Abstract
OBJECTIVE
Randomized, double-blind, placebo-controlled trials of ursodeoxycholic acid (UDCA) in patients with primary biliary cirrhosis (PBC) have not demonstrated improvement in survival during the placebo-controlled phases of these trials. Analyses purporting to demonstrate a survival advantage of UDCA are largely dependent on data obtained after the placebo phases were terminated, and placebo-treated patients were offered open-label UDCA. After completion of our 2-yr placebo-controlled trial of UDCA in which we observed no survival benefit for UDCA, we provided the patients with open-label UDCA to see if delay in providing UDCA for 2 yr had any effect on subsequent liver transplantation or death without liver transplantation.
METHODS
In our previously reported 2-yr placebo-controlled trial, 151 patients with PBC were randomized to receive either UDCA (n = 77) or placebo (n = 74). The number of patients who progressed to liver transplantation or death without transplantation were similar in both the groups, 12 (16%) in the UDCA-treated and 11 (15%) in placebo-treated patients. All the patients were then offered open-label UDCA, with 61 original UDCA and 56 original placebo-treated patients now taking UDCA in an extended open-label phase of the trial.
RESULTS
No significant differences were observed in the number of patients who underwent liver transplantation or died without liver transplantation in the open-label phase of the trial. Moreover, no difference in the time to these endpoints was seen over the period of observation of as long as 6 yr from the time of initial randomization.
CONCLUSIONS
Results of open-label extensions of previous conducted placebo-controlled trials of UDCA in PBC leave uncertain whether UDCA impacts significantly on liver transplantation and death without liver transplantation in patients with PBC.
INTRODUCTION
The impression that ursodeoxycholic acid (UDCA) prolongs survival in patients with primary biliary cirrhosis (PBC) is largely based on the data of Poupon and co-workers (1). In their 2-yr randomized, double-blind trial of UDCA versus placebo, the incidence of liver transplantation or death without transplantation was very low, and not different in the two treatment groups (2). At the end of this 2-yr period, patients originally on UDCA were continued on UDCA for 2 more yr, whereas the placebo group was now offered open-label UDCA, and most of this group took UDCA for the next 2 yr. During this latter 2-yr period of open-label UDCA, a striking increase was observed in the number of patients who underwent or were referred for liver transplantation in the treatment group that originally received placebo for 2 yr, then open-label UDCA for the next 2 yr compared to the group originally randomized to receive UDCA for 2 yr, and then received open-label UDCA for 2 additional yr (1). These observations, when combined with the experiences of the Mayo Clinic (3), and of the Canadian group that also compared UDCA to placebo (4) led to the conclusion that UDCA prolongs survival in PBC patients, even though neither the Mayo Clinic trial nor the Canadian trial independently demonstrated a statistically significant effect of UDCA on survival. It was only when the results of these three independent trials were combined (5), that a positive UDCA effect was said to exist, and then much of the data were obtained from periods when UDCA and placebo were not compared simultaneously.
We, too, compared the effects of UDCA versus placebo in PBC patients randomized and treated in a 2-yr doubleblind clinical trial (6). Although we, like almost all others who have assessed UDCA in PBC patients, found impressive effects of UDCA on improving markers of liver inflammation and cholestasis, and some aspects of liver histology, we did not observe any advantage of UDCA in preventing liver transplantation or death without liver transplantation during the 2 yr of our placebo-controlled trial.
At the completion of our placebo-controlled trial, all the patients were offered UDCA, and virtually all continued to take UDCA thereafter in the open-label phase of our study. The current report presents our observations on the incidence of liver transplantation or death without transplantation over the ensuing years in this study group. Our findings have been presented earlier in part in abstract form (7).
MATERIALS AND METHODS
In our controlled trial, 151 patients with PBC satisfying specified inclusion and exclusion criteria were randomized at six treatment centers to receive UDCA or placebo at a dose of 10–12 mg/kg/day taken orally once a day at bedtime. In this 2-yr double-blinded trial, 74 patients were randomized to placebo and 77 to UDCA. Demographic, laboratory, and histologic characteristics at entry were comparable in both groups. Similar numbers of patients in each group completed the trial, 60 (80%) placebo and 63 (82%) UDCA. Despite major improvements in laboratory tests and some histologic features in the UDCA-treated group, the number of patients who progressed to liver transplantation or death without transplantation was similar in both groups (11 (15%) in the placebo group, and 12 (16%) in UDCA-treated patients). Only three placebo-treated and two UDCA-treated patients withdrew from the trial (6).
Upon completion of the placebo-controlled trial, all the patients were offered UDCA. Four placebo patients did not take it. One was being assessed for transplantation, which was provided within 6 months of completion of the randomized trial; one with recently diagnosed breast cancer, and two others withdrew from the open trial. Two patients originally on UDCA decided not to continue in the open-label trial. Thus, 56 original placebo and 61 original UDCA-treated patients took UDCA in the extended open-label phase of the trial.
Data from the open-label trial were collected retrospectively and were complete for death, liver transplantation, and development of major complications, i.e., variceal bleeding, ascites, and encephalopathy. Data for other markers of possible treatment failures as defined in our original protocol, i.e., development of varices, histologic progression, doubling of serum bilirubin, and marked worsening of symptoms (pruritus, fatigue) were incomplete in the open-label study, because after completion of the controlled trial, bilirubin levels were not measured at regular time intervals, biopsy and upper endoscopy were not performed at a uniform time point for all the patients, and no systematic efforts were made prospectively to collect information on severity of fatigue and pruritus.
In order to compare our findings with those of Poupon et al., the data from our double-blind trial (0–2 yr) and the open-label extension (2–4 yr) were combined in order to compare the two groups as initially randomized with respect to the incidence of transplantation and death without transplantation. The data were analyzed according to the intent-to-treat principle. Thus, in Table 1, the group labeled UDCA received UDCA for 4 yr from the time of randomization, while the placebo group received placebo for 2 yr, followed by UDCA for 2 yr. Moreover, patients accepted into the trial had been stratified into four strata on the basis of (i) a serum bilirubin of less than 2 mg/dl, or 2 mg/dl or greater; and (ii) liver histology, either stages I and II or stages III and IV as defined by Ludwig et al. (8). Patients in stratum I had a serum bilirubin less than 2 and stage I or II histology; stratum 2, a bilirubin less than 2 and stage III or IV histology; stratum 3, a bilirubin of 2 or greater and stage I or II histology; stratum 4, a bilirubin of 2 or greater and stage III or IV histology. Data are presented for stratum 1, stratum 2, strata 3–4, and for all strata.
Table 1.
Transplantation or Death Without Transplantation in the Combined Randomized and Open-Label Studies—4-Yr Follow-Up Data
| Strata 3–4
|
All Patients
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratum 1
|
Stratum 2
|
Placebo | UDCA | Log Rank p Value | Placebo | UDCA | Log Rank p Value | |||
| Placebo* | UDCA† | Placebo | UDCA | |||||||
| No. of patients | 19 | 26 | 33 | 26 | 22 | 25 | 74 | 77 | ||
| 02 yr‡ (placebo controlled) | ||||||||||
| Transplants | 0 | 0 | 1 | 1 | 7 | 7 | 0.706 | 8 | 8 | 0.902 |
| Death | 0 | 0 | 0 | 1 | 3 | 3 | 3 | 4 | ||
| 2–4 yr (open label) | ||||||||||
| Transplants | 0 | 0 | 2 | 2 | 6 | 4 | 8 | 6 | ||
| Death | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 3 | ||
| 0–4 yr (combined data) | ||||||||||
| Transplants | 0 | 0 | 3 | 3 | 13 | 11 | 0.999 | 16 | 14 | 0.839 |
| Death | 1 | 0 | 0 | 2 | 3 | 5 | 4 | 7 | ||
Placebo group received placebo for 2 yr, then open label UDCA for 2 yr.
UDCA group received UDCA for 2 yr, then open label UDCA for 2 yr.
These data were previously reported in Table 4, Reference (6).
The numbers of transplants and deaths without transplantation for the first and second 2-yr periods and for the cumulative 4-yr period are summarized in Table 1. The original randomized 2-yr placebo-controlled trial, which was approved by our respective IRBs, began at the end of December 1988. The cut-off date for the retrospective collection of information was July 1995. Thus, a number of patients had more than 4 yr of follow-up from initial randomization. Their findings are summarized in Table 2.
Table 2.
Transplantation or Death Without Transplantation Occurring after 0–4 Yr
| Stratum 1
|
Stratum 2
|
Strata 3–4
|
All Patients
|
|||||
|---|---|---|---|---|---|---|---|---|
| Placebo | UDCA | Placebo | UDCA | Placebo | UDCA | Placebo | UDCA | |
| After 0–4 yr | ||||||||
| Transplants | 0 | 0 | 4 | 1 | 0 | 1 | 4 | 2 |
| Death | 0 | 0 | 0 | 1 | 2 | 0 | 2 | 1 |
| Total no. of events 0–6 years | ||||||||
| Transplants | 0 | 0 | 7 | 4 | 13 | 12 | 20 | 16 |
| Death | 1 | 0 | 0 | 3 | 5 | 5 | 6 | 8 |
The times to all of these endpoints are presented in Figure 1 in a survival analysis utilizing the Kaplan–Meier method using time from randomization to liver transplantation, or death without liver transplantation. For those who did not experience liver transplantation or death, the dates when these patients were last seen by our investigators pertain to the censoring times.
Figure 1.
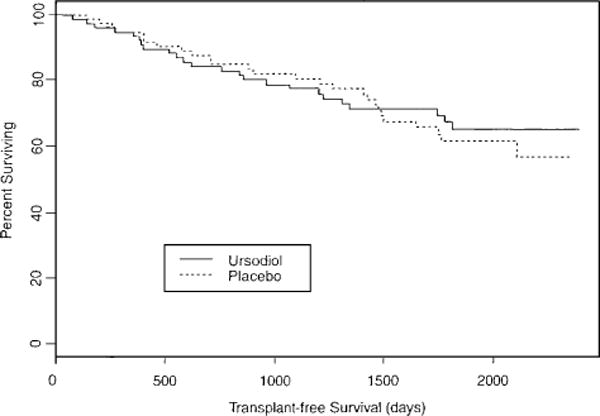
Kaplan–Meier assessment of survival using time from randomization to liver transplantation or death without transplantation. There were 26 observed events in the treatment group originally randomized to placebo (n = 74), then after 2 yr continued on open-label UDCA therapy. The expected number of events in this group was 24.6. Twenty-four events were observed in the group originally randomized to UDCA (n = 77) and continued on UDCA thereafter. Expected events in this latter group were 25.4. χ2 = 0.2 on one degree of freedom, p = 0.688.
Statistical Analyses
The rates of treatment failure in the two groups were estimated by the Kaplan–Meier method, and compared by logrank χ2 tests. The p-values less than 0.05 were considered to be statistically significant.
RESULTS
No significant differences were observed in the number of patients who underwent liver transplantation, or died without transplantation, either during the first 2 yr of the placebo-controlled trial, during the second 2 yr of the open-label phase, or for the combined 4 yr of the trial, in any of the strata or in the composite findings of all strata (see Table 1). Similarly, no differences were noted in the number of events occurring after 4 yr of initial randomization (see Table 2).
The Kaplan–Meier assessment of transplant-free survival revealed no differences between the two groups in time to these endpoints over the period of observation of as long as 6 yr from the time of randomization (see Fig. 1).
Overall, the causes of death prior to transplantation were related predominantly to liver disease and its complications in 9 of the 10 patients initially grouped in strata 3–4. The one death in a stratum 1 patient was due to pneumonia; and of three deaths in stratum 2 patients, one was due to a myocardial infarction, one to heart failure, and one was attributed to liver disease.
DISCUSSION
The inference of Poupon and associates’ data (1) that a delay of 2 yr in providing UDCA for PBC patients would result in sufficient worsening of PBC, so that transplantation, referral for transplantation, and death without transplantation would ensue at an enhanced rate is not supported by our current trial, by the Canadian trial of Heathcote and associates (9), or by the Swedish multicenter trial of Eriksson and associates (10). In all of these trials, patients were randomized to receive either UDCA or placebo for 2 yr. Subsequently, all or many of the patients were offered open-label UDCA. This was accepted by virtually all of the patients in the current and Eriksson’s trials (10), and by a good number but not all of the patients in the Canadian trial (9). Subsequent analysis showed no differences in survival defined as transplantation or death without transplantation based on intent-to-treat from initial randomization to either UDCA or placebo. Other than indicating that a 2-yr delay in initiation of UDCA for therapy of PBC does not affect long-term survival, it is not clear what other implications can be derived from these findings. Why Poupon’s data are so different from the other, similar studies, is not clear; the possibilities of differences in patient responses due to chance, or exuberance in referral for transplantation are major considerations.
The patients followed by Lindor et al., who did not demonstrate statistically significant improvements in transplantation and death during their controlled trial (3), are reported to show improvements during the open-label phases of their trial when placebo controls are no longer present (11). One hundred and eighty patients were recruited for the controlled trial over a period of 4 yr with 91 randomized to placebo and 89 to UDCA. The study was stopped 2 yr after the 132nd patient was entered. Thus, many randomized patients were followed for over 2 yr, and 48 patients for less than 2 yr, but the numbers of such patients in the respective treatment arms at any given time point are not cited. When the incidence of death or transplantation and the time until death or liver transplantation were compared between the two treatment arms in the placebo-controlled period of their trial, no significant differences were found (p values for log-rank tests comparing time until event = 0.30 for death, = 0.41 for transplantation, and = 0.18 for death or transplantation). It is stated in reference (3) “If the difference in time until death or transplantation between groups observed in this trial were in fact the true difference, it would take 7.3 yr of patient accrual at the rate attained in this study to provide 80% power to detect such a difference at the alpha = 0.05 level.” The implication is that a larger number of patients would have been needed to resolve the question of a treatment effect when death and transplantation are the clinical endpoints.
At the end of the blinded, controlled phase of the Lindor trial, open-label drug was offered, and all the patients then received ursodiol (11). Because of the crossover of patients receiving placebo, their median follow-up was 2.3 yr (0.1–4.2 yr) whereas, the median follow-up was 4.6 yr (0.4–7.0 yr) for the ursodiol group. Two analyses were performed, i.e., an efficacy analysis and an intent-to-treat analysis. In the efficacy analysis, patients were censored at the time of drug discontinuation whatever the reason for stoppage. In the intent-to-treat analysis, patients were followed up without regard to withdrawal or crossover. The only exception was that all the patients receiving placebo were censored approximately 1–2 months after the 132nd randomized patient had been followed for 2 yr. In these extended analyses, adapted to include open-label UDCA-treated information, with death or liver transplantation as events, the risk of an event in the placebo group versus the ursodiol group was 2.60 with p = 0.04 for the efficacy analysis, and 2.45 with p = 0.04 for the intent-to-treat analysis. These extended analyses which provide comparative information for placebo treatment for as long as 4.2 yr may explain the survival benefit (death or transplantation) for patients with PBC treated with ursodiol compared with placebo-treated patients. The numbers of patients in the respective treatment groups at each year since randomization were not presented in this publication, however. It becomes difficult, therefore, to comment on the power of the reported observations.
Thus, in all of the controlled studies in which placebo was switched to UDCA, the time of simultaneous comparison of UDCA to placebo was severely limited, and in our judgment conclusions about efficacy of UDCA, particularly with regard to preventing transplantation or death without transplantation are highly suspect.
It can be proposed that the differences in results in the switch-over to open-label trials are due to different dosages and preparations of the UDCA administered. Thus, Eriksson’s group administered UDCA in a dose of 7.7 mg/kg/day. We provided 10–12 mg/kg/day, whereas Poupon et al., Lindor et al., and Heathcote et al. administered 13–15 mg/kg/day. Nevertheless, the enrichment of the bile acid pool was virtually identical in our and the Mayo Clinic trials, when bile acids were measured in a blinded fashion in the same laboratory of Dr. Alan Hoffman (see Fig. 2). Moreover, the Canadian trial which used the same preparation and dose of UDCA as the Mayo Clinic group found no significant effects on survival either during their 2-yr controlled trial, and in the case of the Canadian group after patients were offered and many accepted open-label UDCA.
Figure 2.
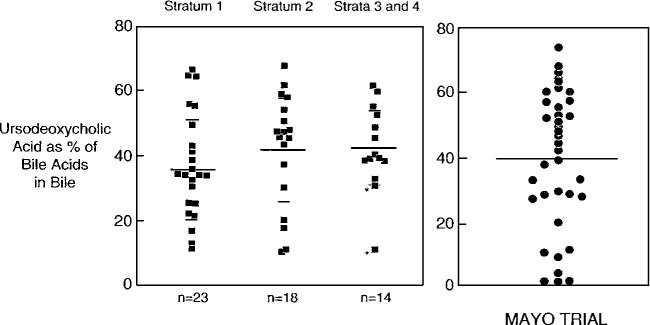
Comparison of results of UDCA as percent of bile acids in fasting bile obtained at 2 yr in the placebo-controlled trials of Combes et al. (6) and Lindor et al. (3). The figure represents a composite of Figure 5 previously published in Reference (6), and Figure 6 published in Reference (3). Permission was obtained from the copyright owners, i.e., the American Association for the Study of Liver Diseases and the American Gastroenterological Association, to reproduce this material. Administered doses of UDCA were 10–12 mg/kg/day (6) and 13–15 mg/kg/day (3) in these respective trials. Mean percent ± SD was 37.0 ± 16.3 for patients in stratum 1; 42.1 ± 17.4 for stratum 2; and 42.7 ± 12.9 for strata 3 and 4 (6). The mean UDCA value for the Mayo Clinic trial was 39.5% ((3), data shown on right-hand side of Figure 2.)
Subsequent conclusions based on efforts to combine some of the data of the Poupon, Mayo Clinic, and Canadian experiences which are felt to support a survival advantage to UDCA are in our judgment suspect since all prospectively randomized patients assigned to receive UDCA or placebo were not studied for comparable periods of time. The recent detailed meta-analysis of Goulis et al. (12), which assesses all of the trials cited above, including the switch-over periods, concludes that a therapeutic benefit of UDCA in PBC has yet to be proven. In our judgment, all of the controlled trials, including our own, were terminated too soon because of the impressive effects of UDCA on (i) results of liver tests which assess cholestasis and hepatic inflammation, and (ii) on certain aspects of liver histology.
Unfortunately, we are still left with uncertainty as to whether UDCA will impact significantly on liver transplantation and death without transplantation in patients with PBC.
Acknowledgments
We are indebted to Renate Davis (UT Southwestern) for invaluable administrative support and preparation of this manuscript; to Clinical Coordinators Judy Otey (UT Southwestern), Sharon Westerberg (Thomas Jefferson), Jenny McCarthy (Nebraska), and to Melissa Loyet (Washington University, St. Louis) for superior interactions with patients; and to Willis C. Maddrey, M.D. (UT Southwestern) for critical review of the manuscript. This project was supported in part by a research grant from Ciba-Geigy; NIH General Clinical Research Center grants to UT Southwestern (M01-RR00633), Yale (M01-RR00125), Medical College of Virginia (M01-RR00065), Washington University, St. Louis (M01-RR00036); NIH grants Yale (P30-DK34989); institutional funds: Thomas Jefferson-Louis A. Rosen Fund for Liver Research; and Nebraska-Clinical Research Funds.
References
- 1.Poupon RE, Poupon R, Balkau B, for the UDCA-PBC Study Group Ursodiol for the long-term treatment of primary biliary cirrhosis. New Engl J Med. 1994;330:1342–7. doi: 10.1056/NEJM199405123301903. [DOI] [PubMed] [Google Scholar]
- 2.Poupon RE, Balkau B, Eschwege E, et al. The UDCA-PBC Study Group A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. New Engl J Med. 1991;324:1548–51. doi: 10.1056/NEJM199105303242204. [DOI] [PubMed] [Google Scholar]
- 3.Lindor KD, Dickson ER, Baldus WP, et al. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284–90. doi: 10.1016/0016-5085(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 4.Heathcote EJ, Cauch-Dudek K, Walker V, et al. The Canadian multicenter double-blind randomized controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1994;19:1149–56. [PubMed] [Google Scholar]
- 5.Poupon RE, Lindor KD, Cauch-Dudek K, et al. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology. 1997;113:884–90. doi: 10.1016/s0016-5085(97)70183-5. [DOI] [PubMed] [Google Scholar]
- 6.Combes B, Carithers RL, Jr, Maddrey WB, et al. A randomized, double-blind, placebo-controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology. 1995;22:759–66. [PubMed] [Google Scholar]
- 7.Carithers RL, Jr, Luketic VA, Peters M, et al. Extended follow-up of patients in the U.S multicenter trial of ursodeoxycholic acid for primary biliary cirrhosis. Gastroenterology. 1996;110:A1163. doi: 10.1111/j.1572-0241.2004.04047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis) Virchow’s Arch A. 1978;379:103–12. doi: 10.1007/BF00432479. [DOI] [PubMed] [Google Scholar]
- 9.Kilmurry MR, Heathcote EJ, Cauch-Dudek K, et al. Is the Mayo model for predicting survival useful after the introduction of ursodeoxycholic acid treatment for primary biliary cirrhosis? Hepatology. 1996;23:1148–53. doi: 10.1002/hep.510230532. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson LS, Olsson R, Glauman H, et al. Ursodeoxycholic acid treatment in patients with primary biliary cirrhosis. A Swedish multicentre, double-blind, randomized controlled study. Scand J Gastroenterol. 1997;32:179–86. doi: 10.3109/00365529709000190. [DOI] [PubMed] [Google Scholar]
- 11.Lindor KD, Therneau TM, Jorgensen RA, et al. Effect of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology. 1996;110:1515–8. doi: 10.1053/gast.1996.v110.pm8613058. [DOI] [PubMed] [Google Scholar]
- 12.Goulis J, Leandro G, Burroughs AK. Randomised controlled trials of ursodeoxycholic-acid therapy for primary biliary cirrhosis: A meta-analysis. Lancet. 1999;354:1053–60. doi: 10.1016/S0140-6736(98)11293-X. [DOI] [PubMed] [Google Scholar]


