlecan, which are capable of binding laminin and collagen concurrently. We speculate that when sufficient LARGE-glycan is available, these collateral linkages facilitate compaction of the basement membrane layers (Extended Data Fig. 10a, b). We also note that basement membranes from different tissues vary in both thickness and elasticity28,29, and that α-DG is differentially glycosylated in a manner that affects ligand-binding characteristics (due mainly to differences in LARGE-glycan) across many tissues15,30(Extended Data Fig. 10c, d). It is thus possible that the capacity to fine-tune extension of the α-DG LARGE-glycan contributes to diversity in basement membrane structures across mammalian tissues.
METHODS
Experimental replicates
Unless otherwise stated, all experiments were repeated in the laboratory three times. Data reported are representative.
Animals
Animal care, ethical usage and procedures were approved and performed in accordance with the standards set forth by the National Institutes of Health and the Animal Care Use and Review Committee at the University of Iowa. At the University of Iowa all mice are socially housed (unless single housing is required) under specific pathogen-free conditions in an AAALAC accredited animal facility. Housing conditions are as specified in the Guide for the Care and Use of Laboratory Animals (NRC). Mice are housed on Thoren brand, HEPA filtered ventilated racks, in solid bottom cages with mixed paper bedding. A standard 12/12-h light/dark cycle was used. Standard rodent chow (or special diet if required) and water is available ad libitum. C57BL6/J and Largemyd mice were obtained from The Jackson Laboratory. LargeKD mice were generated at TaconicArtemis GmbH. CAG-Large transgenics were generated by ligating a 1.7-kb fragment of the CAG promoter to a 2.3-kb fragment corresponding to positions +174 through +2444 of the Large (NM_010687) cDNA, plus the rabbit β-globin polyadenylation signal. The linearized 4.6-kb SalI-StuI CAG-Large construct was injected into pronuclei of fertilized zygotes from C57BL/6NTac mice and transferred to pseudopregnant females. Offspring were screened for genomic integration of this fragment by PCR of tail DNA, using the following CAG-Large-specific primers (PCR product size of 2.5 kb): forward 5′-CCTACAGCTCCTGGGCAACGTGCTGGTT-3′, reverse 5′-AGAGGGAAAAAGATCTCAGTGGTAT-3′. Mice were generated by breeding F1 heterozygous transgenic males to wild-type females.
Mice were bred onto a C57BL6/J background (backcross 6 or greater). All mouse studies were performed on adult (8–15-week-old) mice raised on a standard mouse diet. Whereas within each experiment all subjects were sex- and age-matched (littermates where possible), both male and female mice were used and found to respond similarly with the exception of the downhill running assessment (Fig. 2a) wherein only male mice were used as performance variability was noted between the sexes. Previous experience with standard deviation of given techniques and pilot studies using naive animals were used to determine required power for experiments where appropriate with all efforts made to ensure animals for a given experiment were littermates or within 1 week of age. All treatment group designations (randomization) were assigned based on mice identification number and genotype information before experimenter’s observation of the animals to exclude any bias based on animal appearance. 10 μM CTX (Accurate Chem & Sci Co) in phosphate buffered saline (PBS) was used for intramuscular injections, at the following volumes: tibialis anterior, 25 μl; gastrocnemius, 50 μl. Injections of PBS alone caused no observable muscle damage. Injections were unilateral unless otherwise noted. Induction of knockdown was achieved by replacing the normal mouse diet with an equivalent chow containing 1 g kg−1 doxycycline (Bio-Serv) at the time of CTX injection (day 0). Animals were exercised by downhill running (15° grade), using a variable speed belt treadmill (Omnipacer, AccuScan Instruments, Inc.) by an experimenter blinded to the genotypes of individual animals. Warm-up: 5 min, 3 metres per min (m.p.m.); running at the following paces; 10 m.p.m., 15 m.p.m., 20 m.p.m. for 5 min each, and 25 m.p.m. for 10 min or to exhaustion. Exhaustion was scored as 10 consecutive seconds of non-performance.
Human subjects and samples
Muscle biopsies were originally collected for diagnostic purposes and were obtained and tested according to the guidelines set out by the Human Subjects Institutional Review Board of the University of Iowa; informed consent was obtained from all subjects or their legal guardians.
Cell culture
C2C12 mouse myoblasts (freshly purchased from ATCC) were maintained in DMEM containing 10% FBS, 1% L-glutamine at 37 °C in 5% CO2, and kept to a maximum of eight passages. For differentiation, cells were grown to confluence (designated differentiation day 0, DD0) and then switched to DMEM with 2% donor equine serum. AraC (cytosine β-D-arabinofuranoside hydrochloride, 10 nM final, Sigma) was added to differentiating C2C12 cells after myotubes formed, to limit the proliferation of differentiation-incompetent myoblasts. Satellite cell isolation was performed as per ref. 31. Satellite cells were plated on BD Matrigel-coated dishes and activated to differentiate into myoblasts in DMEM-F12, 20% fetal bovine serum (FBS), 40 ng ml−1 basic fibroblast growth factor (R&D Systems, 233-FB/CF), 1× non-essential amino acids, 0.14 mM β-mercaptoethanol, 1× penicillin/streptomycin and Fungizone. Myoblasts were maintained with 10 ng ml−1 basic fibroblast growth factor and differentiated in DMEM-F12, 2% FBS, 1× insulin-transferrin-selenium. For AChR-aggregation assays, primary myoblasts were seeded on BD Matrigel-coated glass coverslips.
Muscle analysis
Immunofluorescence and haematoxylin and eosin staining were carried out as described previously9. All images are representative from larger analysis of at least three animals per group, per condition. Fibre size diameter (minimal Feret’s diameter) was assessed using ImageJ software (version 1.45s), based on β-DG immunofluorescence. Necrosis was assessed using anti-mouse IgG fluorescent antibody (detects immune-cell infiltration of degenerating fibres). qRT-PCR for mouse Dag1 and associated glycosyltransferases was performed using a BioRad MyIQ system, with protocols and primer sets previously described32. The following primer sets were also used: Ispd1 forward 5′-TGGTGTGGATTAGGGGGTTA-3′, reverse 5′-TGGCTGCACTTTGTCCTAAA-3′; Tmem5 forward 5′-GAGAACAGTGGCAGCCTCA-3′, reverse 5′-CAAAGGAGCAGGCCTCATAG-3′; Sgk196 forward 5′-GCTGTCCTGTGAAGAGCTGA-3′, reverse 5′-GGGAGAGAGCGACTTTGTGT-3′; Ignt1 forward 5′-ACATTTGACGAACGCTTTC-3′, reverse 5′-CCTCCTTTTGGGGATGGAAC-3′; Itga7 forward 5′-TTGCTGTTAGCCACGATCAG-3′, reverse 5′-CGCCAGAGAAGAAGAGTTGC-3′; Col6a forward 5′-CTCTCCTGGTTCACCCATGT-3′, reverse 5′-CCCGACTCTACCGAGATTGA-3′; Lama2 forward 5′-CCAAGAAGGAGGCTGCATAG-3′, reverse 5′-CCAGGTGTTGGGAAGACACT-3′; Lamb1 forward 5′-GTTCGAGGGAACTGCTTCTG-3′, reverse 5′-GTTCAGGCCTTTGGTGTTGT-3′; perlecan(Hspg2) forward 5′-GAGCGGACTGTACCTTGGTC-3′, reverse 5′-ACCAGTTGCACACAGCTCAC-3′; agrin forward 5′-CAGTGGGGGACCTAGAAACA-3′, reverse 5′-ACCTTTCCAATCCACAGCAC-3′.
Fold changes were calculated using the ΔΔCt method. Failed reactions, those with Ct values within 1 Ct value of water control, were excluded. Mouse and human protein samples were obtained by 1% Triton X-100 solubilization of physically disrupted tissue. Glycoprotein enrichment via WGA agarose beads (Vector Labs) was performed as described previously9. Densitometry was performed using LiCor Odyssey Software, V3.0, of triplicate blots. Laminin overlay and solid-phase assays were performed as previously described3,9. For analysis of NMJs, Alexa-488-conjugated α-bungarotoxin was used to stain 4% paraformaldehyde-fixed tibialis anterior muscle cut into thirds longitudinally. Labelled muscle was cleared using glycerol and flattened. Maximal-intensity projections were obtained using an FV1000 Olympus Scanning Confocal laser microscope and z-stack sections compiled with FluoViewer-1.5 (Olympus). Next, individual NMJs from each image were assigned a number, cropped and copied onto a scoring PowerPoint file in a randomized fashion before scoring. Individual NMJs were scored by three blinded observers, using criteria established previously33. NMJs were scored as ‘fragmented’ if they were comprised of 5 or more AChR islands, and ‘irregular” if they were abnormally shaped (either shallow folds and involutions or none at all). NMJs throughout the muscle were sampled, from 3 animals per group. CAG-Large mice were analysed for fibre size diameter (minimal Feret’s), fibre cross-sectional area and density from images acquired using a VS120-S5-FL slide scanner microscope (Olympus) with VS-ASW (version 2.6). Analysis was carried out using VS-Desktop software version 2.6.
Statistics
All experimental data are representative of repeated experiments. Statistical analysis was performed using SigmaPlot software which determines if testing assumptions are met (Shapiro–Wilk normality and equal variance testing). Figure 1a used the Student’s t-test, two sided. Figures 1e, f, 2a and 4c–e were analysed by two-way ANOVA. Post-hoc, pairwise multiple comparison procedures used the Holm–Sidak method with correction for multiple testing. Figure 3c data were analysed by one-way ANOVA on ranks.
Malachite green phosphate assay and sample preparation
C2C12 cells were differentiated on three 15-cm dishes and collected by physical scrapping. Cells were homogenized with 1% Triton X-100 by vortexing for 5 min, and insoluble material was centrifuged and discarded. An initial Lowry protein assay determined that starting protein was equivalent in each sample. DG pull-down was carried out using a β-DG-specific monoclonal antibody (8D5, Developmental Studies Hybridoma Bank) pre-bound to protein-A agarose beads (Santa Cruz Biotechnology). After extensive washing with 0.1% Triton X-100 in Tris-buffered saline, DG and the associated proteins were eluted using 0.1 M β-DG peptide. Samples were then dialysed exhaustively in ddH2O (6,000–8,000 molecular mass cutoff, 5 days, 12+ 4 l changes). Concentrations relative to that of the α-DG core protein were determined by ELISA. Equal starting amounts of α-DG were then treated with 150 units calf intestinal alkaline phosphatase (CIP, New England Biolabs) for 30 min at 37 °C, to remove organic phosphate modifications such as the O-linked N,N′-diacetyllactosamine modification recently described for α-DG34. To release phosphate associated with the LARGE-glycan, samples were incubated with ice-cold 48% aqueous hydrofluoric acid at 0 °C for 20 h. The reagent was removed under a steady stream of nitrogen gas while on ice. Control samples were prepared by the same procedure, except that ice-cold water was used in place of hydrofluoric acid. Re-suspended samples were then assessed for phosphate content using the Malachite Green Phosphate Detection kit (R&D Systems).
LARGE-glycan preparation for the binding assays
0.2 mM biotinylated GlcA-MU (custom synthesis by Sussex Research Laboratories Inc.) was incubated with LARGEdTM7 10 mM UDP-glucuronic acid (GlcA), 10 mM UDP-xylose (Xyl), 10 mM MgCl2 and 10 mM MnCl2 in 100 mM 2-(N-morpholino)ethanesulphonic acid (MES) buffer (pH 6.5) at 37 °C for 18 h. The enzymatic reaction was terminated by boiling in the presence of 50 mM EDTA. After centrifugation (20,000g for 10 min), the supernatant was fractionated using gel-filtration chromatography (Superdex peptide 10/300GL, GE Healthcare) as described previously7. The peaks eluted from retention time 75–97 min, and from 97–170 min were pooled as low-molecular-mass and high-molecular-mass LARGE repeats, respectively, and then lyophilized. The LARGE repeats in these two pools were dissolved in water and then analysed using a Bruker UltrafleXtreme MALDI-TOF/TOF mass spectrometer in negative reflection mode. α-Cyano-4-hydroxycinnamic acid (saturated in a mixture of acetonitrile and 0.1% TFA in 1:1 v/v) was used as the matrix as described7. To measure the concentration of MU-linked glycan, the glycan samples and known amounts of GlcA-MU were hydrolysed in 1 M of HCl at 95 °C for 20 min. The amounts of 4-methylumbelliferone released by acid hydrolysis were measured using a microplate reader Synergy4 (BioTek) (excitation, 350 nm; emission, 450 nm). GlcA-MU and low- and high-molecular-mass LARGE repeats (0.08 nmol per well) were immobilized on a NeutraAvidin coated 96-well plate (Pierce) according to the manufacturer’s protocol. The plates were used for the solid-phase binding assays as described below.
Solid-phase binding assays
Myotube culture samples were collected by scraping and homogenized as described above. Total protein was quantified by Lowry protein assay, and equal amounts of protein were used to enrich for glycoproteins, using WGA agarose beads (Vector Labs) as described previously9. The use of equivalent amounts of DG was confirmed by ELISA to α-DG core protein and western blotting for β-DG. Samples were diluted with Tris-buffered saline 1:50, and bound to Costar 96-well, High Bind EIA/RIA plates (50 μl per well) overnight at 4 °C. Unbound sample was carefully aspirated and the plate blocked with 3% bovine serum albumin in laminin binding buffer (LBB; 746 mM triethanolamine, 140 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, pH 7.6) for 2 h at room temperature. A series of ligand dilutions was prepared in LBB with a final concentration of 3% bovine serum albumin and 2 mM CaCl2. Laminin 111 (Invitrogen), the perlecan V domain35, the α1LG4-5 domain (isolated from conditioned medium from HEK293 cells secreting Flag-tagged α1LG4-5 domain, collected and enriched as described36), and AEBSF-laminin 111 were used in separate binding assays. To generate AEBSF-laminin 111, 10 mM serine protease inhibitor p-aminoethylbenzenesulphonyl fluoride AEBSF, HCl (Sigma) was incubated overnight on ice with 1 mg mouse laminin protein (Invitrogen) and used to inactivate laminin polymerization. AEBSF-treated laminin was dialysed to remove free AEBSF with 5 LBB changes of one litre each. Binding solutions were incubated with bound sample for 1 h at room temperature. Mixtures were aspirated completely and washed three times with 200 μl of 1% bovine serum albumin in LBB (wash solutions were aspirated each time). Primary antibodies to detect ligands were prepared in 3% bovine serum albumin in LBB and incubated for 1 h at room temperature. Antibody solutions were aspirated and washed before adding horseradish peroxidase conjugated secondary antibody (diluted in 3% bovine serum albumin in LBB) and incubating for 30 min at room temperature. After washing as before, sample reactions were developed using 100 μl pre-warmed 1-Step Ultra TMB-ELISA solution (Thermo Scientific) and stopped with 100 μl 2 M sulphuric acid. Absorbance was read at 450 nm, using a BioTek Synergy 4 microplate reader. For each ligand concentration, non-α-DG ligand binding was determined by blocking any LARGE-glycan bound to the plate with IIH6 antibody. The absorbance value from IIH6-blocked samples was subtracted from those of non-blocked samples to determine the specific binding activity of α-DG. Data from triplicate samples were assessed and fitted to a ligand-binding curve using SigmaPlot software. IIH6 ELISAs were completed similarly.
Tissue-specific α-DG ligand binding
Brains (cerebrum), hearts and quadriceps were collected from nine C57BL6/J male mice (9–10 weeks of age) and rinsed with ice-cold PBS. Samples were homogenized by Brinkmann Plytron (cardiac and skeletal muscle) or Wheaton Overhead Stirrer (brain) in 7.5 volumes of 20 mM sodium pyrophosphate, 20 mM sodium phosphate monobasic, 1 mM MgCl2, 0.303 M sucrose, 0.5 M EDTA, pH 7.0. Samples were spun (15 min, 14,000g, 4 °C) and the supernatant was passed through cheesecloth to remove non-homogenized material. Total microsomes were obtained from the supernatant through ultra-centrifugation (37 min, 142,000g, 4 °C) and the microsomes were washed twice with 0.303 M sucrose, 0.6 M KCl, 20 mM Tris-maleate, pH 7.0 (recollecting microsomes by repeating ultra-centrifugation each wash) to remove loosely associated proteins. Total KCl washed microsomes were re-suspended in 0.303 M sucrose, 20 mM Tris-maleate, pH 7.0 and solubilized with 1% Triton X-100, 0.1% SDS. Non-solubilized material was removed by centrifugation (30 min, 30,000g, 4 °C) and the solubilized proteins (supernatant) were enriched for glycoproteins by WGA-agarose as described above. Equivalent loading for solid-phase binding assays was determined by preparing dilution curves and performing a β-DG ELISA assay. Solid-phase assays were performed as described above.
Glycosidase treatment
Treatment with glycosidases was as described previously7, with sialidase (Prozyme) added to the Enzymatic Protein Deglycosylation kit (Sigma-Aldrich) containing: PNGaseF, O-glycosidase, β-N-acetylglucosaminidase, β(1-4)-galactosidase. Glycosidase treatment was carried out on WGA-enriched total cell lysates after the native enzymes were heat inactivated (5 min incubation at 94 °C). Control (non-glycosidase treated) samples were treated identically without adding glycosidases.
AChR aggregation
The AChR aggregation assay was performed on DD4 myotubes differentiated on Matrigel-coated glass cover slides. Twelve hours before fixation, agrin (R&D Systems, 200 pM) and/or laminin 111 (7.5 nM) was added to the differentiation medium, to stimulate AChR aggregation. Samples were incubated for 30 min with Alexa-488-conjugated α-bungarotoxin (Invitrogen, 1:500), washed twice with cytoskeleton stabilizing buffer37 (CSB) and fixed with 2% paraformaldehyde in CSB for 20 min at room temperature. Samples were blocked with 10% FBS, 0.3% Triton X-100 in PBS for 10 min and incubated overnight with IIH6 (1:50) and additional Alexa-488-conjugated α-bungarotoxin (1:1,000). Samples were imaged by confocal microscopy. Maximal intensity projections were obtained from confocal z-stacks and quantified using ImageJ software (version 1.45s).
Transmission electron microscopy
Tibialis anterior muscles were isolated from mice 10 days after CTX injection, after cardiac perfusion with PBS, and were treated with 4% paraformaldehyde in PBS. Samples were fixed in 2.5% glutaraldehyde, 2% paraformaldehyde, 1% tannic acid in 0.1 M Na cacodylate buffer (pH 7.3) for 1 h at 4 °C. They were then washed in 0.1 M Na cacodylate buffer and post-fixed with 1% osmium tetroxide for 1.5 h at 4 °C. After serial alcohol dehydration (50%, 75%, 95% and 100%), the tissue samples were embedded in Epon 12 (Ted Pella). Ultramicrotomy was performed, and ultrathin sections (65 nm) were post-stained with uranyl acetate and lead citrate. Samples were examined and imaged using a JEOL 1230 transmission electron microscope. Regions of interest, which had undergone regeneration due to CTX damage, were identified based on the presence of centrally localized nuclei and/or an accumulation of mitochondria at the sarcolemma.
Antibodies
The following antibodies have been described previously and were obtained from the listed sources: IIH638 monoclonal antibody (Campbell laboratory); monoclonal β-DG antibodies 8D539, MANDA G2 7D1140 (Campbell laboratory); polyclonal anti-laminin (L9393) (Sigma-Aldrich); anti-Flag (Sigma-Aldrich); laminin-2 (α2 chain) 4H8-2 (Enzo Life Sciences); perlecan Ab-1 (NeoMarker); DHPR rabbit polyclonal antibody41 to α2 subunit (Campbell laboratory); affinity purified β-DG rabbit polyclonal AP8342 (Campbell laboratory); embryonic myosin heavy chain developed by H. Blau (F1.653, obtained from the Developmental Studies Hybridoma Bank under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology); core α-DG antibody, RbtG631722 (Campbell laboratory); anti-collagen type VI (70R-CR009x, Fitzgerald Industries International); anti-β-actin (clone AC-74, Sigma-Aldrich); and anti-Flag antibody (F7425, Sigma-Aldrich).
Supplementary Material
Acknowledgements
M.M.G. was supported by a NIH/NIAMS Ruth L. Kirschstein National Research Science Award (F32 AR057289-01) and NIH grant (T32-DK07690-16). The work was supported by American Reinvestment and Recovery Act Grant (1RC2NS069521-01), a Muscular Dystrophy Association Research Grant (157538), and a Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant (1U54NS053672). F.S. and K.M. were supported by an Intramural Research Grant (23-5) for Neurological and Psychiatric Disorders of NCNP (Ministry of Health and Welfare, Japan) and a MEXT Grant-in-Aid for Scientific Research (C 23591256, 24501357, 25430075). We thank P. Yurchenco for his gift of α1LG4-5 producing cells; J. Levy for microscopy expertise; M. B. Zimmerman for assisting with statistical analysis; R. Crawford for technical expertise; H. Nguyen for illustrations; J. Hartner for oversight of LargeKD mouse targeting; J. Shao and R. Nessler of the University of Iowa Central Microscopy Core for their contributions to imaging; and J. Sanes, G. Valdez, D. Glass, C. Blaumueller and Campbell laboratory colleagues for discussions. K.P.C. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contributions M.M.G. co-designed the project, carried out the experimental work, analysed and interpreted the data and co-wrote the manuscript. B.W. conducted experimental work and analysed the data. D.V. generated essential reagents. T.Y.-M. generated LARGE-glycan repeat chains and performed binding assays thereon. F.S. and K.M. generated the CAG-Large transgenic mouse model. SA.M. compiled patient biopsies and edited the manuscript. K.P.C. co-designed the project, co-wrote the manuscript and supervised the research. All authors discussed the data and the manuscript.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
References
- 1.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Inamori K, et al. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 2012;335:93–96. doi: 10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanagawa M, et al. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 2004;117:953–964. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Muntoni F, Torelli S, Wells DJ, Brown SC. Muscular dystrophies due to glycosylation defects: diagnosis and therapeutic strategies. Curr. Opin. Neurol. 2011;24:437–442. doi: 10.1097/WCO.0b013e32834a95e3. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez-Mallebrera C, et al. A comparative study of alpha-dystroglycan glycosylation in dystroglycanopathies suggests that the hypoglycosylation of alpha-dystroglycan does not consistently correlate with clinical severity. Brain Pathol. 2009;19:596–611. doi: 10.1111/j.1750-3639.2008.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanagawa M, et al. Residual laminin-binding activity and enhanced dystroglycan glycosylation by LARGE in novel model mice to dystroglycanopathy. Hum. Mol. Genet. 2009;18:621–631. doi: 10.1093/hmg/ddn387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida-Moriguchi T, et al. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 2010;327:88–92. doi: 10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara Y, et al. Like-acetylglucosaminyltransferase (LARGE)-dependent modification of dystroglycan at Thr-317/319 is required for laminin binding and arenavirus infection. Proc. Natl Acad. Sci. USA. 2011;108:17426–17431. doi: 10.1073/pnas.1114836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michele DE, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 10.Willer T, Valero MC, Tanner W, Cruces J, Strahl S. O-mannosyl glycans: from yeast to novel associations with human disease. Curr. Opin. Struct. Biol. 2003;13:621–630. doi: 10.1016/j.sbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Barresi R, et al. LARGE can functionally bypass α-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nature Med. 2004;10:696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- 12.Sato S, et al. Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nature Neurosci. 2008;11:923–931. doi: 10.1038/nn.2160. [DOI] [PubMed] [Google Scholar]
- 13.Brown SC, et al. Abnormalities in α-dystroglycan expression in MDC1C and LGMD2I muscular dystrophies. Am. J. Pathol. 2004;164:727–737. doi: 10.1016/s0002-9440(10)63160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leschziner A, et al. Neural regulation of α-dystroglycan biosynthesis and glycosylation in skeletal muscle. J. Neurochem. 2000;74:70–80. doi: 10.1046/j.1471-4159.2000.0740070.x. [DOI] [PubMed] [Google Scholar]
- 15.Ibraghimov-Beskrovnaya O, et al. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- 17.Jurado LA, Coloma A, Cruces J. Identification of a human homolog of the Drosophila rotated abdomen gene (POMT1) encoding a putative protein O-mannosyl-transferase, and assignment to human chromosome 9q34.1. Genomics. 1999;58:171–180. doi: 10.1006/geno.1999.5819. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida A, et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev. Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 19.Brockington M, et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin α2 deficiency and abnormal glycosylation of α-dystroglycan. Am. J. Hum. Genet. 2001;69:1198–1209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willer T, Amselgruber W, Deutzmann R, Strahl S. Characterization of POMT2, a novel member of the PMT protein O-mannosyltransferase family specifically localized to the acrosome of mammalian spermatids. Glycobiology. 2002;12:771–783. doi: 10.1093/glycob/cwf086. [DOI] [PubMed] [Google Scholar]
- 21.Longman C, et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of α-dystroglycan. Hum. Mol. Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- 22.Willer T, et al. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nature Genet. 2012;44:575–580. doi: 10.1038/ng.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohn RD, et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–648. doi: 10.1016/s0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- 24.Beedle AM, et al. Mouse fukutin deletion impairs dystroglycan processing and recapitulates muscular dystrophy. J. Clin. Invest. 2012;122:3330–3342. doi: 10.1172/JCI63004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostrominova TY, Tanzer ML. Temporal and spatial appearance of α-dystroglycan in differentiated mouse myoblasts in culture. J. Cell. Biochem. 1995;58:527–534. doi: 10.1002/jcb.240580416. [DOI] [PubMed] [Google Scholar]
- 26.Montanaro F, et al. Laminin and α-dystroglycan mediate acetylcholine receptor aggregation via a MuSK-independent pathway. J. Neurosci. 1998;18:1250–1260. doi: 10.1523/JNEUROSCI.18-04-01250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barresi R, et al. LARGE can functionally bypass α-dystroglycan glycosylation defects in distinct congenital muscular dystrophies. Nature Med. 2004;10:696–703. doi: 10.1038/nm1059. [DOI] [PubMed] [Google Scholar]
- 28.Osawa T, Onodera M, Feng XY, Nozaka Y. Comparison of the thickness of basement membranes in various tissues of the rat. J. Electron Microsc. 2003;52:435–440. doi: 10.1093/jmicro/52.4.435. [DOI] [PubMed] [Google Scholar]
- 29.Buxboim A, Ivanovska IL, Discher DE. Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells ‘feel’ outside and in? J. Cell Sci. 2010;123:297–308. doi: 10.1242/jcs.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satz JS, et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J. Neurosci. 2010;30:14560–14572. doi: 10.1523/JNEUROSCI.3247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerletti M, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groh S, et al. Sarcoglycan complex: implications for metabolic defects in muscular dystrophies. J. Biol. Chem. 2009;284:19178–19182. doi: 10.1074/jbc.C109.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valdez G, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc. Natl Acad. Sci. USA. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breloy I, et al. O-linked N,N′-diacetyllactosamine (LacdiNAc)-modified glycans in extracellular matrix glycoproteins are specifically phosphorylated at subterminal N-acetylglucosamine. J. Biol. Chem. 2012;287:18275–18286. doi: 10.1074/jbc.M111.280297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanagawa M, et al. Disruption of perlecan binding and matrix assembly by post-translational or genetic disruption of dystroglycan function. FEBS Lett. 2005;579:4792–4796. doi: 10.1016/j.febslet.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 36.Harrison D, et al. Crystal structure and cell surface anchorage sites of laminin α1LG4-5. J. Biol. Chem. 2007;282:11573–11581. doi: 10.1074/jbc.M610657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trendelenburg AU, et al. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 38.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 39.Masaki T, et al. Expression of dystroglycan complex in satellite cells of dorsal root ganglia. Acta Neuropathol. 2001;101:174–178. doi: 10.1007/s004010000276. [DOI] [PubMed] [Google Scholar]
- 40.Pereboev AV, Ahmed N, thi Man N, Morris GE. Epitopes in the interacting regions of beta-dystroglycan (PPxY motif) and dystrophin (WW domain) Biochim. Biophys. Acta. 2001;1527:54–60. doi: 10.1016/s0304-4165(01)00147-7. [DOI] [PubMed] [Google Scholar]
- 41.Gurnett CA, Kahl SD, Anderson RD, Campbell KP. Absence of the skeletal muscle sarcolemma chloride channel CIC-1 in myotonic mice. J. Biol. Chem. 1995;270:9035–9038. doi: 10.1074/jbc.270.16.9035. [DOI] [PubMed] [Google Scholar]
- 42.Williamson RA, et al. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum. Mol. Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 43.Hsu NY, et al. Matrix-assisted laser desorption/ionization mass spectrometry of polysaccharides with 2′,4′,6′-trihydroxyacetophen one as matrix. Rapid Commun. Mass Spectrom. 2007;21:2137–2146. doi: 10.1002/rcm.3072. [DOI] [PubMed] [Google Scholar]
- 44.Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J. Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Behrens DT, et al. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J. Biol. Chem. 2012;287:18700–18709. doi: 10.1074/jbc.M111.336073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature. 1995;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- 47.Han R, et al. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of α-dystroglycan. Proc. Natl Acad. Sci. USA. 2009;106:12573–12579. doi: 10.1073/pnas.0906545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.