Abstract
Cerebellar deficits and subsequent impairment in procedural learning may contribute to both motor difficulties and reading impairment in dyslexia. We used quantitative magnetic resonance imaging to investigate the role of regional variation in cerebellar anatomy in children with single-word decoding impairments (N=23), children with impairment in fluency alone (N=8), and typically developing children (N=16). Children with decoding impairments (dyslexia) demonstrated no statistically significant differences in overall grey and white matter volumes or cerebellar asymmetry; however, reduced volume in the anterior lobe of the cerebellum relative to typically developing children was observed. These results implicate cerebellar involvement in dyslexia and establish an important foundation for future research on the connectivity of the cerebellum and cortical regions typically associated with reading impairment.
Keywords: Developmental dyslexia, Decoding, Fluency, Cerebellar anatomy, Structural imaging, Magnetic resonance imaging
Dyslexia is a common developmental disorder affecting 5 % to 15 % of school-aged children. By definition, dyslexia is characterized by difficulties acquiring accurate and fluent single-word reading skills [1]. Almost 50 % of all children receiving special education services in the U.S. do so for reading problems [2]. Given that reading is a fundamental skill required for learning other academic material, individuals with dyslexia often perform poorly in school [3], are more likely to drop out of school, and are more likely to experience adjustment difficulties than youth with typical reading skills [4].
Various anatomical magnetic resonance imaging (MRI) studies have attempted to determine the neural correlates of dyslexia by focusing on differences between the brains of individuals with and without reading difficulties. Although findings and methodologies vary, a pattern of differences in brain structures has emerged that implicates the inferior frontal gyrus, temporal–parietal region, medial occipital lobe, and cerebellar anterior and posterior lobes [5]. Some of these findings are not surprising given the demands of reading on graphophonemic processing, language comprehension, and working memory capacities. Cerebellar findings, however, have generated both a “theory” of dyslexia and controversy involving the role of implicit learning on reading acquisition, particularly given that processing speed and motor deficits are often observed in children with dyslexia and other learning and attention disorders [6].
Cerebellar Theory of Dyslexia
Proponents of the cerebellar theory of dyslexia argue that the cerebellum is active during early stages of skill acquisition [7, 8], a process with which some children with dyslexia struggle [9]. The resulting procedural learning deficits prevent automatization of accurate word decoding and phonological processing. This theory has been evaluated by comparing children with dyslexia and typically developing children on neuropsychological and cognitive tasks presumably associated with the cerebellum [10, 11]. However, the results are controversial because the children with dyslexia were defined in part by performance on these tasks. In other studies, these same cerebellar tasks have not been strongly related to reading or to reading difficulties [12]. In addition, there have been efforts to train the cerebellum through exercises, with claims that reading and cerebellar functions improved [9, 13]. These findings have been controversial because of questions about how the children with dyslexia were identified and the methods used to train the children and evaluate outcomes [14]. It is not clear why implicit learning as mediated by the cerebellum would be involved in decoding difficulties as opposed to problems with automaticity associated with fluent reading, especially given the role of the cerebellum in automaticity [15].
Cerebellar Anatomy in Adults with Dyslexia
Purkinje cells in the medial areas of the posterior cerebellar cortex, which receive information from peri- and intra-oral structures, were found to be significantly larger in the brain specimens of individuals with dyslexia a postmortem study of dyslexia [16]. Anatomical MRI studies have yielded inconsistent findings. Leonard et al. [17] and Rae et al. [18] found leftward asymmetry of the anterior lobe of the cerebellum in adults with dyslexia, which they hypothesized was due to a reduction in the volume of the right anterior lobe. Additional support for cerebellar anomalies has been found in posterior and medial regions [19, 20]. Other studies have found no differences in cerebellar grey matter volume between adults with and without dyslexia [21–23], while Laylock et al. [23] found that the group with dyslexia had a larger volume of white matter in both cerebellar hemispheres.
Results from the adult literature are variable and highlight differences in participant selection and image analysis methods. First, the studies varied with respect to age of participants, gender composition, and inclusion criteria. While some studies required that participants meet criteria for dyslexia as set forth in the Diagnostic and Statistical Manual of Mental Disorders, fourth ed. [19, 21] or International Classification of Diseases [22], others included participants that only had a familial history of dsylexia and demonstrated no normative deficits [20]. Second, some studies used manual segmentation approaches [17] while others used automated methods for volumetric analysis [20–23].
Cerebellar Anatomy in Children with Dyslexia
One of the first studies of children with dyslexia was conducted to replicate cerebellar findings in college students [17, 24]. Sagittal images (1.2 mm) were acquired using a 1.5 Tesla scanner in 18 children in grades 4–6 with at least a standard deviation discrepancy in their prorated verbal IQ and at least one reading or spelling measure. The volume of the anterior lobe of the cerebellum was estimated from manual tracings of a single mid-sagittal slice as a proxy. Participants with dyslexia had a smaller right anterior cerebellar lobe, right and left pars triangularis, and whole brain volume. The right anterior cerebellar lobe and right pars triangularis made unique contributions in predicting group membership even with cerebral volume and asymmetry included in the regression [24].
Noting potential weaknesses in image analysis methodology, this group conducted another analysis to determine if an automated approach using voxel-based morphometry (VBM) would identify anatomical differences in the right anterior cerebellar lobe and right and left pars triangularis in the same group of children [25]. The VBM approach revealed grey and white matter estimates for the right anterior cerebellar lobe that were smaller for the group with dyslexia. However, these findings were no longer statistically significant after correcting for multiple comparisons.
Based on previous findings, Leonard, Eckert, Given, Berninger, and Eden [26] developed and utilized a quantitative anatomical risk index (ARI) in an attempt to predict behavioral profiles in a heterogeneous sample of children with reading and language impairments. The index was based on an asymmetry profile of the planum temporale, anterior cerebellar lobe, cerebral volume, and surface area of Heschl’s gyrus. Children were categorized as having a positive or negative index, where a positive index score represented children with more leftward asymmetry of the planum temporale and anterior cerebellar lobe. Twenty-two children 11–16 years old identified by the school system as having reading and language impairments were scanned using a 1.5 Tesla scanner and 1 mm thick slices. Blind raters traced every fourth sagittal image for cerebral hemispheres and every slice for cerebellar hemispheres. While children with negative risk indices had more deficits in general and were more likely to have deficits in both expressive and receptive oral language, children with positive risk indices (relatively smaller volume of the anterior lobe of the cerebellum) were more likely to have word reading deficits with comprehension relatively spared. Notably, there was no relation between rapid naming speed and anterior cerebellar lobe measures or the anatomical risk index, a relation one might expect if the cerebellar hypothesis were correct [26].
Kibby and Hynd [27] examined cerebellum morphology and its relation to cognition in a heterogeneous group of children between the ages of 8 and 12 years with dyslexia and attention-deficit/hyperactivity disorder. An MRI was obtained with a 0.6 Tesla scanner with 3.1 mm slices. The cerebellar hemispheres were manually traced on the coronal plane using every other slice. The group of typical readers displayed greater “rightward asymmetry” (greater volume in the right cerebellar hemisphere relative to the left cerebellar hemisphere) than those with dyslexia [27]. In this group, cerebellar hemisphere volume was associated with phonological awareness (Reversals) and short-term memory (Digit Span), while the anterior vermis was correlated with phonological awareness (both Reversals and Elision). Rapid naming speed was also related to asymmetry. In the children with dyslexia, rapid naming errors correlated with left and right hemispheric volumes of the cerebellum [27].
Rationale and Hypotheses
Based on this body of research, it appears that reduced volume in the right anterior lobe of the cerebellum may be reliably identified in heterogeneous groups of reading impaired children relative to the volume of the same region in typical readers. Similarly, typical readers ought to demonstrate more robust rightward cerebellar asymmetry. These findings are consistent with functional MRI research in which the right hemisphere of the cerebellum has been linked to the speed–accuracy trade off in cue-dependent decision-making [28]. Additionally, the anterior portion of the cerebellum is associated with precise motor function and timing [29]. The finding that measures of phonological awareness, rapid naming (although not consistently), and short-term memory are associated with this differential pattern of regional volume and asymmetry between children with and without reading impairment is compelling and lends support to the cerebellar hypothesis. However, the strength of the associations appears to vary based on the imaging analysis methodology and how the groups of children with dyslexia were identified. No study has focused on children with the hallmark characteristic of dyslexia (word decoding and spelling difficulties) with an approach to segmentation of the entire cerebellum.
We used cerebellar segmentation methods outlined by Pierson et al. [30] that were further modified by Juranek et al. [29]. These methods utilized both contemporary terminology and surface-landmark-based identification of two-dimensional slices developed exclusively for MR images [31]. We also included a group of children with reading impairments characterized by accurate but slow decoding speed because of the possibility that automaticity deficits would be more likely mediated by the cerebellum.
If the cerebellar theory is correct, children with decoding problems would be expected to differ from children with reading fluency problems and typical readers on at least some facets of cerebellar anatomy. Available research suggests that children with dyslexia should exhibit (a) no statistically significant differences in cerebellar grey matter volume but increased white matter volume relative to typically developing children, (b) decreased cerebellar asymmetry in regions primarily comprised of grey matter (right–left hemisphere volume)/total volume), and (c) reduced volume in the right anterior lobe of the cerebellum. An alternative hypothesis is that differences will be apparent in children with reading fluency deficits even when decoding is accurate but not necessarily in children with word reading/decoding difficulties or dyslexia. This alternative hypothesis reflects the view that cerebellar abnormalities are more related to the automaticity of word-reading.
Methods
Participants
Participants in this study were part of a large-scale, middle-school reading intervention study [32]. A subset of 53 children volunteered for the MRI component of the study. These children completed a structural MRI at baseline prior to intervention as part of their participation in the functional magnetoencephalography imaging study [33]. From these children, three groups were formed. Children in the group with decoding impairment (dyslexia) [N=23 (11, female (f); 12, male (m)), mean age=13.7 years] scored below the 26th percentile on a measure of single-word decoding. Most of these children also had measureable fluency deficits. Children in the group with fluency impairment [N=8 (6 f. 2 m), mean age=13.7] scored above the 25th percentile on the decoding measure but below the 26th percentile on the fluency measure. Typically, developing children [N=16 (5 f. 11 m), mean age=11.7] did not show impairment on either measure and had no history of reading difficulties. The 25th percentile was selected because it has been commonly employed in studies of people with learning disabilities [34].
All children had a composite IQ score of at least 70 on the Kaufman Brief Intelligence Test-second (KBIT-2) edition [35] or Stanford-Binet Intelligence Scales-4 [36]. IQ-discrepancy definitions were not used because of the lack of evidence of validity of these methods in comparisons of cognitive [37] and neuroimaging [38] correlates. Handedness information was obtained based on the Edinburgh handedness procedure, which requires the child to demonstrate how they would perform different manual tasks. Four non-right-handed participants were included in the analysis (two with dyslexia and two typical readers). Because these subsamples were too small for parametric analysis, their structural data were examined on a case-by-case basis and was consistent with that of right-handed participants in the study. The study was conducted with approval from the Institutional Review Board of the University of Houston and University of Texas Health Science Center at Houston Committees for the Protection of Human Subjects, and all participants were provided with the approved informed consent.
Table 1 provides a summary of group characteristics on IQ and reading measures. A set of planned t tests revealed that, on average, the typical readers were younger than the children with dyslexia by 1.96 years, t (1, 37)=4.06, p<0.001, and younger than the children with fluency impairments by 1.95 years, t (1, 22)=2.70, p<0.05. The IQ differences were expected based on previous literature in which the average IQ of children with dyslexia is lower than normative expectations, presently viewed more as a consequence of underlying cognitive difficulties related to both reading achievement and IQ [2, 38]. The reading differences are consistent with how the groups were formed.
Table 1.
Descriptive data (standard scores)
| Full-scale IQ
|
Verbal IQ
|
Nonverbal IQ
|
Letter–word identification
|
TOWRE fluency
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Dyslexia N=23 (11 f. 12 m) | 88 | 11 | 85 | 10 | 93 | 16 | 79 | 10 | 83 | 13 |
| Fluency N=8 (6 f. 2 m) | 100 | 10 | 98 | 12 | 101 | 6 | 96 | 5 | 85 | 4 |
| Typical N=16 (5f, 11 m) | 104 | 11 | 100 | 13 | 109 | 17 | 111 | 14 | 97 | 16 |
A subset of these children received the Stanford Binet and Woodcock Johnson-III Reading Fluency measures instead of the Kaufman Brief Intelligence Test-2 and Test of Word Reading Efficiency (TOWRE) Fluency
Measures
Decoding
Word reading accuracy was assessed with the Woodcock-Johnson III Letter-Word Identification (LWI). This measures has been widely used in studies of dyslexia [34] and has excellent reliability (LWI r=0.918) [39].
Fluency
A composite score combining the Sight Word Efficiency (SWE) and Phonemic Decoding Efficiency (PDE) subtests from the Test of Word Reading Efficiency (TOWRE) was used to assess real and pseudowords. Reliability for these measures is also excellent (SWE r=0.91, PDE r=0.90, total WRE r=0.93) [40]. A portion of the group of typical readers with archival data received the Reading Fluency measure of the Woodcock Johnson-III [39].
Intelligence
The KBIT-2 is an individually administered intellectual function measure used primarily for descriptive purposes. The Matrices subtest was administered pre-intervention, and Verbal Knowledge subtest was administered post-intervention and prorated for the verbal domain score. The reliability of the Verbal (r=0.90) and Nonverbal (r=0.86) scores is high among children and adolescents aged 4–18 years and excellent for the IQ Composite among ages 10 to 18 years (r=0.93) [35]. The subgroup of typically developing children with archival data received the Stanford-Binet Intelligence Scales-4; reliabilities range from 0.94 to 0.96 for children under age 17 years [41].
MRI Acquisition
T1-weighted MR images were collected on a 3 T Philips Intera system with an eight-channel SENSE (Sensitivity Encoding) technology head coil. Images were acquired in the coronal plane with voxel dimensions of 0.9375×0.9375×1 mm and a matrix of 256×256. Repetition time was either 8.5 or 8.6 ms; echo time was either 3.9 or 4.0 ms; flip angle was 6.0 degrees.
MR Image Analysis
The methods for analyzing the acquired MR images, obtaining cerebellum parcellation units, and co-localization of anatomical landmarks were based on the procedures outline in Juranek et al. [29], although some modifications were necessary. All MRI analysis procedures were performed by a rater blind to the adolescent’s group membership. Although not usually problematic in T1 sequences, all scans were checked for motion artifacts, and none were excluded from analysis.
Cerebellar Parcellation Units
A four-compartment model (one WM and three principally GM) was used to parcellate the cerebellum into the following regions: corpus medullare, anterior lobe, superior–posterior lobe, and inferior–posterior lobe. As described by Pierson et al. [30], each cerebellar parcellation unit was defined according to the following anatomical features: (a) corpus medullare: central white matter and output nuclei; (b) anterior lobe: lobules I–V, bounded by the most posterior point of the fourth ventricle, corpus medullare, and primary fissure; (c) superior–posterior lobe: lobe VI and crus I of VIIA, bounded by the primary fissure, corpus medullare, and horizontal fissure; and (d) inferior–posterior lobe: crus II of VIIA, VIIB, VIII, IX, and X, bounded by the most posterior point of the fourth ventricle, corpus medullare, and horizontal fissure. Localization of anatomical landmarks in each individual brain was guided by the cerebellum atlas published by Schmahmann et al. [31]. Nevertheless, inter-individual variation in cerebellar topography (e.g., fissures and lobes) was preserved as no spatial transformations to a standardized template were implemented. This procedure was completed for both the left and right cerebellar regions to obtain separate volumetric data for each region by hemisphere. Figure 1a–c illustrates the masks created for each of these regions.
Fig. 1.
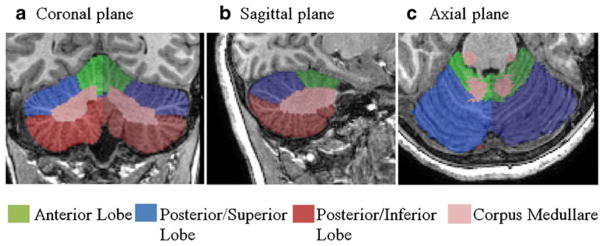
a–c Parcellation units
Co-localization of Anatomical Landmarks and Manual Fissure Tracings
Primary and horizontal fissures were manually delineated in the plane that optimized our ability to follow the entire trajectory of each fissure (e.g., sagittal plane for horizontal fissure and para-sagittal and axial planes for primary fissure). Each fissure marker was readily visible in all three cardinal planes, independent of the plane selected for tracing. This process facilitated the manual tracing of the boundaries of each parcellation unit which was then assigned to a new unique mask overlying the T1-weighted image.
Statistical Analyses
Analysis of covariance (ANCOVA) was utilized to test each of the hypotheses, controlling for age, gender, and/or cerebellar volume as needed. To test the first hypothesis that cerebellar WM volume, but not GM volume, is greater for the group with impaired decoding, the models had a single between-subjects factor (group) with three levels (typical readers, decoding impairment, and fluency impairment) and a single dependent variable (cerebellar WMV and GMV, respectively).
To test the second hypothesis that asymmetry in cerebellar GM volume would be greater in the typical readers relative to the group with dyslexia, an estimate of asymmetry was obtained by summing raw volume for each of the hemispheric regions primarily comprised of grey matter and calculating a difference [(right hemisphere−left hemisphere)/total volume] score for each participant. This model also had a single between-subjects factor (group) with the three levels mentioned above and a single dependent variable (difference score).
Finally, a mixed-model ANOVA was used to test the third hypothesis that the group with decoding impairments (dyslexia) would demonstrate reduced volume in the right anterior lobe of the cerebellum relative to typical readers. The model included a single between-subjects factor (group) and two within-subjects factor (hemisphere and region).
Results
Table 2 presents descriptive statistics for estimated volumes of each cerebellar region of interest by group. Gender was moderately correlated to total cerebellar volume, r=0.41, p<0.01. Males had larger cerebellar volumes than females, t (1, 45)=−3.00, p<0.01. Age, r=−0.31, p<0.05, was moderately related to the asymmetry index and to the left anterior lobe of the cerebellum, r=−0.36, p<0.05, although not to our primary region of interest (the right anterior lobe), r=−0.18, p>0.05.
Table 2.
Neuroanatomical means and standard deviations by group (cubic millimeters)
| Dyslexia (n=23)
|
Fluency (n=8)
|
Typical (n=16)
|
|||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Anterior | Left | 7,302.03 | 1,578.94 | 8,188.20 | 1,509.85 | 9,271.35 | 2,224.31 |
| Right | 7,587.96 | 1,768.88 | 8,216.09 | 2,839.27 | 8,913.58 | 1,872.23 | |
| Superior | Left | 23,555.56 | 3,286.17 | 23,748.65 | 5,659.04 | 23,435.78 | 2,918.17 |
| Right | 23,638.17 | 3,013.26 | 24,765.26 | 5,312.05 | 22,878.27 | 3,971.77 | |
| Inferior | Left | 31,039.45 | 3,928.25 | 30,494.56 | 6,253.00 | 33,166.14 | 4,086.74 |
| Right | 30,648.53 | 3,961.52 | 30,175.87 | 6,112.59 | 32,223.13 | 4,638.29 | |
| WMV | Total | 18,219.92 | 1,901.68 | 19,134.17 | 3,548.31 | 19,156.88 | 1,123.98 |
| GMV | Total | 123,771.69 | 12,163.12 | 125,588.64 | 25,971.20 | 129,888.26 | 14,314.87 |
White matter volume includes volume from right and left corpus medullare. Grey matter volume includes volume from right and left anterior lobe and the superior and inferior regions of the posterior lobe
Hypothesis 1: Cerebellar Grey and White Matter Volumes
ANCOVA, controlling for gender, revealed no statistically significant differences in total cerebellar volume among children with decoding impairments, fluency impairments, and typically developing children, F (2, 41)=0.78, p>0.05, η2=0.03. Additionally, no significant group differences were noted in total cerebellar grey matter volume, F (2, 41)=0.57, p>0.05, η2=0.02 or cerebellar white matter volume, F (2, 41)=0.90, p>0.05, η2=0.04, again controlling for gender.
Hypothesis 2: Index of Asymmetry
Results of the anatomical asymmetry index are summarized in Table 3. No statistically significant differences were found in degree of asymmetry among the three groups controlling for age and gender, F (2, 41)<1, p>0.05. The proportion of variance accounted for by group was small, η2=0.02, indicating that the null result was not due to insufficient power.
Table 3.
Cerebellar asymmetry (cubic millimeters)
| N | Raw asymmetry
|
Asymmetry index
|
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Decoding | 23 | 22.3783 | 1,528.9300 | 0.0002 | 0.0127 |
| Fluency | 8 | −725.7952 | 1,551.1900 | −0.0049 | 0.0127 |
| Typical | 16 | 1,858.2900 | 3,491.7200 | 0.0154 | 0.0304 |
Raw asymmetry scores were obtained by subtracting left from right GMV. The asymmetry index is a standardized difference score calculated with the following formula: (R GMV−L GMV)/total GMV
Hypothesis 3: Regional Variation by Group
A mixed-model ANOVA using data corrected for cerebellar volume did not demonstrate the hypothesized group× region×hemisphere interaction, F (4, 88)<1. The group by hemisphere interaction was also not statistically significant, F (2, 44)<1. However, the group by region interaction was significant, F (4, 88)=5.04, p<0.01. The group with decoding impairment demonstrated an unexpected reduction in both anterior regions of the cerebellum, not the hypothesized right anterior difference. In addition, the volume difference tended to be greater in the left than right anterior lobe (Table 2).
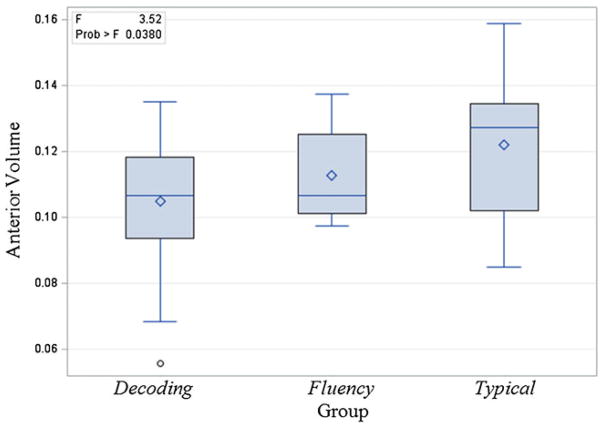
Linear regression analyzing anterior lobe volume by group demonstrated a statistically significant difference between the three groups in anterior lobe volume, F (2, 44)=3.52, p<0.05, η2=0.14 as presented in Fig. 2. Pairwise contrasts using a Tukey’s adjustment for multiple comparisons demonstrated a statistically significant difference only between the decoding impaired children and typical readers, F (1, 38)=7.04, p<0.05, η2=0.14.
Fig. 2.
Distribution of volume (corrected for cerebellar volume) in anterior lobe of cerebellum by group
Post hoc analyses
In post hoc analyses, it was predicted that anterior lobe volume would account for performance on a decoding measure despite group structure. Pearson correlations of regional volumes corrected for cerebellar volume are summarized in Table 4. As predicted, these analyses revealed a significant relation between LWI and the anterior lobe of the cerebellum, r=0.34, p<0.05 and right anterior region of the cerebellum, r=0.41, p<0.05. The regression analysis controlling for age was not statistically significant F (1, 44)=3.27, p>0.05, but the effect size was moderate, η2=0.07.
Table 4.
Pearson correlates of behavioral measures and cerebellar regions
| All groups
|
Decoding
|
Fluency
|
Typical
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| LWI | Fluency | LWI | Fluency | LWI | Fluency | LWI | Fluency | ||
| Anterior | 0.3443* | 0.2012 | −0.19915 | −0.21399 | 0.27107 | 0.51019 | 0.3198 | 0.2163 | |
| Left | 0.2160 | 0.1625 | −0.23758 | −0.11175 | 0.38768 | 0.45723 | 0.2406 | 0.1721 | |
| Right | 0.4113* | 0.2044 | −0.12724 | −0.30831 | −0.07839 | 0.38892 | 0.3244 | 0.2118 | |
| Superior–posterior | −0.0005 | −0.0041 | −0.19428 | −0.19738 | 0.1657 | −0.29637 | 0.2056 | 0.0588 | |
| Left | −0.2105 | −0.2898* | −0.20411 | −0.21629 | 0.09523 | −0.3389 | 0.2878 | 0.0899 | |
| Right | −0.2098 | −0.2228 | −0.17168 | −0.16629 | 0.22918 | −0.24601 | 0.0224 | −0.0031 | |
| Inferior–posterior | 0.0074 | 0.1721 | 0.36007 | 0.41847 | −0.04637 | −0.37451 | −0.4388 | −0.2488 | |
| Left | −0.0286 | 0.1556 | 0.4071* | 0.4755* | −0.23947 | −0.54993 | −0.3734 | −0.1882 | |
| Right | 0.0426 | 0.1680 | 0.27645 | 0.31915 | 0.11648 | −0.18562 | −0.4731 | −0.2990 | |
| Asymmetry index | 0.1933 | 0.1179 | 0.02099 | −0.27107 | 0.11415 | 0.1333 | −0.1204 | −0.0551 | |
| GMV | 0.2501 | 0.1565 | 0.27023 | 0.26886 | 0.7953* | −0.34554 | 0.0034 | −0.0453 | |
| WMV | −0.0005 | −0.0041 | −0.02744 | −0.16543 | −0.47753 | 0.56479 | 0.0089 | 0.0905 | |
| Total volume | 0.2597 | 0.1633 | 0.27656 | 0.2617 | 0.7749* | −0.30471 | 0.0018 | −0.0422 | |
Fluency scores include those of both the TOWRE and WJ Fluency subsets
p<0.05
Within-group correlations revealed a statistically significant correlation between the left inferior–posterior lobe of the cerebellum and LWID, r=0.41, p<0.05 and Fluency measures r=0.48, p<0.05 in the group of children with decoding deficits. In the group of children with fluency deficits, statistically significant correlations were identified between LWID and grey matter volume r=0.80, p<0.05, and total cerebellar volume, r=0.77, p<0.05 respectively. No within group correlations were found between the behavioral reading measures and anatomical regions of the cerebellum in typically developing children.
Discussion
The cerebellar theory of dyslexia has been proposed as an explanation for the diverse impairments observed in individuals with dyslexia, suggesting that cerebellar abnormalities contribute to ineffective procedural learning of both word reading and motor skills. This study examined the hypothesis at three levels of morphology: total cerebellar grey and white matter volume, degree of asymmetry, and regional variability among children with dyslexia, those with fluency impairments, and typically developing children. Results suggested that children with dyslexia did not differ significantly from typically developing children in grey matter volume or degree of cerebellar asymmetry. However, there was a significant bilateral reduction in the anterior lobe of the cerebellum in those with decoding impairments. Little evidence was found in support of an alternative hypothesis that the group of children with fluency impairment would represent a unique subgroup group of children with cerebellar abnormalities.
Cerebellar Grey Matter and White Matter Volumes
These results were consistent with those of Menghini et al. [21], who found no general structural reductions in the grey matter of adults with dyslexia. They were also consistent with those of Raschle et al. [42], who found no differences in cerebellar volume among pre-reading children with a family history of dyslexia. Interpretation of these data is limited by the lack of information available on how children were identified with dyslexia. Many studies of cerebellar morphology in dyslexia fail to report data on total cerebellar grey matter volume, although most studies accounted for individual variations in cerebellar volume in their analysis. The only statistically significant difference in grey matter volume in the present study was found with regard to sex, with males having larger cerebellar volume than females. When controlling for this variable, no statistically significant differences were noted. Results were consistent with those of Rae et al. [18], who found white matter to be symmetrical between groups of adult males with and without a history of dyslexia; they were inconsistent with those of Laylock et al. [23], who reported that adult males with dyslexia demonstrated larger bilateral white matter volumes than controls.
Index of Asymmetry
No group differences were found with respect to the asymmetry index. This finding was inconsistent with previously published studies in children [27] and adults [17, 18]. The Kibby and Rae studies, however, were limited by the image resolution (3.1 and 5 mm slices, respectively). Interpretation of this finding is tempered by the fact that hemispheric differences can be accounted for by regional variations in volume rather than global differences in hemispheric volume. Whether asymmetry continues to be a useful index in the future will depend on whether measurements of asymmetry provide information above and beyond that of known regional correlates.
Regional Volume
These findings were inconsistent with the findings of Eckert et al. [24] and Leonard et al. [17], who studied children and college students with reading disabilities, respectively. These studies reported reduced right anterior lobe volume in individuals with dyslexia relative to typically developing individuals using a manual tracing technique similar to that employed in this study. In contrast, the present study found a statistically significant reduction in bilateral anterior lobe volume. Our results are difficult to compare with Leonard et al. [26], who utilized a positive risk index defined by leftward asymmetry of the anterior lobe among other variables to evaluate relations with poor word reading skills in a heterogeneous group of children with reading and specific language impairment that may not have met criteria for dyslexia.
Interestingly, the results of the present study complement Juranek et al. [29]. They found that after correcting for total cerebellum volume, and relative to a group of children with typical development, the anterior lobe was significantly enlarged in children with spina bifida meningomyelocele, who commonly present with strong development of word reading skills and preservation of vocabulary and grammar [43]. The anterior lobe is also associated with regions of the cortex related to motor function. These findings are consistent with the hypothesis that cerebellar deficits may underlie early speech problems and subsequent difficulty with phonological processing [16, 44].
Relations to Behavioral Data
Based on the previous findings, it was hypothesized that (removing the group structure) word reading would be associated with the right anterior cerebellum. This hypothesis was supported and found to be consistent with previous research on children with dyslexia. Eckert et al. [24] found that the right anterior lobe measurement significantly correlated with real word reading, pseudoword reading, spelling, and rapid-automatic naming. We did not find a relation between word reading fluency and the anterior lobe. Rather, we found a correlation between word reading fluency and the superior–posterior lobe of the cerebellum. For the decoding group, both the decoding and fluency measures were related to the inferior–posterior lobe of the cerebellum.
These findings are notable because posterior regions have been linked to prefrontal and association cortices of the brain [45] which have an active role in language and memory [46]. Enlarged Purkinje cells have also been found in the medial regions of the posterior lobe in adults with dyslexia in postmortem studies [16]. Although our results did not find any macrostructural differences in posterior regions of the cerebellum between children with dyslexia and typical readers, these findings may highlight an important opportunity to study regional microstructural changes in the cerebellum.
Limitations
The manual tracing approach utilized in the present study and some of the aforementioned studies have potential limitations, including difficulty replicating findings among research groups because of subtle and sometimes arbitrary differences in designated boundaries of certain regions [24]. A shortcoming of morphometric cerebellar research in general is that highly variable terminology and definitions of regional boundaries often prevented direct comparisons of grey and white matter volume differences altogether. This study addressed these limitations by using well-defined, anatomical landmarks found in the atlas published by Schmahmann et al. [31]. However, comparisons could not be reliably made between studies that reported various cerebellar abnormalities in the semilunar lobules [19], cerebellar nuclei [20], cerebellar declive, and right lentiform nucleus [22].
A further limitation of the manual tracing technique is that regions that are not included in the a priori analysis and that may contribute to the understanding of the theory or construct under investigation may be overlooked. A limitation of this present study is that no cortical regions were analyzed in relation to cerebellar abnormalities. Because these data are available, relating cerebellar abnormalities to known cortical markers of dyslexia represents an important opportunity for future investigation.
Like many imaging studies, one of the limiting factors of the present study is sample size. Due to our small sample size, power was likely insufficient to detect some of the more subtle differences in regional cerebellar anatomy. Unfortunately, the group of children with fluency impairments was particularly small (n=8) and likely contributed to the lack of unique findings for this group. Although there was no evidence from the effect size data of large differences, the stepwise pattern of group differences with the fluency-impaired group between the decoding-impaired and the typical group is an interesting observation. This pattern may be consistent with the idea of fluency impairments representing a less severe form of dyslexia characterized by lack of automaticity as opposed to accuracy and is often seen as children improve in proficiency after intervention [2]. It may be worthwhile to replicate this study with a larger sample and additional children that meet criteria for fluency impairments alone.
Additionally, the discrepancy in ages between the group of impaired children and the younger typically developing children may mask the effects of development. The development of the anterior lobe of the cerebellum has an inverted U-shaped trajectory with age at peak volume at 13.5 years for girls and 15.7 years for boys [47]. With a slightly older group of children with dyslexia, therefore, one might expect to see increased anterior lobe volume due simply to the effects of maturation. Not only was this increase in volume not observed, but the volume of the right anterior lobe was smaller in the older group of children with dyslexia. Despite this unexpected finding, age, gender, and total cerebellar volume were used as covariates where appropriate to address discrepancies in group structure. Due to late maturation and sexual dimorphism noted in cerebellar development, care should be taken to conduct age and sex matching in future studies.
Lastly, atypical lateralization of language is sometimes seen in non-right-handers. Although no major volumetric differences were observed in comparing the right-handed and non-right-handed children, it is possible that their inclusion masked more robust findings in the analysis of asymmetry.
Future Directions
Future research should investigate connectivity of the right anterior cerebellum with cortical regions typically associated with reading including the inferior frontal gyrus, temporal–parietal regions, and medial occipital lobe [5]. Laylock et al. [23] suggested that the changes in white matter they detected in addition to an abnormal ratio of metabolites in the right cerebellar hemisphere may indicate either excessive connectivity or abnormal myelination. In a diffusion tensor imaging DTI study, Richards et al. [48] found greater fractional anisotropy in the left and right cerebellum in a group of individuals with dyslexia relative to typical readers. This study was limited by lack of regional segmentation, but it represents an important area for future research. In addition, relations with reading and other cognitive skills should be evaluated. If the cerebellar theory of dyslexia is correct, there should be an association between cerebellar anatomy and the procedural learning of cognitive tasks rather than simple motor tasks.
Conclusion
Results of this study suggest that after correcting for total cerebellum volume children with impaired single-word decoding, as compared with typically developing children and children with reading fluency impairment alone, demonstrate reduced volume bilaterally in the anterior lobe of the cerebellum. Interpretation of these finding is limited by the emerging literature on specific functions of the cerebellum at a regional level. Evidence that the cerebellum is relevant for cognitive function above and beyond motor timing, precision, and balance continues to grow, particularly in the direction of motor learning.
Acknowledgments
This research was supported by grant P50 HD052117 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
Footnotes
Conflict of interest The authors of this manuscript (Vindia G. Fernandez, Karla K. Stuebing, Jenifer Juranek, and Jack M. Fletcher) declare that they have no conflict of interest to disclose.
Contributor Information
Vindia G. Fernandez, Email: vgfernandez@uh.edu, Department of Psychology, Texas Medical Center Annex, University of Houston, 2151 W. Holcombe Blvd., Suite 220, Houston, TX 77204-5053, USA
Karla Stuebing, Department of Psychology, Texas Medical Center Annex, University of Houston, 2151 W. Holcombe Blvd., Suite 204, Houston, TX 77204-5053, USA.
Jenifer Juranek, Department of Pediatrics, University of Texas Health Science Center, 6655 Travis, Houston, TX 77030-1312, USA.
Jack M. Fletcher, Department of Psychology, Texas Medical Center Annex, University of Houston, 2151 W. Holcombe Blvd., Suite 222, Houston, TX 77204-5053, USA
References
- 1.Pennington BF. Diagnosing learning disorders: a neuropsychological framework. 2. New York: Guilford Press; 2009. [Google Scholar]
- 2.Fletcher JM, Lyon GR, Fuchs LS, Barnes MA. Learning disabilities. New York. NY: Guilford Press; 2007. [Google Scholar]
- 3.Lyon GR. Educational resources information center. [Online] ReEducational Resources Information Center; 1998. [cited 2010 June 10. Available from: http://www.eric.ed.gov/ERICDocs/data/ericdocs2sql/content_storage_01/0000019b/80/16/61/c9.pdf. [Google Scholar]
- 4.Daniel SS, Walsh AK, Goldstein DB, Arnold EM, Reboussin BA, Wood FB. Suicidality, school dropout, and reading problems among adolescents. J Learn Disabil. 2006;39(6):507–14. doi: 10.1177/00222194060390060301. [DOI] [PubMed] [Google Scholar]
- 5.Eckert M. Neuroanatomical markers for dyslexia: a review of dyslexia structural imaging studies. Neuroscientist. 2004;10(4):362–71. doi: 10.1177/1073858404263596. [DOI] [PubMed] [Google Scholar]
- 6.Denckla MB, Rudel RG, Chapman C, Krieger J. Motor proficiency in dyslexic children with and without attentional disorders. Arch Neurol. 1985;42(3):228–31. doi: 10.1001/archneur.1985.04060030042008. [DOI] [PubMed] [Google Scholar]
- 7.Nicolson RI, Fawcett AJ. Developmental dyslexia, learning, and the cerebellum. J Neural Transm. 2005;(Suppl):1–18. doi: 10.1007/3-211-31222-6_2. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson RI, Fawcett AJ. Procedural learning difficulties: reuniting the developmental disorders? Trends Neurosci. 2007;30(4):135–41. doi: 10.1016/j.tins.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds D, Nicolson RI, Hambly H. Evaluation of an exercise-based treatment for children with reading difficulties. Dyslexia: An Int J Res Pract. 2003;9(1):48–71. doi: 10.1002/dys.235. [DOI] [PubMed] [Google Scholar]
- 10.Nicolson RI, Fawcett AJ. Reaction times and dyslexia. Q J Exp Psychol. 1994;47(1):29–48. doi: 10.1080/14640749408401142. [DOI] [PubMed] [Google Scholar]
- 11.Nicolson RI, Fawcett AJ. Performance of dyslexic children on cerebellar and cognitive tasks. J Mot Behav. 1999;31(1):68–78. doi: 10.1080/00222899909601892. [DOI] [PubMed] [Google Scholar]
- 12.Barth A, Denton C, Stuebing KK, Fletcher JM, Cirino PT, Francis DJ, et al. A test of the cerebellar hypothesis of dyslexia in adequate and inadequate responders to reading intervention. J Int Neuropsychol Soc. 2010;16:525–36. doi: 10.1017/S1355617710000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds D, Nicolson RI. Follow-up of an exercise-based treatment for children with reading difficulties. Dyslexia. 2006 doi: 10.1002/dys.331. [DOI] [PubMed] [Google Scholar]
- 14.Bishop D. Curing dyslexia and attention-deficit hyperactivity disorder by training motor co-ordination: miracle or myth? J Pediatr Child Health. 2007;43:653–5. doi: 10.1111/j.1440-1754.2007.01225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balsters JH, Ramnani N. Cerebellar plasticity and the automation of first-order rules. J Neurosci. 2011;31(6):2305–12. doi: 10.1523/JNEUROSCI.4358-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finch AJ, Nicolson RI, Fawcett AJ. Evidence for a neuroanatomical difference within the olivo-cerebellar pathway of adults with dyslexia. Cortex. 2002;38:529–39. doi: 10.1016/s0010-9452(08)70021-2. [DOI] [PubMed] [Google Scholar]
- 17.Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzier J, Mohr CM, et al. Anatomical risk factors for phonological dyslexia. Cereb Cortex. 2001;11:148–57. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- 18.Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, et al. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40:1285–92. doi: 10.1016/s0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- 19.Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 2001;56:781–3. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- 20.Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, et al. Regional reductions of grey matter volume in familial dyslexia. Neurology. 2004;63:742–5. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- 21.Menghini D, Hagberg GE, Petrosini L, Bozzali M, Macaluso E, Caltagirone C, et al. Structural correlates of implicit learning deficits in subjects with developmental dyslexia. Ann N Y Acad Sci. 2008;1145:212–21. doi: 10.1196/annals.1416.010. [DOI] [PubMed] [Google Scholar]
- 22.Pernet CR, Poline JB, Demonet JF, Rousselet GA. Brain classification reveals the right cerebellum as the best biomarker of dyslexia. Neuroscience. 2009;10(67) doi: 10.1186/1471-2202-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laylock SK, Wilkinson ID, Wallis LI, Darwent G, Wonders SH, Fawcett AJ, et al. Cerebellar volume and cerebellar metabolic characteristics in adults with dyslexia. Ann N Y Acad Sci. 2008;1145:222–36. doi: 10.1196/annals.1416.002. [DOI] [PubMed] [Google Scholar]
- 24.Eckert MA, Leonard CM, Richards TL, Aylward EH, Thompson J, Berninger VW. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126:482–95. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- 25.Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, et al. Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex. 2005;41:304–15. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- 26.Leonard C, Eckert M, Given B, Berninger V, Eden G. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 2006;129:3329–42. doi: 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- 27.Kibby MY, Hynd GW. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. J Child Neurol. 2008;23(4):368–80. doi: 10.1177/0883073807309235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallesi A, McIntosh AR, Crescentini C, Stuss DT. fMRI investigation of speed-accuracy strategy switching. Hum Brain Mapp. 2011;33(7):1677–88. doi: 10.1002/hbm.21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juranek J, Dennis M, Cirino PT, El-Messidi L, Fletcher JM. The cerebellum in children with spina bifida and Chiari II malformation: quantitative volumetrics by region. Cerebellum. 2010;9(2):240–8. doi: 10.1007/s12311-010-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierson R, Corson PW, Sears LL, Alicata D, Magnotta V, O’Leary D, et al. Manual and semiautomated measurement of cerebellar subregions on MR images. NeuroImage. 2002;17:61–76. doi: 10.1006/nimg.2002.1207. [DOI] [PubMed] [Google Scholar]
- 31.Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI atlas of the human cerebellum. San Diego. CA: Academic Press; 2000. [Google Scholar]
- 32.Vaughn S, Cirino PT, Wanzek J, Wexler J, Fletcher JM, Denton CD, et al. Response to intervention for middle school students with reading difficulties: effects of a primary and secondary intervention. Sch Psychol Rev. 2010;39(1):3–21. [PMC free article] [PubMed] [Google Scholar]
- 33.Rezaie R, Simos P, Fletcher J, Cirino P, Vaughn S, Papanicolaou AC. Temporo-parietal brain activity as a longitudinal predictor of response to educational interventions among middle school struggling readers. J Int Neuropsychol Soc. 2011;17:875–85. doi: 10.1017/S1355617711000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fletcher JM, Shaywitz SE, Shankweiler DP, Katz L, Lieberman IY, Stuebing KK, et al. Cognitive profiles of reading disability: comparisons of discrepancy and low achievement definitions. J Educ Psychol. 1994;85:1–18. [Google Scholar]
- 35.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. 2. Circle Pines: AGS Publishing; 2004. [Google Scholar]
- 36.Thorndike RL, Hagen EP, Sattler JM. Technical manual, Stanford-Binet intelligence scale. 4. Chicago: Riverside; 1986. [Google Scholar]
- 37.Stuebing KK, Fletcher JM, LeDoux JM, Lyon GR, Shaywitz SE, Shaywitz BA. Validity of IQ-discrepancy classifications of reading disabilities: a meta-analysis. Am Educ Res J. 2002;39:469–518. [Google Scholar]
- 38.Tanaka H, Black JM, Hulme C, Stanley LM, Kesler SR, Whitfield-Gabrieli S, et al. The brain basis of the phonological deficit in dyslexia is independent of IQ. Psychol Sci. 2011;22(11):1442–51. doi: 10.1177/0956797611419521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodcock RW, Mather N. WJ-R tests of achievement: examiner’s manual. In: Woodcock RW, Johnson MB, editors. Woodcock-Johnson psycho-educational battery-revised. Itasca, IL: Riverside Publishing; 1989, 1990. [Google Scholar]
- 40.Torgesen JK, Wagner R, Rashotte C. Test of word reading efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- 41.Becker KA. History of the Stanford-Binet intelligence scales: content and psychometrics. Itasca: Riverside Publishing; 2003. [Google Scholar]
- 42.Raschle NM, Chang M, Gaab N. Structural brain alterations associated with dyslexia predate reading onset. NeuroImage. 2010 doi: 10.1016/j.neuroimage.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simos PG, Papanicolaou AC, Martinez Castillo E, Cirino PT, Rezaie R, Fletcher JM. Brain mechanisms for reading and language processing in spina bifida meningomyelocele: a combined magnetic source-and structural magnetic resonance imaging study. Neuropsychology. 2011;25(5):590–601. doi: 10.1037/a0023694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fawcett AJ, Nicolson RI, Dean P. Impaired performance of children with dyslexia on a range of cerebellar tasks. Ann Dyslexia. 1996;46:259–83. doi: 10.1007/BF02648179. [DOI] [PubMed] [Google Scholar]
- 45.Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, et al. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6(31) doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrieli JD, Poldrack RA, Desmond JE. The role of the left pre-frontal cortex in language and memory. Proc Natl Acad Sci U S A. 1998;95:906–13. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tiemer H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. NeuroImage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards T, Stevenson J, Crouch LC, Johnson K, Maravilla P, Stock R, et al. Tract-based spatial statistics of diffusion tensor imaging in adults with dyslexia. Am J Neuroradiol. 2008;29(6):1134–9. doi: 10.3174/ajnr.A1007. [DOI] [PMC free article] [PubMed] [Google Scholar]