Abstract
Our pre-clinical and clinical trials using a replication-defective adenoviral vector expressing IFN-β have shown promising results for the treatment of malignant mesothelioma. Based on the hypotheses that a replication-competent Vesicular Stomatitis Virus (VSV) oncolytic vector would transduce more tumor cells in vivo, that co-expression of the immunostimulatory IFN-β gene would enhance the immune-based effector mechanisms associated both with regression of mesotheliomas and with VSV-mediated virotherapy, and that virus-derived IFN-β would add further safety to the VSV platform, we tested the use of IFN-β as a therapeutic transgene expressed from VSV as a novel treatment for mesothelioma. VSV-IFN-β showed significant therapy against AB12 murine mesotheliomas in the context of both local and loco-regional viral delivery. Biologically active IFN-β expressed from VSV added significantly to therapy compared to VSV alone, dependent in part upon host CD8+ T-cell responses. Immune monitoring suggested that these anti-tumor T-cell responses may be due to a generalised T-cell activation rather than the priming of tumor antigen-specific T-cell responses. Finally, IFN-β also added considerable extra safety to the virus by providing protection from off-target viral replication in non-tumor tissues and protected SCID mice from developing lethal neurotoxicity. The enhanced therapeutic index provided by the addition of IFN-β to VSV therefore provides a powerful justification for the development of this virus for future clinical trials.
Keywords: VSV, interferon-β, mesothelioma, oncolytic virotherapy
INTRODUCTION
Malignant mesothelioma is an aggressive neoplasm of the pleura or peritoneum associated with asbestos exposure (1). Due to a lack of effective therapy few patients survive beyond two years from onset (1), highlighting the need for new therapeutic approaches. In this respect, oncolytic viruses are being developed to replicate selectively in tumor cells leading to tumor cell lysis while sparing normal cells. In theory, use of a replication-competent oncolytic virus would require only low levels of seeding in a tumor to initiate spreading infections to cover the tumor comprehensively (2, 3). We have previously shown that Vesicular Stomatitis Virus (VSV), which replicates in the cytoplasm and is highly lytic, is an effective oncolytic virus in various tumor models (4, 5). The potential of VSV as an oncolytic agent was suggested because VSV infection of normal cells induces Type-I IFN responses (IFN-a/β), blocking viral replication and extinguishing infection. However, many tumor cells have defects in their IFN response (6–8) allowing free-ranging infection and lysis (9–11). VSV is indeed a potent oncolytic agent against a variety of both human and murine tumors (5, 9–17). In addition, we have shown that the efficacy of VSV-mediated virotherapy in immune-competent mice is not solely attributable to direct viral replication and tumor cell lysis but is also dependent upon host-derived CD8+ and NK cells (4). The accessibility of malignant pleural mesothelioma makes it a good candidate for oncolytic viral therapy with the need for only loco-regional delivery of the virus (18). Use of an oncolytic virus extends our previous work in which inclusion of the IFN-β gene into a replication-defective adenoviral vector led to significant therapy following intratumoral injections (19, 20). Expression of IFN-β generated tumor regressions and cures, dependent upon its pleiotropic anti-tumor effects including enhancement of innate immune responses, its antiproliferative activities (21) and the priming of T-cell responses (19, 22, 23) and our resulting clinical trials have generated promising results (24).
Taken together, these data (19, 20, 24, 25) suggest expression of IFN-β (21) will further enhance the anti-tumor immunotherapeutic effects of the replication-competent oncolytic VSV. Moreover, inclusion of IFN-β within the VSV vector should also increase the safety of VSV in the event that it were to infect normal cells (21). Thus, since normal cells have intact responses to IFN-α/β (unlike many tumor cells) which shut down VSV replication rapidly both in vitro and in vivo, by adding further levels of expression of IFN-β from the virus, we predicted the toxicity of VSV-IFN-β would be further diminished without major impact upon the efficacy against tumor. This is an important issue given the findings that at high doses VSV infection can lead to lethal neurotoxicity in some mouse models (especially immune-deficient mice) (10, 21).
Here, we tested IFN-β as a therapeutic transgene expressed from VSV as a novel treatment for mesothelioma, based upon the potential of VSV-IFN-β to increase immunotherapeutic efficacy, enhance tumor cell transduction and increase the safety of previous approaches using VSV alone. We demonstrate VSV-IFN-β efficiently lyses the murine mesothelioma AB12 cell line, and that added expression of IFN-β does not significantly interfere with VSV replication. Loco-regional delivery of VSV-IFN-β generated tumor regressions, at least in part dependent upon virus-induced activation of CD8+ T-cells. Finally, we confirmed that IFN-β expressed from VSV added considerable extra safety to the virus and protected SCID mice from lethal neurotoxicity. These data support the development of the VSV-IFN-β vector for use in patients with malignant mesothelioma.
Materials and Methods
Cell lines
The human mesothelioma line, MSTO-211H, was purchased from the American Tissue Type Collection. The murine mesothelioma cell line, AB12, was provided by Dr. Bruce Robinson (Univ. of Western Australia) (26). B16ova cells, a murine melanoma cell line, were derived from the parental cell by transduction of cDNA encoding chicken ovalbumin gene (27).
Viral strains
The VSV-mIFN-β and VSV-hIFN-β vectors were originally described by Obuchi et al (21). VSV-GFP was generated as previously described (4). Viral stocks were manufactured by the Core Viral Facility of Mayo Clinic. VSV-GFP is referred to as VSV.
MTT assays
MTT assays were performed with VSV-mIFN-β and VSV-GFP. Cells were plated in quadruplicate on 96-well plates and infected with different MOIs. Cell viability was assessed at the indicated time points per manufacture’s instruction (Promega).
ELISpot analysis for IFN-γ secretion
Spleens or tumor-draining lymph nodes were harvested from mice at the indicated times. For ELISpot assays, 1×105 cells were plated in triplicate on a 96-well plate and restimulated for 48h at 37°C under different conditions (all peptides were at 5µg/mL). Peptide-specific IFN-γ–positive spots were detected per manufacturer's protocol (Mabtech Inc.) and quantified by a computer-assisted image analyzer.
The following synthetic peptides were synthesized at the Mayo Foundation Core Facility: EGSRNQDWL, mouse gp100; SIINFEKL, chicken OVA; SVYDFFVWL, mouse TRP-2; and RGYVYQGL, VSV-N protein.
In vivo studies
All procedures were approved by the Mayo Foundation or the University of Pennsylvania Animal Care and Use Committee in compliance with the Guide for the Care and Use of Laboratory Animals. To establish s.c. tumors, 5×105 B16ova cells, 1×106 AB12 cells, or 1×106 MSTO-211H cells in 100µL of PBS were injected into the flank of either the C57Bl/6, BALB/c, or SCID mice, respectively. Once s.c. tumors reached approximately 200mm3 in size, intratumoral injections were performed with saline, 5×108 pfu (C57Bl/6) or 6.6×108 pfu in 100µL of each vector (VSV-mIFN-β and VSV-hIFN-β) once weekly for two (SCID, C57Bl/6) or three consecutive weeks (BALB/c). Tumors were measured twice a week and mice were euthanized if toxicity was evident or tumor burden exceeded 1500mm3. To establish intraperitoneal tumors, 3.5×105 cells were injected i.p. On day 4, growth of the i.p. tumors was confirmed and injections were performed with saline or 6.6×108 pfu in 100µL of each virus (VSV-mIFN-β and VSV-hIFN-β).
In vivo depletion of CD8+ T-cells
BALB/c mice received i.p. injections of 200µg of purified monoclonal antibodies purified from the anti-CD8+ hybridoma 53-6.7 (ATCC). Injections were administered 3 days and 1 day prior to inoculation with AB12 cells. Thereafter, a maintenance dose of antibody was injected i.p. every 7 days throughout the entire experimental period to ensure depletion. CD8+ T-cell depletion was confirmed by flow cytometry of splenic suspensions at the time of tumor injection and weekly afterward. On days 11 and 18 mice received 6.6×108 pfu VSV-mIFN-β or PBS. Tumors were measured twice a week and mice were euthanized if toxicity was evident or tumor burden exceeded 1500mm3.
Flow cytometry and INF-γ intracellular staining assay
For analysis of phenotype, 1×106 cells were washed in PBS containing 0.1% BSA (wash buffer), resuspended in 50µL of wash buffer, and exposed to conjugated primary antibodies for 30min at 4°C. The OVA-iTag-H-2Kb-SIINFEKL-PE conjugated tetramer was used per manufacturer’s protocol (Beckman Coulter). Cells were washed and resuspended in 500µL PBS containing 4% formaldehyde, analyzed by flow cytometry and data were analyzed using FlowJo software. For intracellular staining, single-cell suspensions were prepared from tumors harvested (3 mice/group) at the indicated times. IFN-γ production in response to antigen was measured by incubation with peptides (5µg/mL) in the presence of Golgi Plug for 4h. Cells were stained, fixed, and permeabilized for intracellular staining using a Cytofix/Cytoperm kit (BD Biosciences) per manufacturer's instructions.
Statistical Analyses
For comparison of two individual data points, two-sided Student’s t-test was applied to determine statistical significance. ANOVA with post-hoc testing was used for groups of three or more. Survival curves were plotted according the Kaplan-Meier method, and statistical significance in the different treatment groups was compared using the log-rank test.
RESULTS
VSV-mIFN-β is potently cytotoxic to mesothelioma cells in vitro
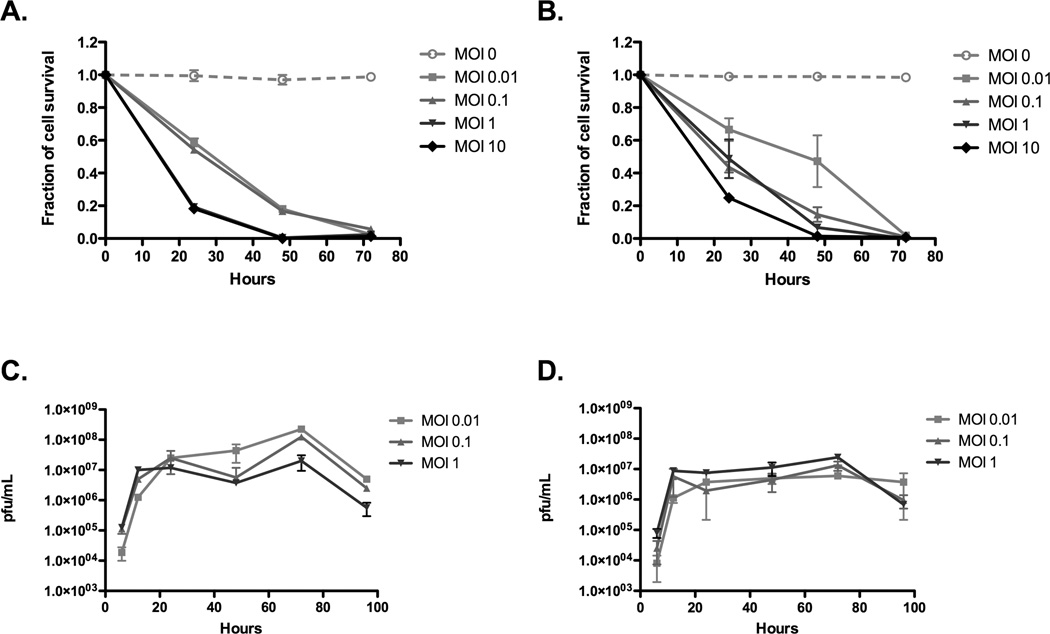
Both VSV (Fig.1A) and VSV-mIFN-β (Fig.1B) induced rapid, and extensive, cell killing following infection of AB12 cells at MOI ranging from 0.01 to 10, associated with ongoing replication of the viruses (Fig.1C&D). We did observe a moderate, but significant, reduction in the rate of viral replication of VSV-mIFN-β compared to VSV at the lowest MOI of infection (Fig.1C&D), additionally reflected in a slightly slower rate of tumor cell killing (Fig.1A&B), suggesting AB12 cells may retain a slight degree of responsiveness to IFN-β-mediated inhibition of viral replication. By 72hr post infection, however, more than 99% of AB12 cells had been eradicated by both viruses (Fig.1A&B).
Figure 1. VSV-IFN-β is potently cytotoxic to mesothelioma cells in vitro.
AB12 cells plated in 96-well plates were infected with various multiplicities of infection with either (A) VSV or (B) VSV-mIFN-β. At specific time points an MTT assay was performed per manufacturer’s instructions. Data are plotted as a fraction of control cell survival with SD. AB12 cells were infected with (C) VSV or (D) VSV-mIFN-β for 1hr at 37°C at indicated MOIs. After incubation, cells were washed and at indicated times points samples were taken for a plaque-forming unit assay.
VSV-IFN-β has anti-tumor activity against established tumors
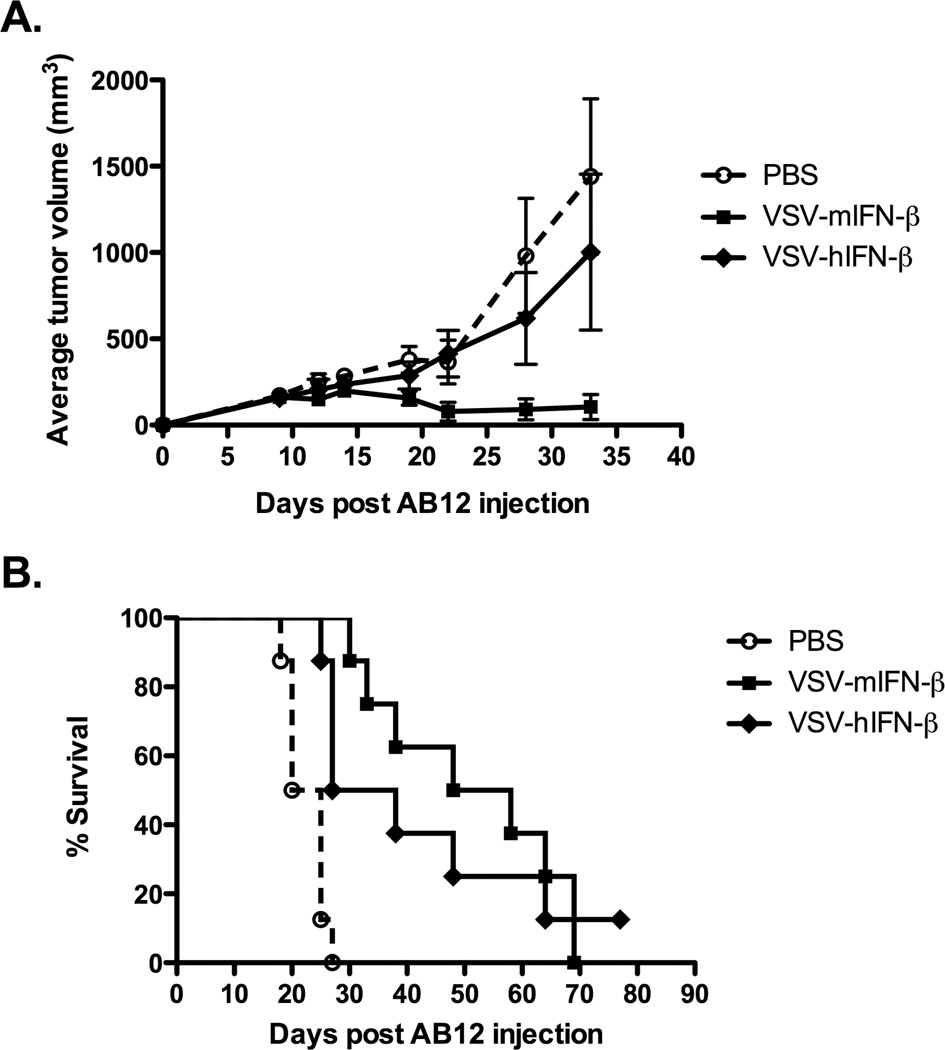
Consistent with the in vitro results of Fig.1, direct injection of VSV-mIFN-β into established subcutaneous AB12 tumors in immune-competent mice also generated significant anti-tumor activity compared to controls (p<0.01 at day 32) (Fig.2A). This reduction in the rate of tumor growth translated into significantly increased survival in VSV-mIFN-β-treated mice with 4/8 mice cured of their tumors (Fig.2A), compared to 0/8 in the PBS-treated group. In addition, all of these long-term survivors rejected a later challenge of live AB12 tumor cells. We predict the anti-tumor immunity observed in Fig.2A is specific against AB12 cells, although we did not have sufficient numbers of survivors to re-challenge these tumor-cured animals with a different, non-mesothelioma tumor cell line.
Figure 2. In vivo therapy is dependent upon biological activity of IFN-β.
Groups (n=8) of BALB/c mice bearing either (A) subcutaneous or (B) intraperitoneal AB12 tumors were treated with saline, VSV-mIFN-β or VSV-hIFN-β at a dose of 6.6×108 pfu, once a week for three weeks. Administration of VSV-mIFN-β led to significant tumor regressions in the subcutaneous model (p<0.01) in comparison to the saline group, while VSV-h-IFN-β does not show any significant therapy. However, both VSV-mIFN-β and VSV-hIFN-β significantly prolong survival in the intraperitoneal model over saline (p<0.0001 and 0.01, respectively).
VSV-mIFN-β was also therapeutically effective in a model of loco-regional virus delivery to treat intraperitoneally seeded AB12 tumors (Fig.2B). Unlike the subcutaneous model, there were no long-term cures. However, survival of tumor-bearing mice treated with VSV-IFN-β (median survival 54 days) was extended significantly over control-treated animals (median survival 26 days) (p<0.01).
Taken together, these data demonstrate VSV-mIFN-β is an effective agent against AB12 mesothelioma tumors both in vitro and in vivo in the contexts of both local and loco-regional delivery.
In vivo therapy is dependent upon biological activity of IFN-β
Based upon our previous studies using a replication-defective adenovirus expressing IFN-β (19, 20, 24, 25), our hypothesis was addition of the IFN-β gene to the replication-competent VSV would further enhance the immunostimulatory activity of virus-mediated tumor cell killing. To test this hypothesis, we constructed a VSV expressing the human IFN-β gene, which is not active in the mouse. Therefore, the efficacy of VSV-mIFN-β and VSV-hIFN-β in immune-competent mice would provide a closely matched comparison between immune-based components of anti-tumor therapy and the effects of direct VSV-mediated oncolysis. We confirmed both the VSV-mIFN-β and VSV-hIFN-β viruses have very similar profiles of replication in, and cytotoxicity to, AB12 cells in vitro (not shown). As before, direct intratumoral injection of VSV-mIFN-β into s.c. AB12 tumors significantly improved anti-tumor therapy compared to PBS alone (Fig.2A) (p<0.01). In contrast, intratumoral injection of VSV-hIFN-β gave no significant therapy over PBS (Fig.2A) (p=0.27), suggesting a major component of the therapy in vivo associated with VSV-IFN-β is contributed by immune reactivity of local IFN-β expression as opposed to direct oncolysis by the virus.
We also observed a trend towards increased efficacy of VSV-mIFN-β compared to VSV-hIFN-β in the model of loco-regional delivery of virus to intraperitoneal tumors, although this difference did not reach statistical significance (p=0.54) (Fig.2B). Both VSV-hIFN-β and VSV-mIFN-β were, however, significantly more therapeutic than PBS (p<0.01 and <0.0001, respectively).
VSV-mediated expression of IFN-β enhances therapy through CD8+ T-cells
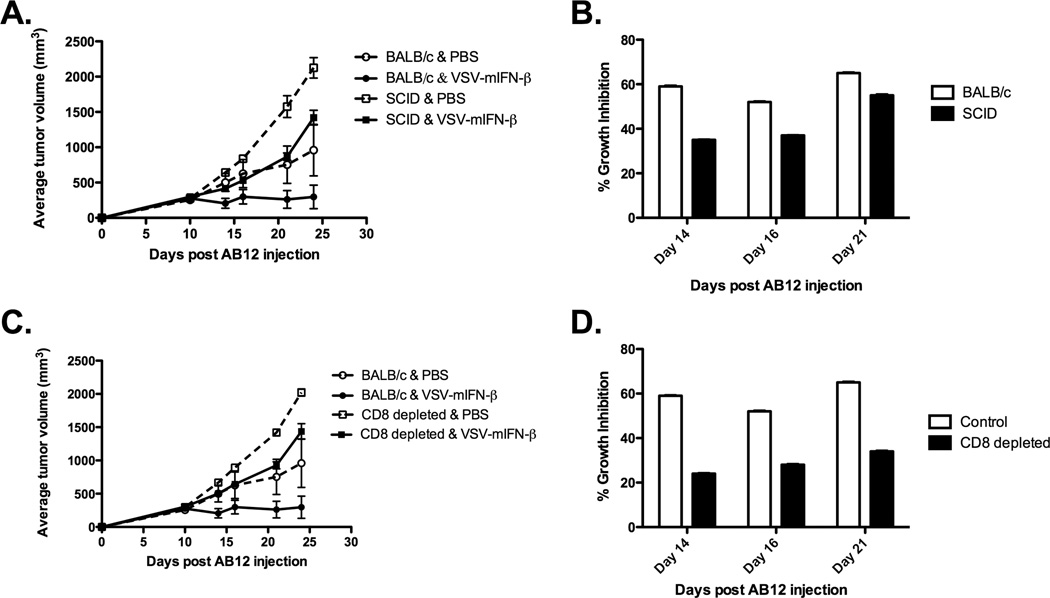
To further define the immune-mediated mechanisms by which IFN-β enhances the therapy associated with intratumoral VSV injection, the experiments of Fig.2 were repeated in SCID mice. Intratumoral injection of VSV-IFN-β significantly reduced the rate of tumor growth in SCID mice compared to control injections (p<0.001) (Fig.3A). However, AB12 tumors develop at a faster rate in SCID mice than in BALB/c mice. Therefore, by measuring the percentage of growth inhibition (GI) between control- and VSV-IFN-β-treated groups, we observed there was a significant difference in the potency of VSV-IFN-β between immune-competent and immune-deficient strains (p<0.0001 for all days) (Fig.3B). Thus, on day 14, post AB12 injection, whereas SCID mice showed a GI of approximately 35% of tumor growth, immunocompetent BALB/c mice had a GI of 60% of tumor growth. Therefore, the absence of T- and/or B-cells in SCID mice was responsible for a loss of approximately 50% of the therapy associated with intratumoral injection of VSV-IFN-β in the AB12 model from 14 days onwards.
Figure 3. VSV-mediated expression of IFN-β enhances therapy through the activity of CD8+ T-cells.
Groups (n=8) of BALB/c (A) SCID or (B) CD8+ T-cell-depleted mice bearing subcutaneous tumors were treated with saline or VSV-mIFN-β (6.6×108 pfu), once a week for two weeks. Because AB12 tumors develop at a faster rate in the SCID and CD8-depleted mice than in the BALB/c mice a percentage of growth inhibition between control- and VSV-IFN-β-treated groups was calculated as: ((Vcontrol–Vvsv-mIFN-β)/Vcontrol)×100%.
Consistent with the results of Fig.3A&B, tumors grown in mice depleted of CD8+ T-cells, and treated with VSV-IFN-β, were approximately 25% smaller than control-treated tumors at day 14 compared to a 60% GI of tumors treated identically in immune-competent mice (p<0.0001) (Fig.3C&D). These data suggest about half of the therapy induced by intratumoral injection of VSV-IFN-β is dependent upon an intact CD8+ T-cell compartment.
Expression of IFN-β does not prime detectable tumor antigen-specific T-cell responses
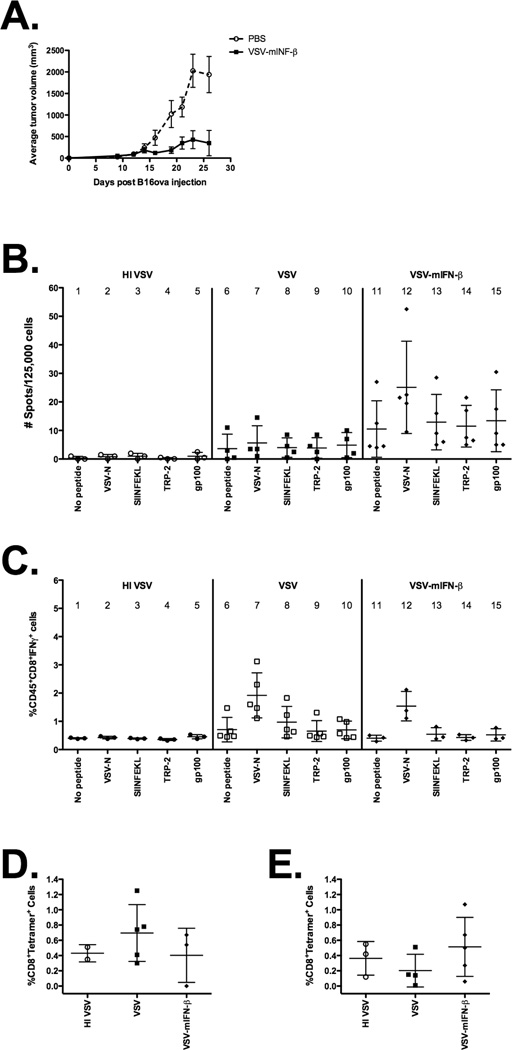
Given this T-cell dependence, it was of interest to further study this INF–β-induced immune response. Since the AB12 cell line has no known specific tumor antigens, we conducted further mechanistic studies using a more well-immunologically characterized cell line (B16ova) that additionally expressed a neo-antigen, chicken ovalbumin, that allowed the use of tetramers. We have previously analyzed the immune responses of this model to wild-type VSV treatment (4). We first showed B16ova tumors responded to VSV-mIFNβ in a similar fashion as AB12 cells (Fig.4A). Next, we assayed splenocytes of VSV- or VSV-IFN-β-treated mice for the magnitude, and specificity of responses against viral or tumor-associated antigens. In order to track antigen responses, we took advantage of the fact that C57Bl/6 mice bearing B16ova tumors generate T-cell specific responses against the well characterised SIINFEKL epitope of the model OVA tumor-associated antigen which can readily be monitored.
Figure 4. Expression of IFN-β does not prime detectable tumor antigen-specific T-cell responses.
(A) Groups (n=8) of C57Bl/6 mice bearing subcutaneous tumors were treated with saline or VSV-mIFN-β (5×108 pfu) on days 8 and 12 post tumor inoculation. Administration of VSV-mIFN-β led to significant tumor regressions in the subcutaneous model (p<0.05) in comparison to the saline group. (B–E) Groups of C57Bl/6 mice (n=4) bearing subcutaneous B16ova tumors were intratumorally treated twice with heat-inactivated VSV, VSV or VSV-mIFN-β at 5×108 pfu. Mice were sacrificed 7 days after the final injection and organs were harvested. (B) Lymph nodes were pulsed either with no peptide, ova, VSV-N, TRP-2 or gp100 peptides and assayed for IFN-γ-producing cells by ELISpot. Data are plotted as an average of duplicates or triplicates from each individual mouse±SD. (C) Tumor cells were incubated for 4h in the presence of Golgi Plug with or without indicated peptides and analyzed by flow cytometry for intracellular IFN-γ. Additionally, (D) tumor and (E) splenocytes cells were analyzed by flow cytometry for H2-Kb-restricted SIINFEKL-tetramer-positive CD8+ T-cells.
Intratumoral injection of VSV into B16ova tumors appeared to prime increased numbers of T-cells specific for the immunodominant epitope of the viral N protein, compared to mice in which tumors were injected with control heat-inactivated VSV (Fig.4B, column 2 vs 7), although there is no statistically significant increase in the N-specific T-cell response compared to the control in mice injected with VSV (Fig.4B, column 6 vs 7). Co-expression of IFN-β from the injected VSV generally enhanced the frequencies of T-cells specific for the viral N protein compared to mice treated with VSV alone (Fig.4B, column 12). However, we also consistently observed splenocytes from mice treated with either VSV or VSV-mIFN-β exhibited a generalised, non-antigen-specific activation, as evidenced by the elevated frequencies of IFN-β-producing spots generated in response to stimulation with no added peptide (Fig.4B, column 11). Relative to the frequencies of antigen non-specific T-cell responses (no stimulating peptide), we did not observe a significant increase in the frequency of OVA-(SIINFEKL)-specific T-cells in the spleens or LN of mice treated with VSV-IFN-β (Fig.4B, column 13). Moreover, frequencies of OVA-specific T-cells were not significantly different in mice whose tumors were treated with VSV-IFN-β compared to those treated with VSV, although between experiments there was a trend towards increased levels of SIINFEKL-specific T-cells (Fig.4B, column 8 vs 13).
Consistent with these data, although intratumoral injection of VSV significantly enhanced the magnitude of the anti-viral T-cell response seen within tumors (Fig.4C, column 6 vs 7), there was no significant increase in the T-cell response to either the non-self OVA-specific, or the self TRP-2- or gp100-specific, tumor-associated antigens (Fig.4C, column 6 vs 8–10). Co-expression of IFN-β from the virus neither enhanced the anti-viral T-cell response (relative to treatment with VSV) nor did it enhance the anti-OVA response within the tumor (Fig.4C, column 8 vs 13). Similarly, although we observed a trend towards increased number of tetramer-positive OVA-specific T-cells infiltrating tumors treated with VSV compared to control-treated animals, this was not significant (Fig.4D). Addition of IFN-β expression from the virus did not increase the frequency of tumor antigen-associated T-cell responses in either the tumor or spleens of treated mice compared to either control- or VSV-treated mice (Figs.4D&E). Taken together, these data indicate VSV intratumoral injection primes viral-specific T-cell responses, which can be enhanced in their frequency by the co-expression of IFN-β from the virus. In addition, VSV intratumoral injection is associated with enhanced levels of T-cell activation but through a non-antigen-specific mechanism. Finally, we could not demonstrate the priming of T-cell responses specifically against either the endogenous TRP-2 or gp100 tumor antigens, nor the artificial non-self OVA tumor antigen, at levels higher than controls.
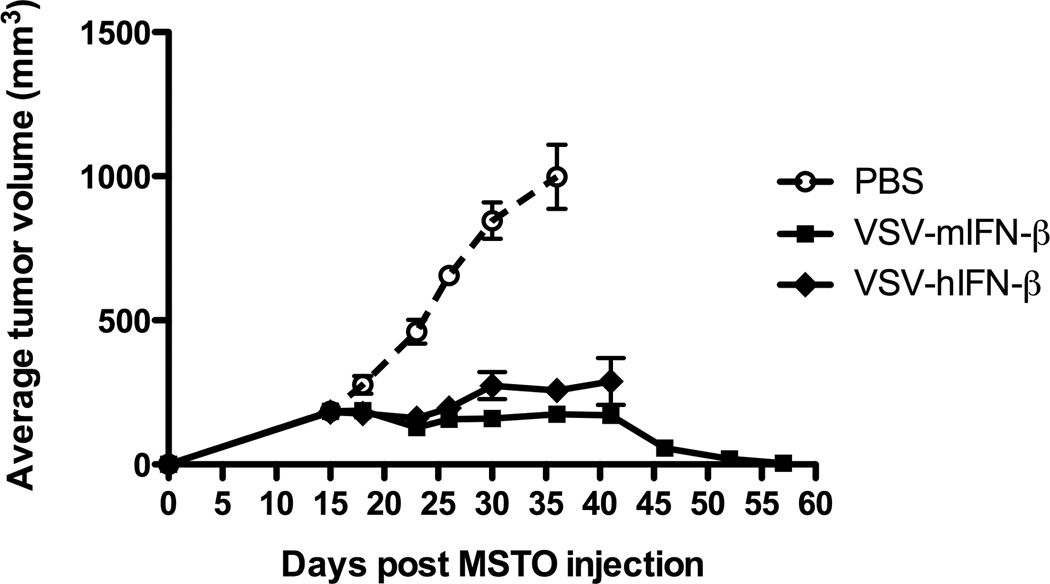
IFN-β provides enhanced safety against VSV-induced neurotoxicity
A principal rationale for the inclusion of the IFN-β gene within the VSV platform was to increase the safety of this virus should it become disseminated within a patient and infect normal cells – in which case the expression of IFN-β would enhance the anti-viral response of normal cells and extinguish extraneous viral replication. The potential toxicities of VSV, including neurovirulence, are most potent when the virus is administered to immune-deficient mice. For this reason, human MSTO-211H mesothelioma tumors were grown in SCID mice and treated with either the VSV-hIFN-β (in which the human IFN-β confers no protection against viral replication in a mouse) or with VSV-mIFN-β (in which the IFN-β will be active in the host mouse at protecting normal tissues from viral replication). Significantly, all tumor-bearing mice treated with VSV-hIFN-β showed significant tumor regressions compared to mice with control-treated tumors (Fig.5), but these mice also exhibited unacceptable neurotoxicity by day 40 necessitating termination of the experiment in that group. In contrast, treatment of tumors with VSV-mIFN-β led to similar levels of tumor growth inhibition by day 40 following tumor seeding (Fig.5) but was not associated with any overt viral-associated toxicities. This lack of toxicity enabled the experiment to be continued, under which circumstances tumor regressions continued and long-term survivors were generated (Fig.5). These results confirm inclusion of a biologically active IFN-β in the VSV platform adds a significant safety benefit of protection against VSV-associated neurological toxicity and allows for increased efficacy to be manifested through protecting the host from adverse side effects.
Figure 5. IFN-β provides enhanced safety against VSV-induced neurotoxicity.
Groups (n=8) of SCID mice bearing subcutaneous MSTO-211H tumors were intratumorally treated with saline, VSV-mIFN-β or VSV-hIFN-β at a dose of 6.6×108 pfu, once weekly for two weeks. Administration of both viruses significantly reduces tumor growth in the mice compared to control (p<0.0001 for both). However, mice treated with VSV-hIFN-β displayed neurotoxicity and were sacrificed around day 40.
DISCUSSION
Based upon the hypothesis that expression of IFN-β would enhance the immunotherapeutic efficacy of VSV while decreasing its systemic toxicity, we tested a replication-competent oncolytic VSV expressing IFN-β in a murine model of mesothelioma. We show here AB12 cells are highly sensitive to both VSV and its IFN-β-expressing derivative in vitro (Fig.1). This sensitivity to VSV-mIFN-β is also reproduced in vivo following both intratumoral, as well loco-regional, delivery (Fig.2). Consistent with our original rationale, a significant proportion of the in vivo therapy is contributed by IFN-β-dependent immune-based mechanisms as shown by comparing VSV-mIFN-β with a closely matched VSV-hIFN-β virus in which the IFN-β is not biologically active in the murine host (Fig.2). This immune component was most marked in the subcutaneous as opposed to the i.p. model of tumor growth (Fig.3A&B). We believe this is probably because tumors growing in the s.c and i.p sites may differ significantly in the access, and activation, of the immune effectors we believe are critical to these anti-tumor mechanisms, such as macrophages, NK (4) cells, and, as shown in Fig.3, CD8+ T-cells. However, further studies will be required to confirm this hypothesis.
We showed previously that inclusion of the IFN-β gene into a replication-defective adenoviral vector led to significant therapy following intratumoral injections through priming of immune-mediated anti-tumor responses (19, 20, 24, 25). Therefore, we hypothesized that addition of IFN-β may lead to similar enhancements in the immunotherapeutic effects of oncolytic VSV. To monitor/track potential tumor antigen-specific responses, we used the B16ova model in which immunological assays are available to both the model non-self OVA antigen, as well as to several endogenous self-melanoma-associated antigens. To justify the similarities between the mesothelioma and melanoma models, we have shown VSV-mIFN-β provides effective therapy against B16ova and that addition of IFN-β to VSV has similar effects as seen in the AB12 model (Fig.4A). Significantly, however, our results indicate that addition of IFN-β to oncolytic VSV did not enhance priming of tumor antigen-specific T-cell responses (Fig.4). While VSV injection into tumors primed specific anti-viral T-cell responses (Fig.4), we were unable to detect antigen-specific responses against either OVA, or the endogenous TRP-2 or gp100 tumor antigens. Mice injected intratumorally with VSV did develop generally elevated levels of T-cell activity, although these were not specific for any stimulating peptide other than virally-derived epitopes (Fig.4B–E and data not shown). Nonetheless, depletion studies showed these apparently antigen non-specific CD8+ T-cell responses are important for therapy. This may be due to the fact that the non-specific immune activation induced by either virus or interferon-β does induce a series of relatively infrequent, but polyclonal, antigen responses against tumor-associated antigens but that these responses are not strong enough to be detected without some sort of amplification. This is consistent with our previous reports that effective anti-ova T-cell responses can be primed optimally in the B16ova model when the tumor antigen (OVA) is expressed from the VSV itself (4). Alternatively, it may be possible that the strong viral responses predominate and over-shadow T-cell responses against antigens expressed within the tumor and dependence of the therapy upon CD8+ T-cells may be associated more with the observed generalised T-cell hyperactivity induced by a potently immunogenic adjuvant rather than by antigen-specific T-cell effectors recognising tumor-associated antigens. Favoring the first possibility is that BALB/c mice cured of established AB12 tumors following intratumoral VSV-IFN-β therapy rejected a subsequent challenge with tumor. Therefore, it will be important to elucidate whether these rejection responses are associated with the concept of non-specific concomitant tumor immunity (28) - compatible with the VSV-induced generalised T-cell reactivity - or with low frequency, genuinely tumor antigen-specific T-cell responses.
Viral-directed expression of IFN-β would be expected to counteract anti-tumor efficacy, compared to VSV alone, by inhibiting viral replication if the tumor cells retain any levels of sensitivity to type-I interferon signaling. However, our data here, as well as other studies (Galivo et al. Submitted), suggest immune effectors are the predominant mechanism of anti-tumor therapy in the immunocompetent model. Therefore, the immune activating benefits of addition of IFN-β to VSV may outweigh the negative therapeutic impact of any reduced viral replication. Further studies on the levels of viral replication in tumors with time will be required to confirm this.
Consistent with this, our data clearly show a predominantly immune-based component of anti-tumor therapy with VSV-IFN-β in the immune-competent AB12 model (Fig.2). However, in the human MSTO/SCID model (Fig.5), therapy was principally associated with oncolyis since both VSV-hIFN-β and VSV-mIFN-β viruses gave equivalent anti-tumor therapy. These results are consistent with the hypothesis that the anti-tumor mechanisms of oncolytic virotherapy represent a balance between immune effectors and direct tumor cell destruction by viral replication and oncolysis. In the fully immune-competent setting, we believe that immune effectors predominate in both viral clearance and in anti-tumor efficacy. In contrast, in the context of SCID mice, the role of direct oncolysis is likely to be both more dominant and more apparent.
We also confirmed that inclusion of IFN-β into VSV as an additional safety feature protects normal cells/tissues from VSV-induced replication and cytotoxicity (Fig.5). Thus, inclusion of the biologically inactive human IFN-β gene into VSV unmasked very significant neurotoxicity of intratumorally injected VSV in the context of SCID mice, in which toxicity of VSV is more readily apparent, compared to the immune-competent counterpart hosts. In contrast, replacing the hIFN-β gene with the murine gene, led to a more prolonged therapeutic window of opportunity without any apparent toxicity during which intratumoral injections of virus were able to achieve significant tumor cures. These data are consistent with our ongoing toxicology studies to support implementation of clinical trials of the VSV-IFN-β virus in patients. In this respect, we have observed that incorporation of a biologically active IFN-β gene into VSV significantly increases the maximum tolerated dose by in excess of two orders of magnitude when administered in rodent models.
In summary, we have shown that VSV-IFN-β has significant therapeutic potential against mesothelioma tumors in the context of both local and loco-regional viral delivery. Addition of IFN-β provides both added efficacy, through immune-mediated mechanisms, which include activation of CD8+ T-cell responses, as well as added safety by providing protection from off-target viral replication in non-tumor tissues. Our original hypothesis was that the use of a replication-competent viral vector expressing IFN-β would be therapeutically more effective than a replication-defective adenoviral vector against established mesothelioma tumors. This was based upon the assumption that the replication-competent VSV-IFN-β virus would transduce more tumor cells and, correspondingly, express higher levels of IFN-β, leading to both higher levels of direct tumor cell killing as well as improved IFN-β-mediated anti-tumor immunity. Our studies here suggest that this initial hypothesis may not necessarily prove to be correct. Thus, we observe only very limited replication of the VSV vector in vivo, which is almost certainly associated with the potent innate immunogenicity of this virus (Galivo et al, Submitted). Moreover, this immunogenicity also resulted in an effect in which adaptive T-cell responses to the virus appear to dominate over those to tumor-associated antigens. In the light of our current results, therefore, a direct head-to-head comparison between the two vector platforms (VSV-IFN-β and Ad-IFN-β) is warranted and these studies are now underway in our laboratory.
ACKNOWLEDGEMENTS
We thank Toni Higgins for expert secretarial assistance. This work was supported by the Richard Schulze Family Foundation, the Mayo Foundation and by NIH Grants CA107082-02, CA130878-01, and CA66726-12.
REFERENCES
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma.[see comment] New England Journal of Medicine. 2005;353(15):1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 2.Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nature Medicine. 2001;7(7):781–787. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 3.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nature Reviews Cancer. 2005;5(12):965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 4.Diaz RM, Galivo F, Kottke T, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Research. 2007;67(6):2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. Journal of Virology. 2002;76(2):895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunology. 2004;17(4):516–527. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- 7.Barber GN. VSV-tumor selective replication and protein translation. Oncogene. 2005;24(52):7710–7719. doi: 10.1038/sj.onc.1209042. [DOI] [PubMed] [Google Scholar]
- 8.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends in Molecular Medicine. 2004;10(5):210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50(2):135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 10.Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nature Medicine. 2000;6(7):821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 11.Stojdl DF, Lichty BD, tenOever BR, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 12.Balachandran S, Porosnicu M, Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. Journal of Virology. 2001;75(7):3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebert O, Harbaran S, Shinozaki K, Woo SL. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Therapy. 2005;12(4):350–358. doi: 10.1038/sj.cgt.7700794. [DOI] [PubMed] [Google Scholar]
- 14.Lichty BD, Stojdl DF, Taylor RA, et al. Vesicular stomatitis virus: a potential therapeutic virus for the treatment of hematologic malignancy. Human Gene Therapy. 2004;15(9):821–831. doi: 10.1089/hum.2004.15.821. [DOI] [PubMed] [Google Scholar]
- 15.Lun X, Senger DL, Alain T, et al. Effects of intravenously administered recombinant vesicular stomatitis virus (VSV(deltaM51)) on multifocal and invasive gliomas. Journal of the National Cancer Institute. 2006;98(21):1546–1557. doi: 10.1093/jnci/djj413. [DOI] [PubMed] [Google Scholar]
- 16.Shinozaki K, Ebert O, Woo SL. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology. 2005;41(1):196–203. doi: 10.1002/hep.20536. [DOI] [PubMed] [Google Scholar]
- 17.Stojdl DF, Abraham N, Knowles S, et al. The murine double-stranded RNA-dependent protein kinase PKR is required for resistance to vesicular stomatitis virus. Journal of Virology. 2000;74(20):9580–9585. doi: 10.1128/jvi.74.20.9580-9585.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10(3):266–283. doi: 10.1111/j.1440-1843.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 19.Odaka M, Sterman DH, Wiewrodt R, et al. Eradication of intraperitoneal and distant tumor by adenovirus-mediated interferon-beta gene therapy is attributable to induction of systemic immunity. Cancer Research. 2001;61(16):6201–6212. [PubMed] [Google Scholar]
- 20.Odaka M, Wiewrodt R, DeLong P, et al. Analysis of the immunologic response generated by Ad.IFN-beta during successful intraperitoneal tumor gene therapy. Molecular Therapy: the Journal of the American Society of Gene Therapy. 2002;6(2):210–218. doi: 10.1006/mthe.2002.0656. [DOI] [PubMed] [Google Scholar]
- 21.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. Journal of Virology. 2003;77(16):8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed CM, Wills KN, Sugarman BJ, et al. Selective expression of nonsecreted interferon by an adenoviral vector confers antiproliferative and antiviral properties and causes reduction of tumor growth in nude mice. Journal of Interferon & Cytokine Research. 2001;21(6):399–408. doi: 10.1089/107999001750277871. [DOI] [PubMed] [Google Scholar]
- 23.Brin E, Atencio I, Helmich BK, Maneval D, Laface D. Adenovirus delivery provides extended interferon-alpha exposure and augments treatment of metastatic carcinoma. Cancer Gene Therapy. 2006;13(7):664–675. doi: 10.1038/sj.cgt.7700942. [DOI] [PubMed] [Google Scholar]
- 24.Sterman DH, Recio A, Carroll RG, et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses.[erratum appears in Clin Cancer Res. 2007 Sep 1;13(17):5226 Note: Kanther, Michelle [added]] Clinical Cancer Research. 2007;13(15 Pt 1):4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 25.Kruklitis RJ, Singhal S, Delong P, et al. Immuno-gene therapy with interferon-beta before surgical debulking delays recurrence and improves survival in a murine model of malignant mesothelioma. Journal of Thoracic & Cardiovascular Surgery. 2004;127(1):123–130. doi: 10.1016/j.jtcvs.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Davis MR, Manning LS, Whitaker D, Garlepp MJ, Robinson BW. Establishment of a murine model of malignant mesothelioma. International Journal of Cancer. 1992;52(6):881–886. doi: 10.1002/ijc.2910520609. [DOI] [PubMed] [Google Scholar]
- 27.Linardakis E, Bateman A, Phan V, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Research. 2002;62(19):5495–5504. [PubMed] [Google Scholar]
- 28.Prehn RT. Two competing influences that may explain concomitant tumor resistance. Cancer Research. 1993;53(14):3266–3269. [PubMed] [Google Scholar]