Abstract
Highly selective angiotensin II (Ang II) type 1 (AT1) receptor blockers (ARBs) are now available. AT1 receptor is a member of the G protein-coupled receptor (GPCR) superfamily and block the diverse effects of Ang II. Several ARBs are available for clinical use. Most ARBs have common molecular structures (biphenyl-tetrazol and imidazol groups) and it is clear that ARBs have “class effects”. On the other hand, recent clinical studies have demonstrated that not all ARBs have the same effects, and some benefits conferred by ARBs may not be class effects, and instead may be “molecular effects”. In addition, each ARB has been clearly shown to have molecular effects in basic experimental studies, and these effects may be due to small differences in the molecular structure of each ARB. However, it is controversial whether ARBs have molecular effects in a clinical setting. Although the presence of molecular effects for each ARB based on experimental studies may not directly influence the clinical outcome, this possibility has not been adequately evaluated. This review focuses on the class effects vs. molecular effects of ARBs from bench to bedside.
Keywords: angiotensin II receptor blockers, common molecular structures, class effects, molecular effects, angiotensin II type 1 receptor
Introduction
Angiotensin II (Ang II) is the major effector peptide of the renin-angiotensin system (RAS). Ang II binds to two receptor subtypes Ang II type 1 and type 2 (AT1 and AT2) receptors, which are members of the G protein-coupled receptor superfamily (GPCRs). AT1 receptor blockers (ARBs) are highly selective for the AT1 receptor and block the deleterious effects of Ang II, such as vasoconstriction, aldosterone release, retention of sodium and water, sympathetic nerve activation and cell proliferation (1). Many ARBs are available for clinical use worldwide. Most ARBs have class (or common) effects because they have common molecular structures [biphenyl-tetrazol and imidazol groups (2)], although recent clinical studies have demonstrated that not all ARBs have the same effects and some benefits conferred by ARBs may not be class effects, but rather molecular (or differential) effects. Each ARB has been shown to have molecular effects in basic experimental studies, and these effects may be due to small differences in the molecular structure of each ARB. The molecular effects most likely may be caused by specific off target effects. However, it is still controversial whether each ARB has molecular effects in a clinical setting. Therefore, this review focused on the class effects vs. molecular effects of ARBs in the field of translational research.
Structures of Ang II and AT1 receptor
Ang II is an octapeptide hormone that binds to AT1 receptor, which contains 359 amino acids and has a molecular mass of 41 kDa, by 4 main unique interactions. Two salt bridges, one between the Ang II side-chain Arg2 and the AT1 residue Asp281 and the other between Ang II α-COOH group of Phe8 and the AT1 residue Lys199, may be important for docking the hormone to the receptor (3,4). These salt-bridge interactions do not play a role in AT1 receptor activation. In addition, we have shown that two important interactions, one between Phe8 of Ang II and His256 in AT1 receptor (5) and the other between Ang II Tyr4 and Asn111, are necessary to activate the receptor (6,7).
Molecular structures of ARBs
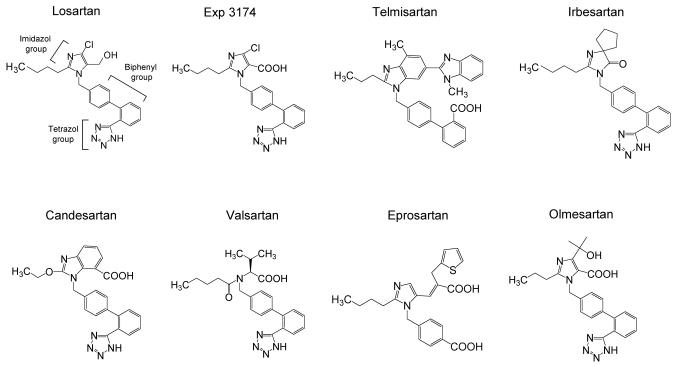
Seven kinds of ARBs are available for clinical use worldwide. Although several peptide types of ARBs have been synthesized since the 1970's, there have been problems with low bioavailability, short duration of action, and partial agonistic activity. The nonpeptidergic ARB losartan was the first to be developed based on imidazole analogues, and was designed by computational modeling (8). Various improved ARBs have been developed since losartan. For example, the chloride group of losartan was changed to a cyclopenthyl group to give irbesartan. Olmesartan contains a hydroxyl group in addition to a α-carboxyl group in the imidazol ring. Since ARBs mimic Ang II, most, including losartan, have common molecular structures (Figure 1), and it is clear that ARBs have class effects.
Figure 1.
Molecular structure of 8 ARBs including Exp3174, which is an active metabolite of losartan.
Binding affinities of ARBs
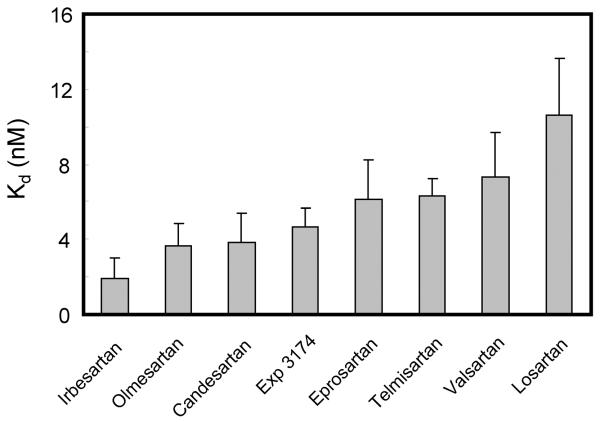
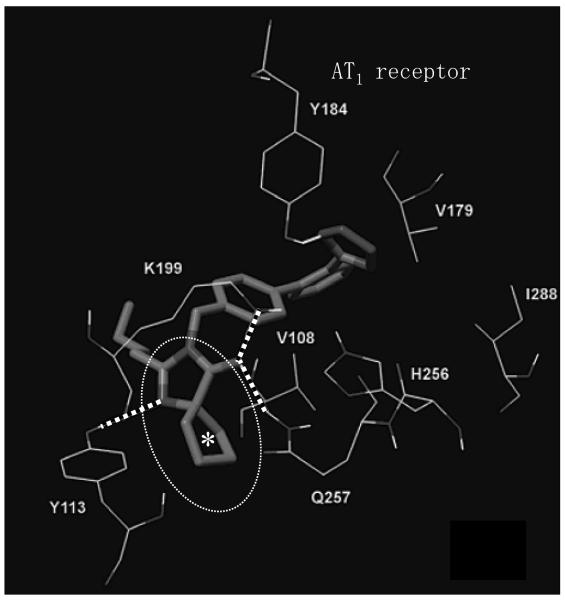
The Kd values of 7 ARBs and Exp3174, which is an active metabolite of losartan, are shown in Figure 2 (our unpublished data). The Kd values of AT1 receptor binding were determined by 125I-[Sar1, Ile8]Ang II-binding experiments under equilibrium conditions, and binding kinetics values were determined. Irbesartan showed the lowest Kd value, indicating that irbesartan may have the highest binding affinity to the AT1 receptor among these 8 ARBs. In particular, since irbesartan contains a cyclopenthyl group instead of the chloride group in both losartan and Exp3174, irbesartan may show higher binding affinity than these two ARBs. Interestingly, the results of computational modeling have suggested that the cyclopenthyl group of irbesartan may bind to a hydrophobic pocket in the AT1 receptor (Figure 3) (9). In addition, since hydrophobic interaction may occur between the cyclopenthyl group of irbesartan and the AT1 receptor using mutagenesis studies (our unpublished data), we refer to this interaction as “pentagon attachment”. Thus, a small difference in the molecular structure may influence various binding affinities.
Figure 2.
Binding affinities (Kd) of 8 ARBs including Exp3174, which is an active metabolite of losartan.
Figure 3.
Docking of irbesartan (*cyclopenthyl group) in the AT1 receptor binding site. Interatomic distances between H-bonded atoms are indicated in dotted lines. Dotted circle indicates hydrophobic pocket in the AT1 receptor (J Med Chem 2006;49:4305–4316).
Fabia et al. studied 36 reports in which blood pressure (BP) was measured using ambulatory BP monitoring for at least 24 hours (10). The antihypertensive activities of ARBs differed, and the magnitude of the reduction in BP did not essentially depend on the initial BP values or on the dose used. In addition, the reduction in mean 24-hour systolic BP with olmesartan was significantly greater than the reductions with losartan and valsartan and equivalent to the reduction with irbesartan (11). On the other hand, magnitude of BP reduction was significantly greater for patients who received olmesartan, but not other ARBs including irbesartan, than for those who received enarapril. Thus, not all ARBs may have the same antihypertensive effects. Although it is natural that the results regarding binding affinities as a molecular effect were not always consistent with those regarding antihypertensive effects, olmesartan and irbesartan showed better binding affinities than the others, and these ARBs may also be better at lowering BP.
Receptor selectivity and insurmountability of ARBs
All ARBs expect for losartan are highly selective for the AT1 receptor. In fact, ARBs show 10,000–30,000 times greater affinity for the AT1 receptor than for the AT2 receptor. This high selectivity implies that the AT2 receptor may be exposed to a higher concentration of Ang II because of renin-angiotensin feedback loop after ARBs treatment. Although AT2 receptor function is still unclear, Ang II-induced AT2 receptor stimulation may cause anti-cell proliferation and vasodilation (12). Surmountable and insurmountable antagonism largely correspond to competitive and non-competitive antagonism. Although losartan acted as a surmountable antagonist in isolated rat aorta, it acts like an insurmountable antagonist in other models (13). All other ARBs are insurmountable antagonists (14). In addition, compared to telmisartan, olmesartan showed a higher degree of insurmountability for AT1 receptor (15). However, since insurmountable antagonists overcome the binding of antagonists to the AT1 receptor only at a high plasma concentration of Ang II, this may not be relevant for the clinical application of ARBs.
Inverse agonism of ARBs
More than 60 wild-type GPCRs have been found to exhibit constitutive activity (16). In most cases, significant levels of constitutive activity are seen in recombinant systems in which GPCR expression levels are relatively high. Although spontaneous mutations have not been reported for the AT1 receptor, we reported that the WT AT1 receptor shows slight but significant constitutive activity (17). An inverse agonist can inhibit the constitutive activity of AT1 receptor. We previously reported that olmesartan and valsartan are stronger inverse agonists than losartan against inositol phosphate production using constitutively active N111G AT1 mutant receptor (17,18). Although WT AT1 receptor shows only slight constitutive activity, Morisset et al. clearly showed that inverse agonists are useful in a therapeutic strategy even if non-mutated receptors are expressed at normal levels in GPCRs, H3 receptor (19).
AT1 receptor mRNA levels were upregulated by myocyte stretching over time; significant increases were evident 6 hours after stretching, maximal levels (2.8-fold) were observed at 12 hours, and these effects were sustained for up to 18 hours (20). In addition, a recent study demonstrated that the AT1 receptor is activated by the mechanical stretching of cultured rat myocytes (21,22) and constriction of the transverse aorta in angiotensinogen knockout mice (21) without the involvement of Ang II, and these adverse effects were suppressed by an inverse agonist. Candesartan had greater effects than losartan. In this way, an inverse agonist for the AT1 receptor may have pharmacotherapeutic relevance, as a molecular effect, for preventing progression of the disease because it takes several decades for hypertension to progress to cardiovascular disease (23).
Several clinical trials have evaluated the effects of ARBs on morbidity and mortality in patients with heart failure (HF). The ELITE II trial suggested that treatment with losartan is not superior to treatment with captopril (24). In the CHARM trial, the benefits of candesartan were demonstrated in patients with HF (25). Although there are important differences in the design and hypotheses of these trials that must be taken into account when comparing their results and interpreting their clinical impact, we should also consider whether these are class effects of ARBs. The inverse agonistic activity of ARBs might also be important for their efficacy in the long-term treatment of heart disease, such as HF including cardiac remodeling, independent of blood pressure-lowering. Although most ARBs have been developed, most ARBs have been simply classified as antagonists. It may important to classify ARBs with regard to their capacity for inverse agonism as a molecular effect.
Anti-inflammatory effects of ARBs
Ang II induces inflammation in vasculature and vascular remodeling, and subsequently promotes atherosclerosis. Ang II stimulates monocyte chemoattractant protein-1 (MCP-1) (26), interleukin (IL)-8, tumor necrotic factor-α and IL-6 production. Irbesartan inhibited basal MCP-1 production in a dose-dependent manner in human monocytes (27). A similar effect was seen with losartan, at concentrations that were twice as high as those with irbesartan. These previous studies also showed that irbesartan decreased basal MCP-1 levels possibly through a mechanism that was independent of binding to the AT1 receptor.
The adipose-specific protein adiponectin has been recently discovered to improve insulin sensitivity and inhibit inflammation. Adiponectin protein expression was markedly stimulated by Ang II, which was inhibited by blockade of the AT2 receptor, and further enhanced by irbesartan (28). Irbesartan-mediated upregulation of adiponectin started beyond the concentrations needed for AT1 receptor blockade and was also present in the absence of Ang II, which suggests that an AT1 receptor-independent mechanism of action may be involved. Telmisartan also stimulated adiponectin protein expression, whereas the non-peroxisome proliferator-activated receptor (PPAR)γ-activating eprosartan had no effect. ARBs have molecular effects against MCP-1 production and PPARγ activation, and these effects may not be mediated by AT1 receptor. There may be another membrane receptor for the irbesartan-induced inhibition of MCP-1 production. Interestingly, both irbesartan and olmesartan may act as antagonists of a theoretical molecular model of C-CChemokine receptor, type-2b (29).
Clinical studies have demonstrated that some ARBs decrease the incidence of new-onset type 2 diabetes (30). In addition, losartan and candesartan increase the plasma levels of adiponectin in patients with essential hypertension (31,32). Although some ARBs have been shown to activate PPARγ, the concentrations used were very high (33,34), and it is doubtful that such concentrations can be achieved in humans. Future large prospective clinical studies that compare PPARγ-activating ARBs to non-activating ARBs will be required to clearly show that a PPARγ-activating phenotype in ARB is superior in patients with type 2 diabetes mellitus and/or hypertension.
Reduction of serum uric acid by ARBs
Generally, ARBs decrease microalbuminuria and proteinuria. With regard to organ protection in the kidney, irbesartan has been studied in two critical large-scale clinical trials [IDNT (Irbesartan Diabetic Nephropathy Trial) (34) and IRMA2 (Irbesartan in patients with type 2 diabetes and microalbuminuria) (35)]. Irbesartan is effective for protecting against the progression of nephropathy due to type 2 diabetes independent of the reduction in BP (35) and has been shown to have a renoprotective effect independent of its BP-lowering effect in patients with type 2 diabetes and microalbuminuria (36). Although the anti-inflammatory effect of irbesartan was stronger than that of losartan, losartan has also been the subject of an important large-scale clinical trial, called RENAAL (37). In that study, losartan conferred significant renal benefits in patients with type 2 diabetes and nephropathy, and although molecular effects did not influence the clinical outcome directly, losartan may have another important molecular effect. Serum uric acid (sUA) is currently recognized as a risk factor for cardiovascular disease as well as chronic kidney disease. Compared to other ARBs, both losartan and irbesartan have been shown to reduce sUA. Interestingly, losartan and telmisartan exhibited cis-inhibitory effects on the uptake of UA by the renal UA transporter (URAT1), and these ARBs reduced uptake in competitive inhibition kinetics (38). On the other hand, candesartan, Exp3174 (a major metabolite of losartan), olmesartan and valsartan did not have similar inhibitory effects. Such differences in the effects of ARBs on URAT1 could be predicted from the partial chemical structures of ARBs and may involve an AT1 receptor-independent mechanism of action. The molecular effects of each ARB may be associated with differences in the strength and weakness of the effect of the ARB, and may not reflect the clinical outcome.
Direct comparison of the efficacies on ARBs in clinical trials
Over the past decade, the efficacies of ARBs have been compared and differences were observed, as shown in Table 1, except with regard to BP-lowering. For example, valsartan is more effective than losartan at reducing left ventricular mass index in hypertensive patients (39) and induces greater renal NO production than losartan in hypertensive patients with chronic renal disease (40). Candesartan, but not losartan, significantly lowered plasma levels of plasminogen activator inhibitor type-1 antigen and MCP-1 in patients with hypertension (41). Exp 3174 is the most efficacious ARB at preventing human coronary artery contraction (42). Valsartan decreased the rate of target lesion revascularization after stenting compared to losartan (43). Olmesartan showed a significant reduction of high-sensitive C-reactive protein after stenting compared to valsartan (44). Changes in serum adiponectin and plasma glucose were significantly greater in the telmisartan group than in the candesartan group in patients with both type 2 diabetes and hypertension (45). Although losartan is numerical inferiority in comparison with other ARBs, losartan reduced human platelet activation significantly greater than valsartan and candesartan (46). These differences in the effects of ARBs are independent of BP-lowering in most studies.
Table 1.
Direct comparison of the efficacies of ARBs in clinical trials.
| Effects | Efficacies | References |
|---|---|---|
| Reducing left ventricular mass | valsartan > losartan | 39 |
| Increasing nitric oxide production | valsartan>losartan | 40 |
| Lowering plasma plasminogen activator inhibitor type-1 antigen and monocyte chemoattractant protein-1 | candesartan>losartan | 41 |
| Preventing coronary artery contractior | Exp3174>candesartan =valsartan>losartan | 42 |
| Preventing coronary restenosis | valsartan>losartan | 43 |
| Reduction of C-reactive protein | olmesartan>valsartan | 44 |
| Reduction of plasma adiponectin and glucose | telmisartan>candesartan | 45 |
| Inhibition of platelet activation | losartan>valsartan >candesartan | 46 |
| Reduction of interleukin-6 and C-reactive protein | olmesartan>telmisartan | 47 |
| Improvement of glucose and lipid profiles | telmisartan>olmesartan | 48 |
Interestingly, when olmesartan was compared to telmisartan, conflicting data were reported (47,48). Although there were no differences between olmesartan and telmisartan with regard to their effects on metabolic parameters including hemoglobinA1c and adiponectin, the decreases in serum IL-6 and hsCRP were more significant with olmesartan (47). On the other hand, telmisartan was more beneficial than olmesartan for improving glucose and lipid profiles (48). Since these trials were relatively small, we must be careful when comparing their results and interpreting their clinical impact, and should also reconsider whether these are molecular effects of ARBs, rather than class effects.
Conclusions
Several clinical trials have shown that ARBs have different degrees of beneficial effects. While most of the benefits conferred by ARBs may be class effects, some may be due to molecular effects. Basic research has clearly demonstrated some molecular effects of ARBs, and an exciting new area in ARB treatment is to determine whether these basic findings can influence the clinical outcome directly or indirectly.
References
- 1.de Gasparo M, Catt KJ, Inagami T, et al. International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 2.Miura S, Fujino M, Saku K. Angiotensin II receptor blocker as an inverse agonist: a current perspective. Curr Hypertens Rev. 2005;1:115–121. [Google Scholar]
- 3.Noda K, Saad Y, Kinoshita A, et al. Tetrazole and carboxylate groups of angiotensin receptor antagonists bind to the same subsite by different mechanisms. J Biol Chem. 1995;270:2284–2289. doi: 10.1074/jbc.270.5.2284. [DOI] [PubMed] [Google Scholar]
- 4.Feng YH, Noda K, Saad Y, et al. The docking of Arg2 of angiotensin II with Asp281 of AT1 receptor is essential for full agonism. J Biol Chem. 1995;270:12846–12850. doi: 10.1074/jbc.270.21.12846. [DOI] [PubMed] [Google Scholar]
- 5.Noda K, Saad Y, Karnik SS, et al. Interaction of Phe8 of angiotensin II with Lys199 and His256 of AT1 receptor in agonist activation. J Biol Chem. 1995;270:28511–28514. doi: 10.1074/jbc.270.48.28511. [DOI] [PubMed] [Google Scholar]
- 6.Feng YH, Miura S, Husain A, et al. Mechanism of constitutive activation of the AT1 receptor: influence of the size of the agonist switch binding residue Asn(111) Biochemistry. 1998;37:15791–15798. doi: 10.1021/bi980863t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miura S, Feng YH, Husain A, et al. Role of aromaticity of agonist switches of angiotensin II in the activation of the AT1 receptor. J Biol Chem. 1999;274:7103–7110. doi: 10.1074/jbc.274.11.7103. [DOI] [PubMed] [Google Scholar]
- 8.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 9.Tuccinardi T, Calderone V, Rapposelli S, Martinelli A. Proposal of a new binding orientation for non-peptide AT1 antagonists: homology modeling, docking and three-dimensional quantitative structure-activity relationship analysis. J Med Chem. 2006;49:4305–4316. doi: 10.1021/jm060338p. [DOI] [PubMed] [Google Scholar]
- 10.Fabia MJ, Abdilla N, Oltra R, Fernandez C, Redon J. Antihypertensive activity of angiotensin II AT1 receptor antagonists: a systematic review of studies with 24 h ambulatory blood pressure monitoring. J Hypertens. 2007;25:1327–1336. doi: 10.1097/HJH.0b013e3280825625. [DOI] [PubMed] [Google Scholar]
- 11.Oparil S, Williams D, Chrysant SG, Marbury TC, Neutel J. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens. 2001;3:283–291. 318. doi: 10.1111/j.1524-6175.2001.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kivlighn SD, Zingaro GJ, Gabel RA, Broten TP, Chang RS, Ondeyka DL, Mantlo NB, Gibson RE, Greenlee WJ, Siegl PK. In vivo pharmacology of an angiotensin AT1 receptor antagonist with balanced affinity for AT2 receptors. Eur J Pharmacol. 1995;294:439–450. doi: 10.1016/0014-2999(95)00564-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Pfaffendorf M, Zhang JS, Van Zwieten PA. A non-competitive type of angiotensin-receptor antagonism by losartan in renal artery preparations. Eur J Pharmacol. 1994;252:337–340. doi: 10.1016/0014-2999(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 14.Shibouta Y, Inada Y, Ojima M, Wada T, Noda M, Sanada T, Kubo K, Kohara Y, Naka T, Nishikawa K. Pharmacological profile of a highly potent and long-acting angiotensin II receptor antagonist, 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylic acid (CV-11974), and its prodrug, (+/−)-1-(cyclohexyloxycarbonyloxy)-ethyl 2-ethoxy-1-[[2'-(1H-tetrazol-5- yl)biphenyl-4-yl]methyl]-1H-benzimidazole-7-carboxylate (TCV-116) J Pharmacol Exp Ther. 1993;266:114–120. [PubMed] [Google Scholar]
- 15.Le MT, Pugsley MK, Vauquelin G, Van Liefde I. Molecular characterization of the interactions between olmesartan medoxomil and telmisartan and the human angiotensin II AT1 receptor. Br J Pharmacol. 2007;151:952–962. doi: 10.1038/sj.bjp.0707323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- 17.Miura S, Kiya Y, Kanazawa T, Imaizumi S, Fujino M, Matsuo Y, Karnik SS, Saku K. Differential bonding interactions of inverse agonists of angiotensin II type 1 receptor in stabilizing the inactive state. Mol Endocrinol. 2008;22:139–46. doi: 10.1210/me.2007-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura S, Fujino M, Hanzawa H, Kiya Y, Imaizumi S, Matsuo Y, Tomita S, Uehara Y, Karnik SS, Yanagisawa H, Koike H, Komuro I, Saku K. Molecular mechanism underlying inverse agonist of angiotensin II type 1 receptor. J Biol Chem. 2006;281:19288–19295. doi: 10.1074/jbc.M602144200. [DOI] [PubMed] [Google Scholar]
- 19.Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, Schunack W, Ganellin CR, Schwartz JC, Arrang JM. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000;408:860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- 20.Kijima K, Matsubara H, Murasawa S, Maruyama K, Mori Y, Ohkubo N, Komuro I, Yazaki Y, Iwasaka T, Inada M. Mechanical stretch induces enhanced expression of angiotensin II receptor subtypes in neonatal rat cardiac myocytes. Circ Res. 1996;79:887–897. doi: 10.1161/01.res.79.4.887. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y, Kiya Y, Imaizumi S, Fujino M, Ito K, Zou Y, Fukuhara S, Kunimoto S, Fukuzaki K, Sato T, Ge J, Mochizuki N, Nakaya H, Saku K, Komuro I. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 2008;9:179–186. doi: 10.1038/sj.embor.7401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 23.Vasan RS, Levy D. The role of hypertension in the pathogenesis of heart failure. A clinical mechanistic overview. Arch Intern Med. 1996;156:1789–1796. [PubMed] [Google Scholar]
- 24.Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm JA, Konstam MA, Riegger G, Klinger GH, Neaton J, Sharma D, Thiyagarajan B. Randomised trial of losartan versus captopril on mortality in patients with symptomatic heart failure: the losartan heart failure survival study - ELITE II. Lancet. 2000;355:15822–15827. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez-Presa M, Bustos C, Ortego M, Tunon J, Renedo G, Ruiz-Ortega M, Egido J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-κB activation, monocyte chemoattractant protein-1 expression and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- 27.Proudfoot JM, Croft KD, Puddey IB, Beilin LJ. Angiotensin II type 1 receptor antagonists inhibit basal as well as low-density lipoprotein and platelet-activating factor-stimulated human monocyte chemoattractant protein-1. J Pharmacol Exp Ther. 2003;305:846–853. doi: 10.1124/jpet.102.047795. [DOI] [PubMed] [Google Scholar]
- 28.Clasen R, Schupp M, Foryst-Ludwig A, Sprang C, Clemenz M, Krikov M, Thöne-Reineke C, Unger T, Kintscher U. PPARgamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- 29.Marshall TG, Lee RE, Marshall FE. Common angiotensin receptor blockers may directly modulate the immune system via VDR, PPAR and CCR2b. Theor Biol Med Model. 2006;3:1. doi: 10.1186/1742-4682-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheen AJ. Prevention of type 2 diabetes mellitus through inhibition of the Renin-Angiotensin system. Drugs. 2004;64:2537–2565. doi: 10.2165/00003495-200464220-00004. [DOI] [PubMed] [Google Scholar]
- 31.Furuhashi M, Ura N, Higashiura K, Murakami H, Tanaka M, Moniwa N, Yoshida D, Shimamoto K. Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension. 2003;42:76–81. doi: 10.1161/01.HYP.0000078490.59735.6E. [DOI] [PubMed] [Google Scholar]
- 32.Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, Kang MH, Ahn TH, Choi IS, Shin EK. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110:3687–3692. doi: 10.1161/01.CIR.0000143085.86697.13. [DOI] [PubMed] [Google Scholar]
- 33.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 34.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 35.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I, Collaborative Study Group Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 36.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 37.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 38.Iwanaga T, Sato M, Maeda T, Ogihara T, Tamai I. Concentration-dependent mode of interaction of angiotensin II receptor blockers with uric acid transporter. J Pharmacol Exp Ther. 2007;320:211–217. doi: 10.1124/jpet.106.112755. [DOI] [PubMed] [Google Scholar]
- 39.Picca M, Agozzino F, Pelosi G. Effects of losartan and valsartan on left ventricular hypertrophy and function in essential hypertension. Adv Ther. 2004;21:76–86. doi: 10.1007/BF02850335. [DOI] [PubMed] [Google Scholar]
- 40.Pool JL, Guthrie RM, Littlejohn TW, 3rd, Raskin P, Shephard AM, Weber MA, Weir MR, Wilson TW, Wright J, Kassler-Taub KB, et al. Dose-related antihypertensive effects of irbesartan in patients with mild-to-moderate hypertension. Am J Hypertens. 1998;11:462–470. doi: 10.1016/s0895-7061(97)00501-3. [DOI] [PubMed] [Google Scholar]
- 41.Krämer C, Sunkomat J, Witte J, Luchtefeld M, Walden M, Schmidt B, Tsikas D, Böger RH, Forssmann WG, Drexler H, Schieffer B. Angiotensin II receptor-independent antiinflammatory and antiaggregatory properties of losartan: role of the active metabolite EXP3179. Circ Res. 2002;90:770–776. doi: 10.1161/01.res.0000014434.48463.35. [DOI] [PubMed] [Google Scholar]
- 42.Pantev E, Stenman E, Wackenfors A, Edvinsson L, Malmsjo M. Comparison of the antagonistic effects of different angiotensin II receptor blockers in human coronary arteries. Eur J Heart Fail. 2002;4:699–705. doi: 10.1016/s1388-9842(02)00166-6. [DOI] [PubMed] [Google Scholar]
- 43.Iwata A, Miura S, Imaizumi S, Kiya Y, Nishikawa H, Zhang B, Shimomura H, Kumagai K, Matsuo K, Shirai K, Saku K. Do valsartan and losartan have the same effects in the treatment of coronary artery disease? Circ J. 2007;71:32–38. doi: 10.1253/circj.71.32. [DOI] [PubMed] [Google Scholar]
- 44.Sugihara M, Miura S, Takamiya Y, Kiya Y, Arimura T, Iwata A, Kawamura A, Nishikawa H, Uehara Y, Saku K. Safety and efficacy of antihypertensive therapy with add-on angiotensin II type 1 receptor blocker after successful coronary stent implantation. Hypertens Res. 2009;32:625–630. doi: 10.1038/hr.2009.66. [DOI] [PubMed] [Google Scholar]
- 45.Yamada S, Ano N, Toda K, Kitaoka A, Shiono K, Inoue G, Atsuda K, Irie J. Telmisartan but not candesartan affects adiponectin expression in vivo and in vitro. Hypertens Res. 2008;31:601–606. doi: 10.1291/hypres.31.601. [DOI] [PubMed] [Google Scholar]
- 46.Nunez A, Gomez J, Zalba LR, Monton M, Jimenez A, Velasco S, Lopez-Blaya A, Uriarte AC, Casado S, Lopez-Farre A. Losartan inhibits in vitro platelet activation: comparison with candesartan and valsartan. J Renin Angiotensin Aldosterone Syst. 2000;1:175–179. doi: 10.3317/jraas.2000.022. [DOI] [PubMed] [Google Scholar]
- 47.Nakayama S, Watada H, Mita T, Ikeda F, Shimizu T, Uchino H, Fujitani Y, Hirose T, Kawamori R. Comparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early-stage type-2 diabetics with hypertension. Hypertens Res. 2008;31:7–13. doi: 10.1291/hypres.31.7. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki T, Noda Y, Yasuoka Y, Irino H, Abe H, Adachi H, Hattori S, Kitada H, Morisawa D, Miyatake K. Comparison of the effects of telmisartan and olmesartan on home blood pressure, glucose, and lipid profiles in patients with hypertension, chronic heart failure, and metabolic syndrome. Hypertens Res. 2008;31:921–929. doi: 10.1291/hypres.31.921. [DOI] [PubMed] [Google Scholar]