Abstract
Reovirus is a promising oncolytic virus, acting by both direct and immune-mediated mechanisms, although its potential may be limited by inactivation after systemic delivery. Our study addressed whether systemically delivered reovirus might be shielded from neutralising antibodies by cell carriage and whether virus-loaded blood or hepatic innate immune effector cells become activated to kill colorectal cancer cells metastatic to the liver in human systems. We found that reovirus was directly cytotoxic against tumour cells but not against fresh hepatocytes. Although direct tumour cell killing by neat virus was significantly reduced in the presence of neutralising serum, reovirus was protected when loaded onto peripheral blood mononuclear cells, which may carry virus after intravenous administration in patients. As well as handing off virus for direct oncolytic killing, natural killer (NK) cells within reovirus-treated blood mononuclear cells were stimulated to kill tumour targets, but not normal hepatocytes, in a Type I interferon-dependent manner. Similarly, NK cells within liver mononuclear cells became selectively cytotoxic towards tumour cells when activated by reovirus. Hence, intravenous reovirus may evade neutralisation by serum via binding to circulating mononuclear cells, and this blood cell carriage has the potential to investigate both direct and innate immune-mediated therapy against human colorectal or other cancers metastatic to the liver.
Keywords: reovirus, colorectal liver metastases, NK cells, interferon-dependent
Reovirus in an unmodified, nonpathogenic, ubiquitous double-stranded RNA virus that selectively targets ras-activated tumour cells for replication and killing.1 Although the virus was initially developed as a direct cytotoxic agent, recent evidence has shown that reovirus, in common with other oncolytic viruses (OV), can also exert therapeutic effects via activation of antitumour immunity.2 However, the immune system can be a hindrance as well as a help in the context of virotherapy for cancer because immune inactivation of OV, in particular by neutralising antibodies (NAB) after systemic intravenous administration, may lead to rapid clearance and impairment of therapy.3
Although reovirus can directly activate purified dendritic cells (DC) to stimulate innate immune effects4 and reovirus-infected melanoma cells support priming of antitumour immunity,5–10 the wider immunological limitations and/or therapeutic potential of systemic reovirus delivery as directly relevant to patient treatment have not been addressed. In particular, the effects of reovirus on the mixed blood cell populations the virus will encounter immediately after intravenous injection have not been characterised. These questions are becoming increasingly relevant clinically, as a number of early Phase I/II studies of systemic reovirus have now been completed and a Phase III study has recently opened in patients with head and neck cancer.11,12
OV can be immunogenic because of the presence of the virus acting as a “danger” signal to alert the immune system for antitumour priming. Activation of the innate immune response, in particular, is consistent with clinical data showing that transient pyrexia and flu-like symptoms are common early side effects after intravenous injection of reovirus,13,14 although whether this immediate immunostimulation has antitumour potential remains unknown. To deal with inactivation of OV by circulating NAB, which are present at baseline in almost all patients treated with reovirus and rise after administration, a variety of ex vivo-loaded cell carriers have been shown in murine models to protect OV from NAB for successful delivery to target tumours.13,14 However, although many viruses bind to blood cell components during human infection, the cellular fate of systemically delivered OV—which may impact on viral protection and tumour delivery—remains unclear.
Reovirus targets tumour cells with activating mutations in the ras pathway, a common occurrence in colorectal cancer (CRC).15 Reovirus causes regression of CRC in murine models16 and has shown potential activity in early-phase human studies, as evidenced by falling tumour marker (CEA) levels in CRC patients.17
The purpose of our study was to test the direct and immune-mediated therapeutic potential of intravenous reovirus in CRC metastatic to the liver with regard to: (i) activity against normal as well as malignant cells; (ii) neutralisation by human serum (HS); (iii) viral carriage and protection after systemic delivery and (iv) viral activation of blood and liver innate effector cells for immune-mediated antitumour therapy.
Material and Methods
Cell lines
The human colon adenocarcinoma cell lines LoVo, LS174T, SW480 and SW620 and the murine fibroblastic cell line, L929, were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, Irvine, UK) supplemented with 10% (v/v) foetal calf serum (FCS) (Biosera, Ringmer, UK) and 1% (v/v) L-glutamine (Sigma) (10% DMEM).
Reovirus
Reovirus Type 3 Dearing Strain was provided by Oncolytics Biotech (Calgary, Canada). Stocks were stored at 4°C for up to 1 month or −80°C for longer term storage.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation from healthy donor blood or patient blood before planned, hepatic resection for colorectal liver metastases. Written, informed consent was obtained from all patients in accordance with local institutional ethics review and approval. Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Sigma) supplemented with 7.5% (v/v) human AB serum (Sera Laboratories International, Hayward’s Heath, UK) and 1% (v/v) L-glutamine (7.5% RPMI).
Isolation of colorectal liver metastatic tumour cells, hepatocytes and liver mononuclear cells
Tumour and normal hepatic parenchyma were taken from patients undergoing routine, planned resection of CRC meta-static to the liver. Written, informed consent was obtained from all patients in accordance with local institutional ethics review and approval. Tissue was dissected into 5-mm cubes before disaggregation using a Cell Dissociation Sieve & Tissue Grinder Kit (Sigma). The resulting cell suspension was passed through a 70-μm cell strainer (BD Biosciences, Oxford, UK) and debris removed by two washes in PBS. For isolation of hepatocytes and liver mononuclear cells (LMC), the liver cell suspension was subjected to Ficoll-Hypaque density gradient centrifugation, before LMC were aspirated from the resulting mononuclear cell layer and hepatocytes were aspirated from above the red blood cell pellet. All cells were cultured in 7.5% RPMI.
Flow cytometry and phenotype analyses
Flow cytometry was performed on a FACS calibur and data were analysed using the CellQuest®Pro Software package (v4.0.1) (both Becton Dickinson, Oxford, UK). For phenotype analyses, cells were stained using the antibodies described below (with appropriate isotype controls) for 30 min at 4°C, before being fixed in 1% paraformaldehyde (PFA; Sigma).
Cell lines
JAM-1-PE (Santa Cruz Biotechnologies, Wembley, UK)
Hepatocytes and colorectal liver metastatic tumour cells
JAM-1-PE, BerEp4-FITC (Dako Cytomation, Stockport, UK) and CEA-FITC (BD Pharmingen, Oxford, UK).
Immune cell JAM-1 expression
PBMC were stained with JAM-1-PE and either: CD4-PerCp, CD8-PerCp, CD14-PerCp, CD19-FITC or CD3-PerCp and CD56-FITC (all BD Biosciences, Oxford, UK).
Immune cell reovirus binding
To detect surface loading of reovirus, PBMC were treated with 0, 1 or 10 plaque forming units (pfu) per cell reovirus in 7.5% RPMI for 4 hr and then washed to remove any unbound virus. Cells were stained with anti-reovirus σ3 capsid protein (DSHB, Iowa City, IA), followed by anti-mouse IgG-FITC (BD Pharmingen), then either: CD4-PerCp, CD8-PerCp, CD14-PerCp, CD19-PE (BD Biosciences) or CD3-PerCp and CD56-PE (Serotec, Kidlington, UK).
Natural killer cell phenotype
PBMC or LMC were treated with 0 or 1 pfu per cell reovirus for 12 hr in 7.5% RPMI and then stained with CD3-PerCP and CD56-FITC and either CD69-PE (BD Pharmingen) or CCR7-PE (R&D Systems, Abingdon, UK).
Assessment of cell viability by propidium iodide staining
Adherent and suspension cells were harvested and stained with 0.05 mg/ml propidium iodide (PI; Sigma) for 15 min at room temperature before immediate acquisition on a FACSCaliber.
Assessment of cytotoxicity and viral replication in CRC cell lines after direct infection with reovirus
Adherent LoVo, LS174T, SW480 and SW620 cells were treated with 0, 1 or 10 pfu per cell reovirus for 24–72 hr in 10% DMEM. Where the effect of NABs was to be determined, cells were also cultured in DMEM supplemented with 1 or 2% (v/v) HS and 1% (v/v) L-glutamine. After each time point, cell viability was determined by PI staining (see above). For viral replication, adherent and suspension cells were harvested and samples of cells/supernatants were taken and stored at −80°C, before preparation of lysates by three cycles of freeze/thaw. Viral titre was determined by standard plaque assay using L929 cells. Fold increase in viral titre was determined by comparison with levels of input virus.
Measurement of intracellular active caspase-3
SW480 and SW620 cells were treated with 0 or 10 pfu per cell reovirus for 72 hr. Apoptotic cell death was measured using the PE-conjugated Active Caspase-3 Apoptosis Kit (BD Pharmingen) as per the manufacturer’s instructions and flow cytometry.
Measurement of inhibition of apoptosis
The irreversible pan-caspase inhibitor, Z-VAD-FMK (Calbiochem, Nottingham, UK), was added to adherent SW480 and SW620 cells at a concentration of 50 μM, 1 hr before reovirus infection at 0 or 10 pfu per cell. After 72 hr, cell viability was determined by PI staining (see above).
Assessment of hepatocyte cell viability after direct infection with reovirus
Hepatocytes were treated with 0 or 50 pfu per cell reovirus for 72 hr before the number of viable cells was determined by Trypan Blue (Sigma) exclusion under a light microscope.
Immune cell carriage (hitch-hiking) of reovirus and delivery to tumour cell targets
PBMC were treated with 0 or 1 pfu per cell reovirus for 4 hr before being subjected to three washes in PBS to remove any unbound virus. Cells were added to adherent SW480 and SW620 cells at a 1:1 ratio for 4 hr. PBMC were then removed from the cultures by gentle washing with PBS and fresh medium was added. Separate SW480 and SW620 targets were adhered and directly infected with reovirus at a dose equivalent to that hitch-hiked on PBMC (PBMC retain on average 0.5% of loading dose, as determined by plaque assay—data not shown). After 120 hr, adherent and suspension cells were harvested, samples of cells/supernatants were taken and plaque assays were performed to determine viral titre (described above). In parallel, PI staining was performed to determine percentage of cell death in hitch-hiked or directly infected SW480 and SW620 target cells. In addition, to determine whether cell carriage of virus was only a transient process, PBMC were loaded with reovirus and washed as described above, before being cultured for 12, 24 or 48 hr. After these time points, PBMC were washed again and cocultures with adherent SW480 and SW620 targets/subsequent assessment of target cell viability were carried out as described above. All steps were performed in the presence of 7.5% HS.
51Cr cytotoxicity assay
Cytotoxicity was measured using a standard 4 hr 51Cr release assay as previously described.18 Briefly, PBMC were treated with 0 or 1 pfu per cell reovirus for 12 hr and then washed to remove any unbound virus, before being cocultured with 51Cr (PerkinElmer, Cambridge, UK)-labelled SW480 and SW620 cells at varying effector:target (E:T) ratios for a further 4 hr. In addition, assays were performed in the presence of 2 mM EGTA (a chelating agent that binds calcium, making it unavailable for granule exocytosis). Where indicated, natural killer (NK) cells were depleted from PBMC using CD56 microbeads (Miltenyi-Biotec, Woking, UK), according to the manufacturer’s instructions. All steps were performed in the presence of 7.5% HS. 51Cr release in supernatants was measured and results expressed as % tumour cell lysis, using the formula: % lysis = 100 × (sample cpm – spontaneous cpm)/(maximum cpm – spontaneous cpm).
CD107 degranulation assay
NK cell degranulation in response to tumour cell recognition was measured by surface expression of CD107, as previously described.8 Briefly, PBMC and LMC were treated with 0 or 1 pfu per cell reovirus for 12 hr, before being cocultured at a 1:1 ratio with SW480 and SW620 target cells in the presence of CD107a/b-FITC (both BD Biosciences). Brefeldin A (Sigma) was added after 1 hr at a concentration of 10 μg/ml. All steps were performed in the presence of 7.5% HS. After 4 hr, cells were stained with CD3-PerCp and CD56-PE before being fixed in 1% PFA and acquired using a FACScaliber (background CD107 expression from effectors in the absence of tumour targets was subtracted from analysis).
Cytokine analysis
PBMC were treated with 0 or 1 pfu per cell reovirus for 12 hr in 7.5% RPMI. Secretion of interferon (IFN)-α and −β in supernatants was determined using antibody-matched pairs for IFN-α (MabTech AB, Buro, Germany) or the IFN-β ELISA Kit (PBL Interferon Source, Newmarket Suffolk, UK), as per the manufacturer’s instructions.
Neutralisation of Interferon
PBMC were treated with 0 or 1 pfu per cell reovirus for 12 hr in 7.5% RPMI in the presence or absence of either antibodies to neutralize the effects of IFNs (IFN Block; all purchased from PBL Interferon Source) or isotype control (IFN Isotype). IFN block consisted of sheep polyclonal anti-human IFN-α, sheep polyclonal anti-human IFN-β (both used at 1.5%) and mouse monoclonal anti-human IFN-α/β receptor chain 2 (used at 2.5%), as previously described.8 Isotype control consisted of sheep serum (Sigma) used at 1.5% and mouse IgG2a (R&D Systems) used at 2.5%. PBMC were then washed and used in CD107 degranulation assays, 51Cr cytotoxicity assays or stained for cell-surface expression of CD69, as described above.
Statistical analysis
p-Values were calculated using paired Student’s t-test or two-way ANOVA with Bonferroni post hoc testing. Statistical significance is denoted by *p < 0.05.
Results
Reovirus induces apoptotic cell death in human CRC lines
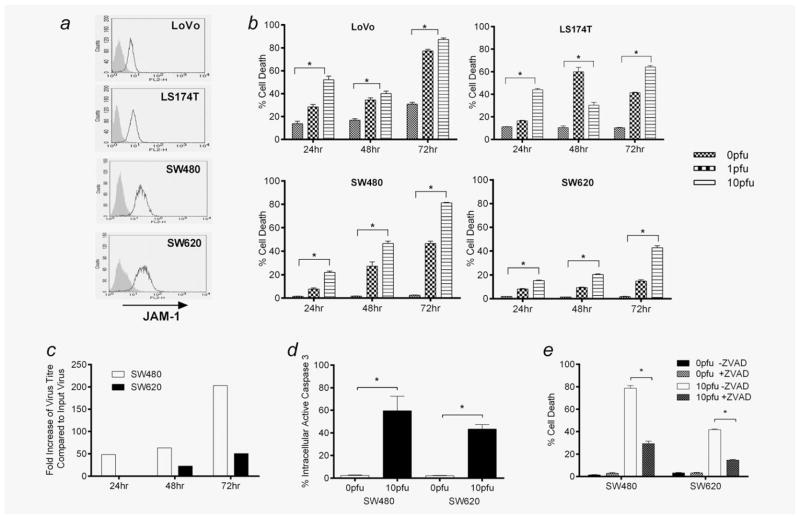
We first tested the direct effects of reovirus on human CRC cells. LoVo, LS174T, SW480 and SW620 were all found to express the reovirus cellular receptor JAM-119 (Fig. 1a) and to be directly killed by reovirus infection, with varying sensitivity (Fig. 1b). We focused further on SW480 and SW620, as these lines were derived from the same patient, from primary tumour and metastases, respectively (both lines are ras mutant20). Both lines supported reovirus replication (Fig. 1c) and levels of cytotoxicity and replication correlated, being greater for SW480 than metastatic SW620. As reovirus can trigger apoptosis as well as cell death because of viral replication/direct lysis,21 we further addressed the mode of killing of SW480/620 after reovirus infection. In both cell lines, there was evidence of apoptosis as measured by cleavage of caspase-3 (Fig. 1d) and inhibition by ZVAD (Fig. 1e), consistent with previous findings in melanoma.5
Figure 1.
Reovirus-induced cytotoxicity in human CRC cell lines. (a) LoVo, LS174T, SW480 and SW620s were assessed for cell-surface expression of JAM-1 using flow cytometry (black line: JAM-1; shaded grey: isotype-matched control). Data are representative of five independent experiments. (b) LoVo, LS174T, SW480 and SW620s were treated with 0, 1 or 10 pfu per cell reovirus for 24–72 hr. Cell viability was assessed by PI staining. Graphs show mean + SEM of five independent experiments. (c) SW480 and SW620s were treated with 1 pfu per cell reovirus. After 24–72 hr, viral replication was determined by plaque assay. Data are representative of five independent experiments. (d) SW480 and SW620s were treated with 0 or 10 pfu per cell reovirus. After 72 hr, production of intracellular active caspase-3 was determined by flow cytometry. Graph shows mean + SEM of five independent experiments. (e) SW480 and SW620s were treated ± 50 μM of Z-VAD-FMK for 1 hr before addition of 0 or 10 pfu per cell reovirus. After 72 hr, cell viability was assessed by PI staining. Graph shows mean + SEM of five independent experiments. Statistical significance is denoted by *p < 0.05.
Reovirus does not kill or replicate in hepatocytes
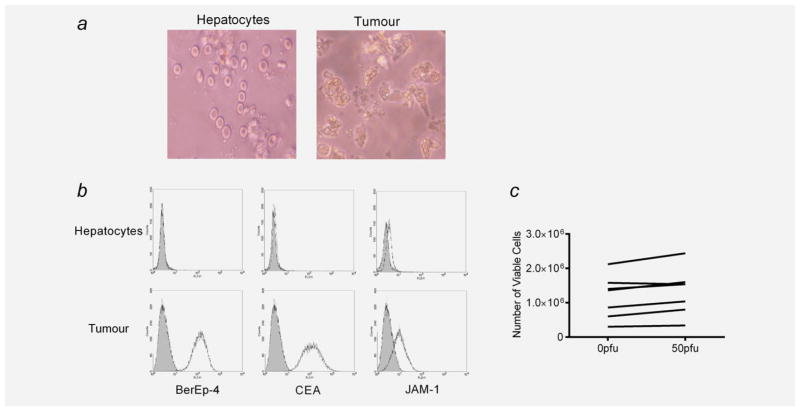
Next, we analysed fresh tissue from patients undergoing resection of CRC liver metastases as part of standard clinical care. Single-cell suspensions of freshly resected tumour cells and hepatocytes (from normal liver excised as a margin around the tumour) were isolated (Fig. 2a) and found to express JAM-1, although levels were consistently higher on tumour cells than on hepatocytes; as expected, only tumour cells expressed the epithelial markers Ber-Ep4 and CEA (Fig. 2b). Although tumour cells survived less than 24 hr in culture, precluding their testing for direct reovirus-mediated cytotoxicity, we were able to maintain freshly resected hepatocytes in vitro for up to 1 week. Importantly, these hepatocytes were not killed by reovirus even at a high dose of 50 pfu per cell (Fig. 2c) and did not support viral replication (data not shown).
Figure 2.
Phenotype of hepatocytes and liver metastatic tumour cells. (a) Single-cell suspensions of hepatocytes and tumour cells were prepared from freshly resected normal liver parenchyma and colorectal liver metastases. Cells were photographed using an Olympus C-7070 camera and light microscope at ×20 magnification. (b) Hepatocytes and tumour cells were examined for cell-surface expression of BerEp4, CEA and JAM-1 using flow cytometry (black line: phenotype marker; shaded grey: isotype-matched control). Plots are representative of five independent experiments. (c) Hepatocytes were treated with 0 or 50 pfu per cell reovirus for 72 hr, and the number of viable cells was determined by Trypan Blue exclusion. Each line represents data from an individual patient.
Human serum blocks reovirus-induced cytotoxicity but does not prevent reovirus binding to blood cells
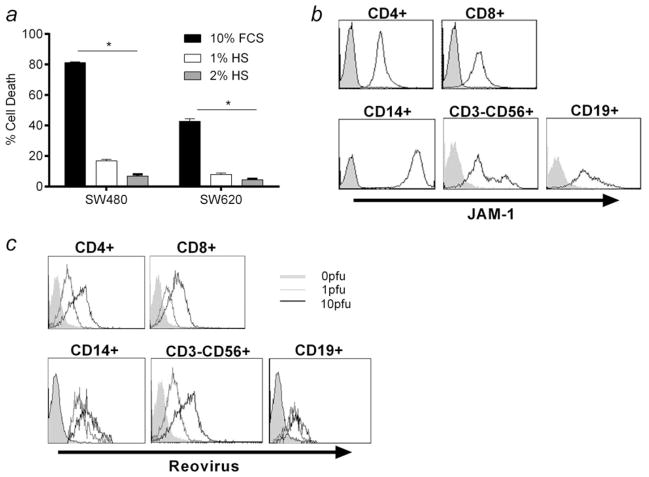
Having shown that reovirus selectively kills CRC tumour cells, we next wished to address the clinical concern that systemically delivered virus may be neutralised by serum, thus preventing access to tumours in patients. Using the SW480 and SW620 cell lines, we found that even low levels of HS (1 and 2%; i.e., much less than the more physiologic level of ~30% HS) did indeed significantly abrogate direct killing by neat reovirus in vitro (Fig. 3a). To address whether components of human blood may be able to carry and protect reovirus from NAB, we first determined whether cells within PBMC express the JAM-1 receptor for potential binding of reovirus. We found that CD4/CD8 T cells, NK cells (CD3-CD56+), B cells (CD19+) and monocytes (CD14+) within PBMC all expressed JAM-1 (Fig. 3b). Moreover, these same populations all stained positive after reovirus pulsing of PBMC in vitro for the reovirus outer capsid σ3 protein, even in the presence of neutralising serum (Fig. 3c). Hence, PBMC are capable of carrying reovirus on the surface of a range of cells despite the presence of NAB although, unlike tumour cells (Fig. 1c), but similar to normal hepatocytes, they do not support viral replication (data not shown). In addition, pulsing PBMC with reovirus did not cause any toxicity to these potential carrier cells (data not shown).
Figure 3.
Human PBMC express JAM-1 and bind reovirus on their cell surface in the presence of neutralising human serum. (a) SW480 and SW620s were treated with 0 or 10 pfu per cell reovirus in the presence of 10% FCS, 1 or 2% HS. After 72 hr, cell viability was assessed by PI staining. Graph shows mean + SEM from three independent experiments. (b) PBMC were isolated from healthy donor blood, and cell-surface expression of JAM-1 within CD4+, CD8+, CD3-CD56+, CD14+ and CD19+ populations was determined by flow cytometry (black line: JAM-1; shaded grey: isotype-matched control). Plots are representative of three independent experiments. (c) PBMC were isolated from healthy donor blood and treated with 0, 1 or 10 pfu per cell reovirus for 4 hr in the presence of 7.5% HS. Cell-surface binding of reovirus σ3 antibody within CD4+, CD8+, CD3-CD56+, CD14+ and CD19+ populations was determined by flow cytometry. Plots are representative of three independent experiments. Statistical significance is denoted by *p < 0.05.
PBMC protect reovirus from neutralising serum to hand off virus to target tumour cells for replication and killing
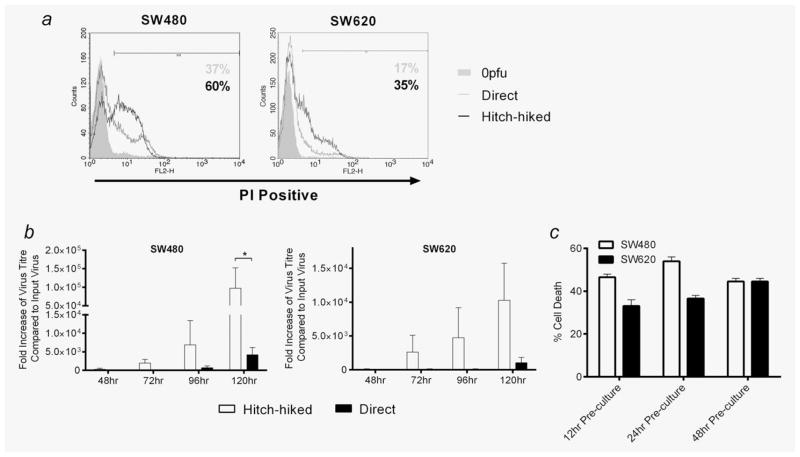
Having demonstrated that reovirus can bind to human PBMC, we next sought to determine whether these clinically relevant potential carrier cells could “hitch-hike” virus to target tumour cells, as previously demonstrated for isolated murine T cells and purified murine/human DC,22–24 even in the presence of NAB. As shown in Figure 4a, coculturing reovirus-loaded PBMC with SW480 or SW620 targets in the presence of neutralising serum led to the death of tumour cells, which was greater than killing by an equivalent dose of direct “neat” virus. Moreover, target tumour cell killing under these neutralising conditions was associated with greater viral replication after hand off from PBMC than with direct virus (Fig. 4b), and hitch-hiking could be demonstrated over time for up to 48 hr after the virus had been loaded onto PBMC and then washed off (Fig. 4c). These data are consistent with a model in which reovirus, carried and retained by PBMC, is protected from NAB for delivery to target CRC to initiate replication and tumour cell death.
Figure 4.
PBMC can hitch-hike and deliver replicating reovirus to SW480 and SW620s in the presence of neutralising human serum. (a) PBMC were isolated from healthy donor blood and treated with 0 or 1 pfu per cell reovirus for 4 hr. Loaded PBMC were cocultured with SW480 or SW620s at a 1:1 ratio for 4 hr. PBMC were then removed from the cultures. Separate SW480 and SW620s were directly infected with an equivalent-to-hitch-hiked dose of reovirus. SW480 and SW620s were incubated for 120 hr before cell viability was assessed by PI staining. Plots are representative of three individual experiments. (b) Duplicate conditions to (a) were set-up and viral replication in samples was determined by plaque assay. Graphs show mean + SEM of three independent experiments. (c) PBMC were treated with 1 pfu per cell reovirus for 4 hr, washed and cultured for 12–48 hr in the absence of any further virus pulsing. After each time point, PBMC were washed again and cocultures with SW480 and SW620s/subsequent assessment of target cell viability were carried out as described in (a). Graphs show mean + SEM of three independent experiments. All steps were performed in the presence of 7.5% HS. Statistical significance is denoted by *p < 0.05.
Reovirus activates NK cells within PBMC to kill CRC targets in the presence of neutralising serum
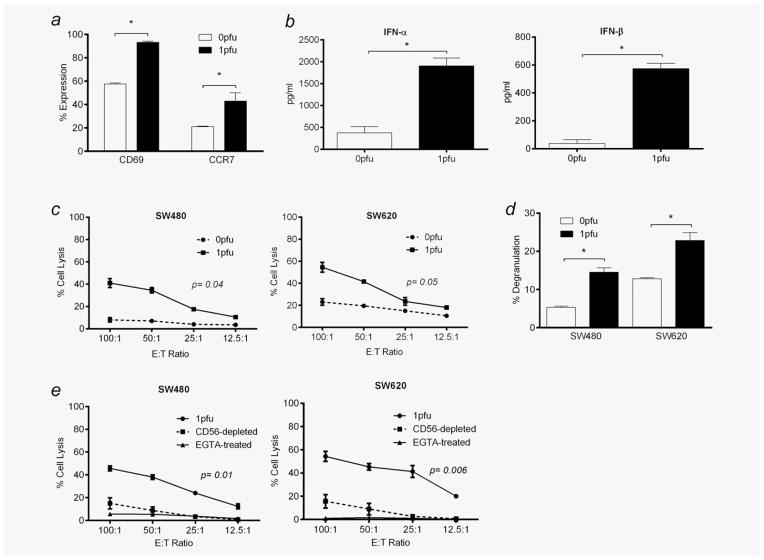
Previous studies have shown that purified human cell populations can acquire innate antitumour immunity after activation by reovirus,6,7,10 although these findings have limited relevance to the virus–blood cell interactions likely to occur after intravenous administration in patients. Nevertheless, early clinical analysis has shown that intravenous reovirus can cause some activation of PBMC constituents in a Phase I study.7 We therefore next investigated the immune-mediated therapeutic potential of the reovirus-carrier PBMC shown to bind and protect reovirus from neutralisation for hand off to tumour cells in Figures 3 and 4. This analysis was performed in the presence of serum without separation of individual PBMC cellular components to mirror the clinical scenario and to allow the essential cross-talk between different types of immune effector cells that is known to be central to immune responses in vivo.25 Figure 5a shows that reovirus activated NK cells within PBMC from normal donors, as shown by upregulation of cell-surface CD69 and CCR7, although expression of other activation markers (DNAM-1, NKp30, NKp44 and NKp46) did not increase (data not shown). Supernatant collected from reovirus-loaded PBMC contained more IFN-α and IFN-β than controls (Fig. 5b), consistent with activation of innate immunity; we also found a trend towards increased MIP-1β and RANTES secretion, although this did not reach statistical significance across all donors (data not shown), whereas TNF-α, MIP-1α, IFN-γ and IL-15 were undetectable and IL-28 was universally low (data not shown). Most importantly, these reovirus-activated PBMC lysed both SW480 and SW620 tumour cell targets to a greater level than untreated PBMC, as measured by chromium release assays (Fig. 5c). The CD3-CD56+ cells within reovirus-pulsed PBMC also expressed the degranulation marker, CD107, on coculture with SW480 and SW620 cells (Fig. 5d), implicating NK cells as the main cytotoxic effectors within PBMC capable of immune-mediated tumour cell killing. This was confirmed by depletion of CD56+ cells within PBMC, which led to significant abrogation of cytotoxicity in chromium release assays (Fig. 5e). To further characterise the mechanism of colorectal tumour cell death, chromium release assays were also performed in the presence of the calcium chelator, EGTA. By rendering calcium unavailable for granule exocytosis, cell lysis of SW480 and SW620 targets was abolished (Fig. 5e), indicating that the mechanism of cell killing was perforin/granzyme-mediated, as expected for NK cell-mediated cytotoxicity. The possibility that this cytotoxic effect of reovirus-loaded PBMC against SW480 and SW620 (Fig. 5c) was due to hand off and direct viral cytotoxicity (as shown in Fig. 4a) was excluded because, over the short time course of this experiment, a high dose of direct reovirus did not kill these targets (data not shown). These data show that PBMC loaded with reovirus in the presence of neutralising serum become activated and lyse tumour cells, potentially providing a further immune-mediated therapeutic mechanism after intravenous virus injection, in addition to cell carriage, hand off and direct viral killing.
Figure 5.
NK cells within healthy donor PBMC are activated by reovirus in the presence of neutralising human serum to become cytotoxic towards SW480 and SW620s. PBMC from healthy donor blood were treated with 0 or 1 pfu per cell reovirus for 12 hr. (a) Cell-surface expression of CD69 and CCR7 within the CD3-CD56+ NK cell population was determined by flow cytometry. (b) Secretion of IFN-α and IFN-β in culture supernatants was determined by ELISA. (c) Cells were cocultured at different E:T ratios with 51Cr-labelled SW480 and SW620s. 51Cr release was quantified and percentage of target cell lysis determined. (d) PBMC were cocultured with SW480 and SW620s, and cell-surface expression of CD107 within the CD3-CD56+ NK cell population was assessed by flow cytometry. (e) Chromium release assays described in (c) were carried out in the presence of the calcium chelator, EGTA, or CD56+ cells were depleted from PBMC prior to the cytotoxicity assays being performed (p-value relates to 1 pfu-treated cells compared to CD56-depleted cells). All experiments were performed in the presence of 7.5% HS. All graphs show mean + SEM from three independent experiments. Statistical significance is denoted by *p < 0.05.
Reovirus activates NK cells within PBMC in a Type I interferon-dependent manner
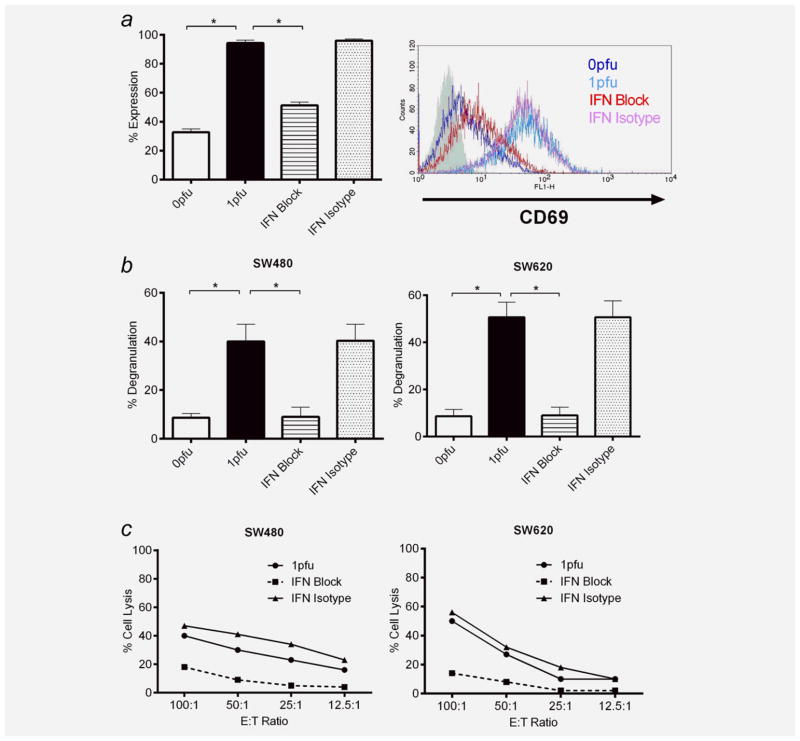
We next sought to address whether the Type I IFN production demonstrated (Fig. 5b) plays a role in the reovirus activation of PBMC for NK-mediated killing of CRC tumour targets (Figs. 5a, 5c and 5d). We therefore repeated the experiments shown in Figure 5 in the presence of NAB against human IFN-α, IFN-β and IFN α/β receptor chain 2. Reovirus-stimulated upregulation of CD69 on the surface of NK cells within PBMC (Fig. 6a) as well as NK degranulation against, and lysis of, SW480/620 tumour targets (Figs. 6b and 6c) were all shown to be inhibited by IFN blockade, confirming Type 1 IFNs as an important mediator of innate antitumour immunity on reovirus activation of PBMC.
Figure 6.
The activation of NK cells within PBMC is Type I interferon-dependent. PBMC from healthy donor blood were treated with 0 or 1 pfu per cell reovirus for 12 hr in the presence or absence of either anti-IFN-α/β neutralising antibodies (IFN block) or isotype control (IFN isotype). (a) Cell-surface expression of CD69 within the CD3-CD56+ NK cell population was determined by flow cytometry. Mean values + SEM from three independent experiments are shown alongside a representative flow cytometry overlay histogram. (b) PBMC were cocultured with SW480 and SW620s, and cell-surface expression of CD107 within the CD3-CD56+ NK cell population was assessed by flow cytometry. Mean values + SEM of three independent experiments are shown. (c) PBMC were cocultured at different E:T ratios with 51Cr-labelled SW480 and SW620s. 51Cr release was quantified and percentage of target cell lysis determined. Graphs are representative of three independent experiments. All experiments were performed in the presence of 7.5% HS. Statistical significance is denoted by *p < 0.05.
Reovirus also activates CRC patients’ PBMC, as well as liver NK cells, to selectively target tumour cells in the presence of neutralising serum
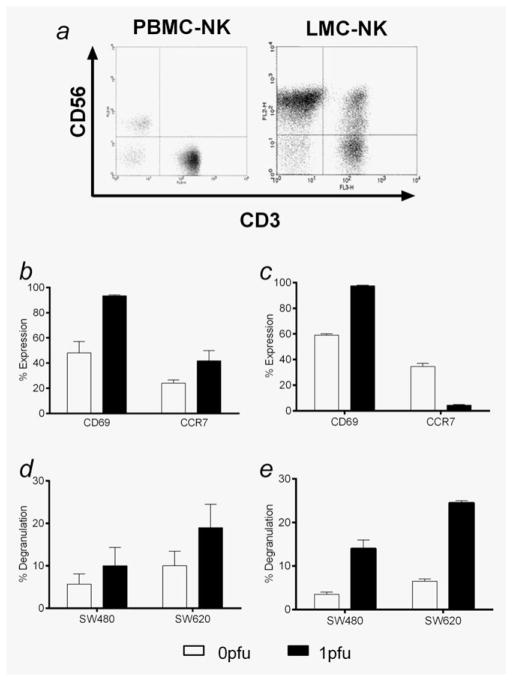
We next wished to test whether the activation of PBMC by reovirus seen in normal donors also applied to cancer patients and whether reovirus could also stimulate corresponding innate effector cells within the liver. Hepatic immune cells may be significant because reovirus is known to access the liver after intravenous administration, as evidenced by the transient transaminitis seen in some patients after treatment. Although this liver injury is mild, transient and has not represented a dose-limiting toxicity,17,26 this clinical observation suggests that systemic reovirus may also stimulate normal liver-resident immune cells to acquire innate antitumour effector function. Therefore, as well as collecting PBMC and hepatocytes from patients undergoing CRC liver metastatectomy, we also isolated LMC.27 Figure 7a shows the characterisation of LMC and PBMC from the same patient into NK (CD3-CD56+), NKT (CD3+CD56+) and T (CD3+CD56−) cells. The relatively higher proportion of NK and NKT cells in LMC, and T cells in PBMC, is consistent with previous studies.28 Patient PBMC-NK were activated by reovirus in the same way as normal donors, with upregulation of both CD69 and CCR7 (Fig. 7b). Although LMC-NK also increased expression of CD69 on addition of reovirus, in marked contrast to PBMC-NK, their CCR7 levels fell (Fig. 7c). This may reflect the different role of LMC-NK as innate cells in situ within the liver, whereas PBMC-NK require migration to lymph nodes to exert their immune effects. However, both reovirus-treated PBMC-NK and LMC-NK degranulated more against tumour cell targets (Figs. 7d and 7e), suggesting that liver NK cells activated by reovirus after systemic delivery, like circulating carrier immune cells, have the potential to target CRC via innate immunity. Reassuringly, no significant degranulation of either PBMC-NK or LMC-NK, with or without prior treatment with reovirus, was seen on coculture with autologous hepatocytes (data not shown), supporting the clinical experience to date that stimulation of innate immunity within the liver, mediated by either carrier cells or in situ effectors, does not lead to significant normal tissue damage.
Figure 7.
NK cells within CRC patient PBMC and LMC are activated by reovirus in the presence of neutralising human serum and degranulate in response to SW480 and SW620s. (a) Autologous PBMC and LMC were isolated from CRC patient blood or normal liver parenchyma, and cell-surface expression of CD3/CD56 was assessed in each population (PBMC-NK and LMC-NK) by flow cytometry. Flow cytometry plots are representative of two independent experiments. (b) Autologous PBMC and (c) LMC were treated with 0 or 1 pfu per cell reovirus for 12 hr. Cell-surface expression of CD69 and CCR7 within each CD3-CD56+ NK cell population was determined by flow cytometry. Graphs show mean + SEM from two independent experiments. (d) Autologous PBMC and (e) LMC were treated with 0 or 1 pfu per cell reovirus for 12 hr. Cell-surface CD107 expression within each CD3-CD56+ NK cell population after coculture with SW480 and SW620s was assessed by flow cytometry. Graphs show mean + SEM from two independent experiments. All experiments were performed in the presence of 7.5% HS.
Discussion
Reovirus and other OV, such as herpes simplex and vaccinia viruses, represent a promising new class of anticancer agents, which have now undergone extensive testing in Phase I and II clinical trials.29 Early results have been encouraging enough for reovirus to progress to Phase III testing in combination with carboplatin and paclitaxel chemotherapy in platinum-resistant head and neck cancer. The high incidence of ras mutations makes CRC another promising target for reovirus and trials of intravenous administration have been initiated.30 Although previous data using purified populations have shown that reovirus therapy can be enhanced by immune-mediated mechanisms as well as direct replication and target tumour cell killing,5,6,8–10 such preclinical studies have not been applied to human CRC or in the context of mixed cell populations such as PBMC, as relevant to clinical systemic delivery.
In our study, we focused on interactions between reovirus, tumour cells and the immune system as specifically relevant to intravenous treatment of CRC metastatic to the liver. We first confirmed that a range of human CRC cell lines expressed the reovirus receptor JAM-1, were sensitive to apoptotic reovirus-mediated killing and supported viral replication (Fig. 1). Despite repeated attempts, we were unable to maintain viable patient liver metastatic CRC cells in vitro long enough to conclusively demonstrate direct killing by reovirus; however, no cytotoxicity against freshly resected normal hepatocytes (which express low levels of JAM-1) was demonstrated, even at a viral load as high as 50 pfu per cell (Figs. 2b and 2c). It is noteworthy that the metastatic cell line, SW620, was less sensitive to direct reovirus-mediated killing and replication than SW480, derived from the primary tumour of the same patient (Fig. 1b). The mechanism(s) responsible for this differential toxicity between primary and secondary tumour cell lines requires further study, because both lines express JAM-1 (Fig. 1a), and both are ras mutant.20
Although reovirus can kill CRC tumour cells (Fig. 1b), there has been significant concern that systemic administration of reovirus will be ineffective because of neutralisation by NAB before the virus can reach its tumour target.3 We confirmed blockade of killing by even low levels of HS added to reovirus-infected CRC cells in vitro (Fig. 3a), an effect of major clinical relevance to intravenous treatment, as reovirus NAB are ubiquitous in the population.31 However, this finding seemed at odds with data from early clinical trials showing that reovirus can access tumour after systemic delivery,17,32 leading us to hypothesise that the virus may be shielded from NAB by cell carriage in the circulation; this theory is supported by recent clinical data showing that patient blood cells, including PBMC, are positive for reovirus by rtPCR shortly after intravenous administration.30 Here, we found that PBMC components expressed JAM-1 and could bind reovirus on their cell surface (Figs. 3c and 3d), but did not support viral replication (data not shown). Significantly, these reovirus-loaded PBMC could “hitch-hike” reovirus to target cells to initiate replication and CRC tumour cell killing, even in the presence of serum (Figs. 4a and 4b). Furthermore, viral hitch-hiking was not a transient process, because PBMC could retain reovirus in the presence of NAB for hand off to tumour cell targets for up to 48 hr after initial loading (Fig. 4c).
Our study also demonstrated that reovirus-loaded PBMC, as well as chaperoning virus to tumour, can exert immune-mediated antitumour effects, as evidenced by their activated status in terms of NK cell phenotype (Fig. 5a), inflammatory cytokine secretion (Fig. 5b) and tumour cell lysis (Fig. 5c). Within PBMC, NK cells were deemed the responsible cytotoxic effectors, as they degranulated on target recognition after pulsing and activation by reovirus (Fig. 5d) and their depletion significantly reduced reovirus-treated PBMC-mediated innate tumour cell killing (Fig. 5e). Moreover, the mechanism of killing was confirmed as perforin/granzyme-mediated, because the addition of the calcium chelator EGTA, which renders NK cells unable to exocytose cytotoxic granules, led to abrogation of tumour cell lysis (Fig. 5e). Interestingly, in both assays used in Figures 5c and 5d, NK-mediated killing of SW620 cells was greater than that of SW480. This was markedly different to their sensitivity to direct viral oncolysis, which was higher in SW480 (Fig. 1b). Although both lines express MHC Class I (a negative regulator for NK-mediated cytotoxicity), its level is lower on SW620 than SW480 (data not shown), consistent with a potential role for Class I in the sensitivity of these lines to NK-mediated lysis. Clinical metastatic CRC expresses less Class I than primary tumours,33 potentially rendering them more NK-sensitive. Hence, Class I-low cells, which are resistant to direct oncolysis, may alternatively be killed by reovirus activation of innate immune effectors. Significantly, we also demonstrated that reovirus-mediated activation of innate antitumour effector function by NK cells within PBMC is Type I IFN-mediated (Fig. 6). Neutralisation of IFN-α and IFN-β from reovirus-treated PBMC greatly reduced activation of CD69 on NK cells within PBMC and abolished their effector functions, because both CD107 degranulation against, and lysis of, CRC tumour targets were lost on IFN blockade. This is consistent with our previous data, where IFN-β played a role in the activation of purified NK cells by DC which had been pulsed with reovirus-infected melanoma.8 Our studies aim to identify which cell populations within whole PBMC (e.g., endogenous plasmacytoid and/or myeloid DC) may be responsible for detecting reovirus and initiating this Type I antiviral response.
In addition to activated innate immune effector cells accessing the tumour as carrier cells from the circulation, there may be virus-sensitive cells within the liver that can be activated in situ by reovirus to stimulate antitumour immune effector function. The potential of this therapeutic mechanism is supported by the clinical data showing mild transient transaminitis in patients treated with intravenous reovirus,17,26 and activation of resident innate immune effector cells may be particularly effective for targeting micrometastatic tumours within the liver which are too small to detect for surgical resection. We found that LMC-NK from patients with metastatic CRC (Fig. 7a), similar to patient and normal donor PBMC-NK, were activated by reovirus to target CRC tumour cells; again metastatic SW620 cells were more susceptible than primary SW480 (Figs. 7d and 7e). Importantly, neither patient reovirus-activated LMC-NK nor PBMC-NK degranulated significantly against autologous hepatocytes (data not shown), suggesting that there may be a useful therapeutic index between OV-mediated innate immune stimulation against tumour and surrounding normal cells within the liver. These data also show that, despite the acknowledged general immunosuppression associated with cancer, both circulating and hepatic innate effectors from these patients with CRC liver metastases can be activated by reovirus to become cytotoxic.
In summary, we have confirmed that CRC is a viable target for reovirus therapy mediated both by direct and innate immune killing. Although serum can neutralise reovirus, our data are consistent with a model in which blood cells can protect and transport reovirus for delivery to target tumour cells after intravenous injection, a concept further supported by recent clinical data.30 These cell carriers, as well as immune cells resident within the liver, can activate in response to reovirus to become antitumour effectors, whilst sparing normal hepatocytes. As well as supporting the further development of reovirus as a systemic treatment for CRC metastatic to the liver, our study suggests that the rapid, detrimental clearance of OV from the circulation, which can restrict therapy in mice, may not inevitably apply in patients treated with intravenous reovirus, where immune cells may act both as protective cell carriers in the circulation and as therapeutic effectors against tumour cells within the liver.
What’s new?
Reovirus can deliver a double whammy to cancer: it hunts down and destroys ras-activated tumor cells, and it can also fire up the immune system against the tumor. The immune system, however, is a fickle ally; in addition to attacking tumor cells, an efficient immune response also rids the body of reovirus, hindering therapy. This study investigated whether therapeutic reovirus could be shielded from immune assault. They showed that loading reovirus onto blood mononuclear cells provides safe transportation to target cells, in this case, colorectal tumor cells, and the reovirus-loaded immune cells themselves also launch an attack against tumor cells. Reovirus appears to be a promising new avenue for cancer treatment.
Acknowledgments
Alan Melcher, Kevin Harrington and Hardev Pandha have previously received commercial research grants from Oncolytics Biotech Inc. This work is supported by a grant from the National Institute of Health (R01CA107082).
Abbreviations
- CRC
colorectal cancer
- DC
dendritic cells
- HS
human serum
- IFN
interferon
- LMC
liver mononuclear cells
- NAB
neutralising antibodies
- NK
natural killer
- OV
oncolytic viruses
- PBMC
peripheral blood mononuclear cells
- PFA
paraformaldehyde
- pfu
plaque forming unit
- PI
propidium iodide
Footnotes
Conflict of Interest: Matt Coffey is an employee of Oncolytics Biotech Inc., with company stock and options.
References
- 1.Yap TA, Brunetto A, Pandha H, et al. Reovirus therapy in cancer: has the orphan virus found a home? Expert Opin Investig Drugs. 2008;17:1925–35. doi: 10.1517/13543780802533401. [DOI] [PubMed] [Google Scholar]
- 2.Prestwich RJ, Harrington KJ, Pandha HS, et al. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–8. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour LW, Cawood R, Fisher KD. Cellular carriers or free viruses? Curr Opin Mol Ther. 2008;10:321–2. [PubMed] [Google Scholar]
- 4.Errington F, Steele L, Prestwich R, et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J Immunol. 2008;180:6018–26. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 5.Errington F, White CL, Twigger KR, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15:1257–70. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prestwich RJ, Errington F, Ilett EJ, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14:7358–66. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White CL, Twigger KR, Vidal L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–20. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 8.Prestwich RJ, Errington F, Steele LP, et al. Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus. J Immunol. 2009;183:4312–21. doi: 10.4049/jimmunol.0901074. [DOI] [PubMed] [Google Scholar]
- 9.Prestwich RJ, Ilett EJ, Errington F, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–81. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steele L, Errington F, Prestwich R, et al. Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is pkr/nf-kappab mediated and supports innate and adaptive anti-tumour immune priming. Mol Cancer. 2011;10:20. doi: 10.1186/1476-4598-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoeckel J, Hay JG. Drug evaluation: reolysin—wild-type reovirus as a cancer therapeutic. Curr Opin Mol Ther. 2006;8:249–60. [PubMed] [Google Scholar]
- 12.Harrington KJ, Vile RG, Melcher A, et al. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–8. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Power AT, Bell JC. Taming the Trojan horse: optimizing dynamic carrier cell/oncolytic virus systems for cancer biotherapy. Gene Ther. 2008;15:772–9. doi: 10.1038/gt.2008.40. [DOI] [PubMed] [Google Scholar]
- 14.Willmon C, Harrington K, Kottke T, et al. Cell carriers for oncolytic viruses: fed ex for cancer therapy. Mol Ther. 2009;17:1667–76. doi: 10.1038/mt.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandan MO, Yang VW. Genetic and chemical models of colorectal cancer in mice. Curr Colorectal Cancer Rep. 2010;6:51–59. doi: 10.1007/s11888-010-0046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirasawa K, Nishikawa SG, Norman KL, et al. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62:1696–701. [PubMed] [Google Scholar]
- 17.Vidal L, Pandha HS, Yap TA, et al. A phase I study of intravenous oncolytic reovirus type 3 dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–37. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 18.Errington F, Jones J, Merrick A, et al. Fusogenic membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and t-cell priming. Gene Ther. 2006;13:138–49. doi: 10.1038/sj.gt.3302609. [DOI] [PubMed] [Google Scholar]
- 19.Barton ES, Forrest JC, Connolly JL, et al. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–51. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt RE, McMarlin A, Kleiner D, et al. Validation of a model of colon cancer progression. J Pathol. 2000;192:446–54. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Clarke P, Tyler KL. Reovirus-induced apoptosis: a minireview. Apoptosis. 2003;8:141–50. doi: 10.1023/a:1022966508671. [DOI] [PubMed] [Google Scholar]
- 22.Qiao J, Kottke T, Willmon C, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive t cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 23.Ilett EJ, Prestwich RJ, Kottke T, et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16:689–99. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilett EJ, Barcena M, Errington-Mais F, et al. Internalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralization. Clin Cancer Res. 2011;17:2767–76. doi: 10.1158/1078-0432.CCR-10-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews DM, Andoniou CE, Scalzo AA, et al. Cross-talk between dendritic cells and natural killer cells in viral infection. Mol Immunol. 2005;42:547–55. doi: 10.1016/j.molimm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 26.Gollamudi R, Ghalib MH, Desai KK, et al. Intravenous administration of reolysin(r), a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2009;28:642–9. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burt BM, Plitas G, Zhao Z, et al. The lytic potential of human liver nk cells is restricted by their limited expression of inhibitory killer Ig-like receptors. J Immunol. 2009;183:1789–96. doi: 10.4049/jimmunol.0900541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Diehl AM. Innate immunity in the liver. Curr Opin Gastroenterol. 2003;19:565–71. doi: 10.1097/00001574-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly OG, Errington-Mais F, Prestwich R, et al. Recent clinical experience with oncolytic viruses. Curr Pharm Biotechnol. 2012;13:1834–41. doi: 10.2174/138920112800958904. [DOI] [PubMed] [Google Scholar]
- 30.Adair RA, Roulstone V, Scott KJ, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selb B, Weber B. A study of human reovirus IgG and IgA antibodies by ELISA and western blot. J Virol Methods. 1994;47:15–25. doi: 10.1016/0166-0934(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 32.Comins C, Spicer J, Protheroe A, et al. Reo-10: a phase 1 study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–72. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Nevot MA, Esteban F, Ferron A, et al. HLA class i gene expression on human primary tumours and autologous metastases: demonstration of selective losses of HLA antigens on colorectal, gastric and laryngeal carcinomas. Br J Cancer. 1989;59:221–6. doi: 10.1038/bjc.1989.45. [DOI] [PMC free article] [PubMed] [Google Scholar]